Fasting as a Therapy in Neurological Disease
Abstract
1. Introduction
2. What Is Fasting?
2.1. Fasting: Origins
2.1.1. Pre-Human Evolutionary Origins of Fasting
2.1.2. Fasting in Human History
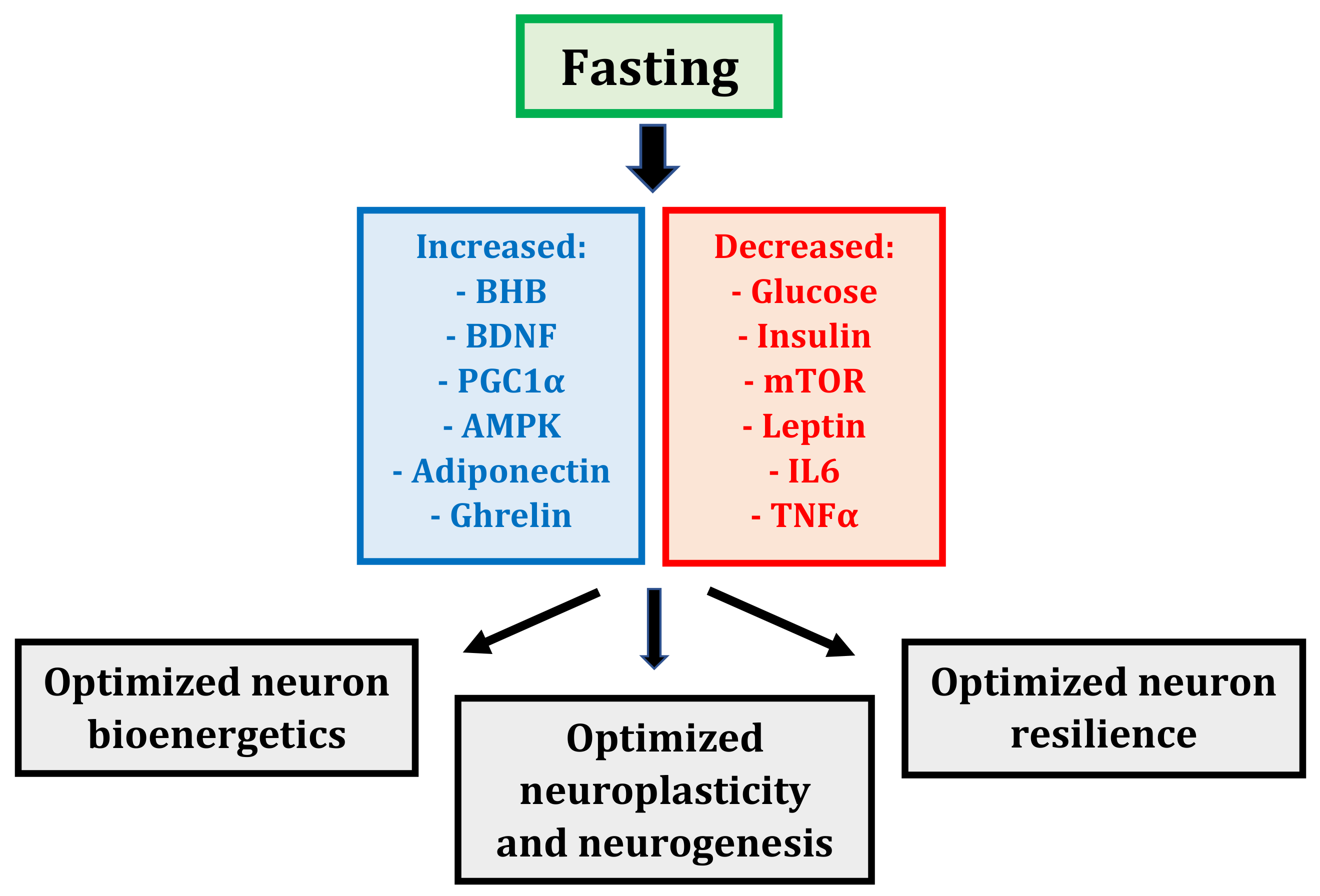
2.2. Fasting: Mechanisms
2.2.1. Fasting: A Whole-Body, Altered Metabolic State
2.2.2. Fasting: More Than Just Calorie Restriction
2.3. Fasting: Regimens
2.3.1. Intensity of the Food and Drink Restriction
2.3.2. Frequency and Duration of the Fasting Periods
3. Evidence Supporting Fasting in Neurological Disease
3.1. Metabolic Syndrome
3.1.1. Fasting as a Therapy in the Metabolic Syndrome: Animal Studies
3.1.2. Fasting as a Therapy in the Metabolic Syndrome: Human Studies
3.2. Cancer
3.2.1. Fasting as a Therapy in Cancer: Animal Studies
3.2.2. Fasting as a Therapy in Cancer: Human Studies
3.3. Neurodegeneration
3.3.1. Fasting as a Therapy in Neurodegeneration: Animal Studies
3.3.2. Fasting as a Therapy in Neurodegeneration: Human Studies
3.4. Stroke
3.4.1. Fasting as a Therapy in Stroke: Animal Studies
3.4.2. Fasting as a Therapy in Stroke: Human Studies
3.5. Epilepsy
3.5.1. Fasting as a Therapy in Epilepsy: Animal Studies
3.5.2. Fasting as a Therapy in Epilepsy: Human Studies
3.6. Multiple Sclerosis
3.6.1. Fasting as a Therapy in Multiple Sclerosis: Animal Studies
3.6.2. Fasting as a Therapy in Multiple Sclerosis: Human Studies
4. Challenges to Implementing Fasting in Neurological Disease
4.1. Potential Contraindications and Adverse Effects of Fasting
4.1.1. Potential Contraindications
4.1.2. Common Adverse Effects
4.1.3. Rare Adverse Effects
4.2. Misconceptions of Fasting
4.2.1. Symptomatic and Metabolic Effects of Fasting Versus Severe Calorie Restriction
4.2.2. Muscle Mass and Exercise Tolerance
4.2.3. Fasting-Induced Insulin Resistance
4.2.4. Compensatory Overeating
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of Intermittent Fasting on Health and Disease Processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Recent Results Cancer Res. 2016, 207, 241–266. [Google Scholar] [PubMed]
- Gonidakis, S.; Finkel, S.E.; Longo, V.D. Genome-wide screen identifies Escherichia coli TCA-cycle related mutants with extended chronological lifespan dependent on acetate metabolism and the hypoxia-inducible transcription factor ArcA. Aging Cell 2010, 9, 868–881. [Google Scholar] [CrossRef]
- Longo, V.D.; Ellerby, L.M.; Bredesen, D.E.; Valentine, J.S.; Gralla, E.B. Human Bcl-2 Reverses Survival Defects in Yeast Lacking Superoxide Dismutase and Delays Death of Wild-Type Yeast. J. Cell Biol. 1997, 137, 1581–1588. [Google Scholar] [CrossRef]
- Longo, V.D.; Shadel, G.; Kaeberlein, M.; Kennedy, B. Replicative and Chronological Aging in Saccharomyces cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef]
- Calixto, A. Life without Food and the Implications for Neurodegeneration. Adv. Genet. 2015, 92, 53–74. [Google Scholar]
- McCue, M.D.; Terblanche, J.S.; Benoit, J.B. Learning to Starve: Impacts of Food Limitation beyond the Stress Period. J. Exp. Biol. 2017, 220, 4330–4338. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Sohal, R.S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age 2012, 34, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Fontan-Lozano, A.; Saez-Cassanelli, J.L.; Inda, M.C.; De los Santos-Arteaga, M.; Sierra-Dominguez, S.A.; Lopez-Lluch, G.; Delgado-Garcia, J.M.; Carrion, A.M. Caloric Restriction Increases Learning Consolidation and Facilitates Synaptic Plasticity through Mechanisms Dependent on NR2B Subunits of the NMDA Receptor. J. Neurosci. 2007, 27, 10185–10195. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.D.; Gross, K.L.; Lowry, S.R. Nutritional and Behavioral Effects of Gorge and Fast Feeding in Captive Lions. J. Appl. Anim. Welf. Sci. 2005, 8, 47–57. [Google Scholar] [CrossRef]
- Crittenden, A.N.; Schnorr, S.L. Current views on hunter-gatherer nutrition and the evolution of the human diet. Am. J. Phys. Anthropol. 2017, 162, 84–109. [Google Scholar] [CrossRef]
- Harari, Y.N. Sapiens: A Brief History of Humankind, 1st ed.; Harper: New York, NY, USA, 2015. [Google Scholar]
- Arbesmann, R. Fasting and Prophecy in Pagan and Christian Antiquity. Traditio 1951, 7, 1–71. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The Influence of Meal Frequency and Timing on Health in Humans: The Role of Fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef]
- Kerndt, P.R.; Naughton, J.L.; Driscoll, C.E.; Loxterkamp, D.A. Fasting: The history, pathophysiology and complications. West. J. Med. 1982, 137, 379–399. [Google Scholar]
- Dewey, E.H. The True Science of Living; The Henry Bill Publishing Company: London, England, 1894. [Google Scholar]
- Buchinger, O. Das Heilfasten; Georg Thieme Verlag: Stuttgart, Germany, 1935. [Google Scholar]
- Longo, V.D. Programmed longevity, youthspan, and juventology. Aging Cell. 2019, 18, e12843. [Google Scholar] [CrossRef]
- World Health Statistics 2018: Monitoring Health for the SDGs. Available online: https://www.who.int/gho/publications/world_health_statistics/2018/en/ (accessed on 12 September 2019).
- Pringsheim, T.; Fiest, K.; Jette, N. The International Incidence and Prevalence of Neurologic Conditions. Neurology 2014, 8, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Korsak, R.A.; Morrow, J.W.; Edmond, J. Fatty Acid Oxidation and Ketogenesis by Astrocytes in Primary Culture. J. Neurochem. 1991, 56, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; Woods, A.; De Ceballos, M.L.; Carling, D.; Guzmán, M. The AMP-Activated Protein Kinase Is Involved in the Regulation of Ketone Body Production by Astrocytes. J. Neurochem. 1999, 73, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Venkatesh, B. Clinical Review: Ketones and Brain Injury. Crit. Care 2011, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Dobson, G.P.; Veech, R.L. The Gibbs–Donnan near-equilibrium system of heart. J. Biol. Chem. 1990, 265, 20321–20334. [Google Scholar]
- Veech, R.L.; Chance, B.; Kashiwaya, Y.; Lardy, H.A.; Cahill, G.F., Jr. Ketone Bodies, Potential Therapeutic Uses. IUBMB Life 2001, 51, 241–247. [Google Scholar]
- Murray, A.J.; Knight, N.S.; Cole, M.A.; Cochlin, L.E.; Carter, E.; Tchabanenko, K.; Pichulik, T.; Gulston, M.K.; Atherton, H.J.; Schroeder, M.A.; et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016, 30, 4021–4032. [Google Scholar] [CrossRef]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor. Science 2013, 339, 211–214. [Google Scholar]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1 and Mitochondrial Metabolism—Emerging Concepts and Relevance in Ageing and Neurodegenerative Disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Lin, J.; Krauss, S.; Tarr, P.T.; Yang, R.; Newgard, C.B.; Spiegelman, B.M. Bioenergetic Analysis of Peroxisome Proliferator-Activated Receptor γ Coactivators 1α and 1β (PGC-1α and PGC-1β) in Muscle Cells. J. Biol. Chem. 2003, 278, 26597–26603. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Eisentraut, A.M.; Madison, L.L. The Effects of Total Starvation Upon the Levels of Circulating Glucagon and Insulin in Man. J. Clin. Investig. 1963, 42, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Veldhuis, J.D.; Johnson, M.L.; Furlanetto, R.; Evans, W.S.; Alberti, K.G.; Thorner, M.O. Fasting Enhances Growth Hormone Secretion and Amplifies the Complex Rhythms of Growth Hormone Secretion in Man. J. Clin. Investig. 1988, 81, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.E.; Katz, A.; Spencer, M.K.; Yan, Z.; Nyomba, B.L. Fasting Inhibits Insulin-Mediated Glycolysis and Anaplerosis in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 1991, 261, E598–E605. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-Day Fasting in Nonobese Subjects: Effects on Body Weight, Body Composition, and Energy Metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef]
- Thissen, J.P. Nutritional Regulation of the Insulin-like Growth Factors. Endocr. Rev. 1994, 15, 80–101. [Google Scholar]
- Merimee, T.J.; Fineberg, S.E. Growth Hormone Secretion in Starvation: A Reassessment. J. Clin. Endocrinol. Metab. 1974, 39, 385–386. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Antunes, F.; Erustes, A.; Costa, A.; Nascimento, A.; Bincoletto, C.; Ureshino, R.; Pereira, G.; Smaili, S. Autophagy and Intermittent Fasting: The Connection for Cancer Therapy? Clinics 2018, 73 (Suppl. 1), e814s. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.; Scherer, P. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Baatar, D.; Patel, K.; Taub, D.D. The effects of ghrelin on inflammation and the immune system. Mol. Cell Endocrinol. 2011, 340, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.; Kim, C.; Sato, T.; Kojima, M.; Park, S. Ghrelin is required for dietary restriction-induced enhancement of hippocampal neurogenesis: Lessons from ghrelin knockout mice. Endocr. J. 2015, 62, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.V.; Phillips, T.M.; Cheng, A.; Morrell, C.H.; Mattson, M.P.; Wan, R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010, 67, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Aksungar, F.B.; Topkaya, A.E.; Akyildiz, M. Interleukin-6, C-Reactive Protein and Biochemical Parameters during Prolonged Intermittent Fasting. Ann. Nutr. Metab. 2007, 51, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Degan, D.; Ornello, R.; Tiseo, C.; Carolei, A.; Sacco, S.; Pistoia, F. The Role of Inflammation in Neurological Disorders. Curr. Pharm. Des. 2018, 24, 1485–1501. [Google Scholar] [CrossRef]
- Weindruch, R. The Retardation of Aging by Caloric Restriction: Studies in Rodents and Primates. Toxicol. Pathol. 1996, 24, 742–745. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; De Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent Fasting Dissociates Beneficial Effects of Dietary Restriction on Glucose Metabolism and Neuronal Resistance to Injury from Calorie Intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The Effects of Intermittent or Continuous Energy Restriction on Weight Loss and Metabolic Disease Risk Markers: A Randomized Trial in Young Overweight Women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The Effect of Intermittent Energy and Carbohydrate Restriction v. Daily Energy Restriction on Weight Loss and Metabolic Disease Risk Markers in Overweight Women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/MTOR Pathway Is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Castets, P.; Lin, S.; Rion, N.; Di Fulvio, S.; Romanino, K.; Guridi, M.; Frank, S.; Tintignac, L.A.; Sinnreich, M.; Rüegg, M.A. Sustained Activation of MTORC1 in Skeletal Muscle Inhibits Constitutive and Starvation-Induced Autophagy and Causes a Severe, Late-Onset Myopathy. Cell Metab. 2013, 17, 731–744. [Google Scholar] [CrossRef]
- Ramamurthy, S.; Chang, E.; Cao, Y.; Zhu, J.; Ronnett, G. AMPK activation regulates neuronal structure in developing hippocampal neurons. Neuroscience 2014, 259, 13–24. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Bloomer, R.J. The Impact of Religious Fasting on Human Health. Nutr. J. 2010, 9, 57. [Google Scholar] [CrossRef]
- Runcie, J.; Thomson, T.J. Prolonged starvation - A dangerous procedure? Br. Med. J. 1970, 3, 432–435. [Google Scholar] [CrossRef]
- Stewart, W.K.; Fleming, L.W. Features of a successful therapeutic fast of 382 days’ duration. Postgrad. Med. J. 1973, 49, 203–209. [Google Scholar] [CrossRef]
- Wilhelmi de Toledo, F.; Buchinger, A.; Burggrabe, H.; Hölz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lützner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting Therapy - an Expert Panel Update of the 2002 Consensus Guidelines. Forsch Komplementmed. 2013, 20, 434–443. [Google Scholar] [CrossRef]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic Use of Intermittent Fasting for People with Type 2 Diabetes as an Alternative to Insulin. BMJ Case Rep. 2018, 2018, bcr-2017-221854. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of Intermittent Fasting on Body Composition and Clinical Health Markers in Humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Di Germanio, C.; Bernier, M.; de Cabo, R. A Time to Fast. Science 2018, 362, 770–775. [Google Scholar] [CrossRef]
- Drenick, E.J.; Swendseid, M.E.; Blahd, W.H.; Tuttle, S.G. Prolonged Starvation as Treatment for Severe Obesity. JAMA 1964, 187, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Thomson, T.J.; Runcie, J.; Miller, V. Treatment of obesity by total fasting for up to 249 days. Lancet 1966, 2, 992–996. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic Syndrome as a Risk Factor for Neurological Disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Grundy, S.M.; Hansen, B.; Smith, S.C., Jr.; Cleeman, J.I.; Kahn, R.A.; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: Report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler. Thromb. Vasc Biol. 2004, 24, e19–e24. [Google Scholar]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-Restricted Feeding and Risk of Metabolic Disease: A Review of Human and Animal Studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef]
- Goodrick, C.L.; Ingram, D.K.; Reynolds, M.A.; Freeman, J.R.; Cider, N.L. Differential Effects of Intermittent Feeding and Voluntary Exercise on Body Weight and Lifespan in Adult Rats. J. Gerontol. 1983, 38, 36–45. [Google Scholar] [CrossRef]
- Wan, R.; Camandola, S.; Mattson, M.P. Intermittent Food Deprivation Improves Cardiovascular and Neuroendocrine Responses to Stress in Rats. J. Nutr. 2003, 133, 1921–1929. [Google Scholar] [CrossRef]
- Pedersen, C.R.; Hagemann, I.; Bock, T.; Buschard, K. Intermittent Feeding and Fasting Reduces Diabetes Incidence in BB Rats. Autoimmunity 1999, 30, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, J.D.; Verpeut, J.L.; Yeomans, B.L.; Yang, J.A.; Yasrebi, A.; Roepke, T.A.; Bello, N.T. Intermittent Fasting Promotes Fat Loss With Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology 2016, 157, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Belkacemi, L.; Selselet-Attou, G.; Hupkens, E.; Nguidjoe, E.; Louchami, K.; Sener, A.; Malaisse, W.J. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int. J. Endocrinol. 2012, 2012, 962012. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmer, C.; Zarrinpar, A.; Di Tacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Mager, D.E.; Wan, R.; Brown, M.; Cheng, A.; Wareski, P.; Abernethy, D.R.; Mattson, M.P. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006, 20, 631–637. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects—A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 17, 4. [Google Scholar] [CrossRef]
- Kul, S.; Savaş, E.; Öztürk, Z.A.; Karadağ, G. Does Ramadan Fasting Alter Body Weight and Blood Lipids and Fasting Blood Glucose in a Healthy Population? A Meta-Analysis. J. Relig. Health 2014, 53, 929–942. [Google Scholar] [CrossRef]
- Sadeghirad, B.; Motaghipisheh, S.; Kolahdooz, F.; Zahedi, M.J.; Haghdoost, A.A. Islamic fasting and weight loss: A systematic review and meta-analysis. Public Health Nutr. 2014, 17, 396–406. [Google Scholar] [CrossRef]
- Varady, K.A. Intermittent versus Daily Calorie Restriction: Which Diet Regimen Is More Effective for Weight Loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef]
- Schübel, R.; Nattenmüller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of Intermittent and Continuous Calorie Restriction on Body Weight and Metabolism over 50 Wk: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.M. Studies Concerning Diabetes. JAMA 1914, LXIII, 939. [Google Scholar] [CrossRef]
- Gilliland, I.C. Total Fasting in the Treatment of Obesity. Postgrad. Med. J. 1968, 44, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.M.D.; Mckiddie, M.; Buchanan, K. Effect of Fasting on Glucose and Insulin Metabolism of Obese Patients. Lancet 1969, 293, 285–287. [Google Scholar] [CrossRef]
- Williams, K.V.; Mullen, M.L.; Kelley, D.E.; Wing, R.R. The Effect of Short Periods of Caloric Restriction on Weight Loss and Glycemic Control in Type 2 Diabetes. Diabetes Care 1998, 21, 2–8. [Google Scholar] [CrossRef]
- Halberg, N.; Henriksen, M.; Söderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of Intermittent Fasting and Refeeding on Insulin Action in Healthy Men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef]
- Eshghinia, S.; Mohammadzadeh, F. The Effects of Modified Alternate-Day Fasting Diet on Weight Loss and CAD Risk Factors in Overweight and Obese Women. J. Diabetes Metab. Disord. 2013, 12, 4. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate Day Fasting for Weight Loss in Normal Weight and Overweight Subjects: A Randomized Controlled Trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef]
- Goldhamer, A.; Lisle, D.; Parpia, B.; Anderson, S.V.; Campbell, T. Medically Supervised Water-Only Fasting in the Treatment of Hypertension. J. Manip. Physiol. Ther. 2001, 24, 335–339. [Google Scholar] [CrossRef]
- Goldhamer, A.C.; Lisle, D.J.; Sultana, P.; Anderson, S.V.; Parpia, B.; Hughes, B.; Campbell, T.C. Medically Supervised Water-Only Fasting in the Treatment of Borderline Hypertension. J. Altern. Complement. Med. 2002, 8, 643–650. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. Ueber den stoffwechsel der tumoren. Biochem. Z. 1924, 152, 319–344. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Epstein, T.; Gatenby, R.A.; Brown, J.S. The Warburg Effect as an Adaptation of Cancer Cells to Rapid Fluctuations in Energy Demand. PLoS ONE 2017, 12, e0185085. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H. Nutrition Needs of Mammalian Cells in Tissue Culture. Science 1955, 122, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.R.; Thompson, C.B. Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a Metabolic Disease: Implications for Novel Therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Hursting, S.D.; Dunlap, S.M.; Ford, N.A.; Hursting, M.J.; Lashinger, L.M. Calorie Restriction and Cancer Prevention: A Mechanistic Perspective. Cancer Metab. 2013, 1, 10. [Google Scholar] [CrossRef]
- O’Flanagan, C.H.; Smith, L.A.; McDonell, S.B.; Hursting, S.D. When Less May Be More: Calorie Restriction and Response to Cancer Therapy. BMC Med. 2017, 15, 106. [Google Scholar] [CrossRef]
- Rous, P. The Influence of Diet on Transplanted and Spontaneous Mouse Tumors. J. Exp. Med. 1914, 20, 433–451. [Google Scholar] [CrossRef]
- Lv, M.; Zhu, X.; Wang, H.; Wang, F.; Guan, W. Roles of Caloric Restriction, Ketogenic Diet and Intermittent Fasting during Initiation, Progression and Metastasis of Cancer in Animal Models: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e115147. [Google Scholar] [CrossRef]
- Colman, R.J.; Anderson, R.M.; Johnson, S.C.; Kastman, E.K.; Kosmatka, K.J.; Beasley, T.M.; Allison, D.B.; Cruzen, C.; Simmons, H.A.; Kemnitz, J.W.; et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science 2009, 325, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci. Transl. Med. 2012, 4, 124ra127. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.C.; Grossmann, M.E. The Manner in Which Calories Are Restricted Impacts Mammary Tumor Cancer Prevention. J. Carcinog. 2011, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Cleary, M.P.; Jacobson, M.K.; Phillips, F.C.; Getzin, S.C.; Grande, J.P.; Maihle, N.J. Weight-cycling decreases incidence and increases latency of mammary tumors to a greater extent than does chronic caloric restriction in mouse mammary tumor virus-transforming growth factor-alpha female mice. Cancer Epidemiol. Biomark. Prev. 2002, 11, 836–843. [Google Scholar]
- Cleary, M.P.; Hu, X.; Grossmann, M.E.; Juneja, S.C.; Dogan, S.; Grande, J.P.; Maihle, N.J. Prevention of mammary tumorigenesis by intermittent caloric restriction: Does caloric intake during refeeding modulate the response? Exp. Biol. Med. 2007, 232, 70–80. [Google Scholar]
- Rogozina, O.P.; Bonorden, M.J.L.; Grande, J.P.; Cleary, M.P. Serum Insulin-like Growth Factor-I and Mammary Tumor Development in Ad libitum–Fed, Chronic Calorie–Restricted, and Intermittent Calorie–Restricted MMTV-TGF-α Mice. Cancer Prev. Res. 2009, 2, 712–719. [Google Scholar] [CrossRef]
- Magee, B.A.; Potezny, N.; Rofe, A.M.; Conyers, R.A. The Inhibition of Malignant Cell Growth by Ketone Bodies. Aust. J. Exp. Biol. Med. Sci. 1979, 57, 529–539. [Google Scholar] [CrossRef]
- Zhou, W.; Mukherjee, P.; Kiebish, M.A.; Markis, W.T.; Mantis, J.G.; Seyfried, T.N. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr. Metab. 2007, 4, 5. [Google Scholar] [CrossRef]
- Fine, E.J.; Miller, A.; Quadros, E.V.; Sequeira, J.M.; Feinman, R.D. Acetoacetate Reduces Growth and ATP Concentration in Cancer Cell Lines Which Over-Express Uncoupling Protein 2. Cancer Cell Int. 2009, 9, 14. [Google Scholar] [CrossRef]
- Siegel, I.; Liu, T.L.; Nepomuceno, N.; Gleicher, N. Effects of Short-Term Dietary Restriction on Survival of Mammary Ascites Tumor-Bearing Rats. Cancer Investig. 1988, 6, 677–680. [Google Scholar] [CrossRef]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a Diet Very High in Vegetables, Fruit, and Fiber and Low in Fat on Prognosis Following Treatment for Breast Cancer: The Women’s Healthy Eating and Living (WHEL) Randomized Trial. JAMA 2007, 298, 289. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Zuccoli, G.; Marcello, N.; Pisanello, A.; Servadei, F.; Vaccaro, S.; Mukherjee, P.; Seyfried, T.N. Metabolic Management of Glioblastoma Multiforme Using Standard Therapy Together with a Restricted Ketogenic Diet: Case Report. Nutr. Metab. 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, A.M.A.; Bary, M.A.; Abdelzaher, E.; Elnaggar, M.; Kalamian, M.; Mukherjee, P.; Seyfried, T.N. Management of Glioblastoma Multiforme in a Patient Treated with Ketogenic Metabolic Therapy and Modified Standard of Care: A 24-Month Follow-Up. Front. Nutr. 2018, 5, 20. [Google Scholar] [CrossRef]
- Safdie, F.M.; Dorff, T.; Quinn, D.; Fontana, L.; Wei, M.; Lee, C.; Cohen, P.; Longo, V.D. Fasting and Cancer Treatment in Humans: A Case Series Report. Aging 2009, 1, 988–1007. [Google Scholar] [CrossRef]
- de Groot, S.; Vreeswijk, M.P.; Welters, M.J.; Gravesteijn, G.; Boei, J.J.; Jochems, A.; Houtsma, D.; Putter, H.; van der Hoeven, J.J.; Nortier, J.W.; et al. The Effects of Short-Term Fasting on Tolerance to (Neo) Adjuvant Chemotherapy in HER2-Negative Breast Cancer Patients: A Randomized Pilot Study. BMC Cancer 2015, 15, 652. [Google Scholar] [CrossRef]
- Dorff, T.B.; Groshen, S.; Garcia, A.; Shah, M.; Tsao-Wei, D.; Pham, H.; Cheng, C.; Brandhorst, S.; Cohen, P.; Wei, M.; et al. Safety and Feasibility of Fasting in Combination with Platinum-Based Chemotherapy. BMC Cancer 2016, 16, 360. [Google Scholar] [CrossRef]
- Onyango, I.G. Mitochondria in the Pathophysiology of Alzheimer’s and Parkinson’s Diseases. Front. Biosci. 2017, 22, 854–872. [Google Scholar] [CrossRef]
- Schapira, A.H.; Cooper, J.; Dexter, D.; Jenner, P.; Clark, J.; Marsden, C. Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef]
- Borghammer, P.; Chakravarty, M.; Jonsdottir, K.Y.; Sato, N.; Matsuda, H.; Ito, K.; Arahata, Y.; Kato, T.; Gjedde, A. Cortical Hypometabolism and Hypoperfusion in Parkinson’s Disease Is Extensive: Probably Even at Early Disease Stages. Brain Struct. Funct. 2010, 214, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, S. Oxidative Energy Metabolism in Alzheimer Brain. Studies in Early-Onset and Late-Onset Cases. Mol. Chem. Neuropathol. 1992, 16, 207–224. [Google Scholar] [CrossRef]
- De la Monte, S.M. Type 3 Diabetes Is Sporadic Alzheimer’s Disease: Mini-Review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.; Zuo, Z. Chronic Intermittent Fasting Improves Cognitive Functions and Brain Structures in Mice. PLoS ONE 2013, 8, e66069. [Google Scholar] [CrossRef] [PubMed]
- Kuhla, A.; Lange, S.; Holzmann, C.; Maass, F.; Petersen, J.; Vollmar, B.; Wree, A. Lifelong Caloric Restriction Increases Working Memory in Mice. PLoS ONE 2013, 8, e68778. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Bakshi, V.; Lin, A. Early Shifts of Brain Metabolism by Caloric Restriction Preserve White Matter Integrity and Long-Term Memory in Aging Mice. Front. Aging Neurosci. 2015, 7, 213. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Talani, G.; Licheri, V.; Biggio, F.; Locci, V.; Mostallino, M.C.; Secci, P.P.; Melis, V.; Dazzi, L.; Carta, G.; Banni, S.; et al. Enhanced Glutamatergic Synaptic Plasticity in the Hippocampal CA1 Field of Food-Restricted Rats: Involvement of CB1 Receptors. Neuropsychopharmacology 2016, 41, 1308–1318. [Google Scholar] [CrossRef]
- Duan, W.; Guo, Z.; Jiang, H.; Ware, M.; Li, X.; Mattson, M.P. Dietary Restriction Normalizes Glucose Metabolism and BDNF Levels, Slows Disease Progression, and Increases Survival in Huntingtin Mutant Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 2911–2916. [Google Scholar] [CrossRef]
- Duan, W.; Mattson, M.P. Dietary Restriction and 2-Deoxyglucose Administration Improve Behavioral Outcome and Reduce Degeneration of Dopaminergic Neurons in Models of Parkinson’s Disease. J. Neurosci. Res. 1999, 57, 195–206. [Google Scholar] [CrossRef]
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.; Yan, S.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Umberger, G.; McFall, R.; Mattson, M.P. Food Restriction Reduces Brain Damage and Improves Behavioral Outcome Following Excitotoxic and Metabolic Insults. Ann. Neurol. 1999, 45, 8–15. [Google Scholar] [CrossRef]
- Halagappa, V.K.M.; Guo, Z.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent Fasting and Caloric Restriction Ameliorate Age-Related Behavioral Deficits in the Triple-Transgenic Mouse Model of Alzheimer’s Disease. Neurobiol. Dis. 2007, 256, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson Disease with Diet-Induced Hyperketonemia: A Feasibility Study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Murtagh, D.K.; Gilbertson, L.J.; Asztely, F.J.; Lynch, C.D. Low-Fat versus Ketogenic Diet in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and Efficacy Data from a Ketogenic Diet Intervention in Alzheimer’s Disease. Alzheimers Dement. 2018, 4, 28–36. [Google Scholar] [CrossRef]
- Castellano, C.; Nugent, S.; Paquet, N.; Tremblay, S.; Bocti, C.; Lacombe, G.; Imbeault, H.; Turcotte, É; Fulop, T.; Cunnane, S.C. Lower Brain 18F-Fluorodeoxyglucose Uptake but Normal 11C-Acetoacetate Metabolism in Mild Alzheimer’s Disease Dementia. J. Alzheimers Dis. 2015, 43, 1343–1353. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Yu, Z.F.; Mattson, M.P. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: Evidence for a preconditioning mechanism. J. Neurosci. Res. 1999, 57, 830–839. [Google Scholar] [CrossRef]
- Manzanero, S.; Erion, J.R.; Santro, T.; Steyn, F.J.; Chen, C.; Arumugam, T.V.; Stranahan, A.M. Intermittent Fasting Attenuates Increases in Neurogenesis after Ischemia and Reperfusion and Improves Recovery. J. Cereb. Blood Flow Metab. 2014, 34, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Roberge, M.; Messier, C.; Staines, W.; Plamondon, H. Food Restriction Induces Long-Lasting Recovery of Spatial Memory Deficits Following Global Ischemia in Delayed Matching and Non-Matching-to-Sample Radial Arm Maze Tasks. Neuroscience 2008, 156, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.M.; Pauly, J.R.; Readnower, R.D.; Rho, J.M.; Sullivan, P.G. Fasting Is Neuroprotective Following Traumatic Brain Injury. J. Neurosci. Res. 2008, 86, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Plunet, W.T.; Streijger, F.; Lam, C.K.; Lee, J.H.; Liu, J.; Tetzlaff, W. Dietary restriction started after spinal cord injury improves functional recovery. Exp. Neurol. 2008, 213, 28–35. [Google Scholar] [CrossRef]
- Prins, M.L.; Lee, S.M.; Fujima, L.S.; Hovda, D.A. Increased Cerebral Uptake and Oxidation of Exogenous BetaHB Improves ATP Following Traumatic Brain Injury in Adult Rats. J. Neurochem. 2004, 909, 666–672. [Google Scholar] [CrossRef]
- Fisher, R.S.; Boas, W.V.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic Seizures and Epilepsy: Definitions Proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Landgrave-Gómez, J.; Mercado-Gómez, O.F.; Vázquez-García, M.; Rodríguez-Molina, V.; Córdova-Dávalos, L.; Arriaga-Ávila, V.; Miranda-Martínez, A.; Guevara-Guzmán, R. Anticonvulsant Effect of Time-Restricted Feeding in a Pilocarpine-Induced Seizure Model: Metabolic and Epigenetic Implications. Front. Cell. Neurosci. 2016, 10, 296. [Google Scholar] [CrossRef]
- Yum, M.S.; Ko, T.; Kim, D.W. Anticonvulsant Effects of β-Hydroxybutyrate in Mice. J. Epilepsy Res. 2012, 2, 29–32. [Google Scholar] [CrossRef]
- Yum, M.S.; Ko, T.; Kim, D.W. β-Hydroxybutyrate Increases the Pilocarpine-Induced Seizure Threshold in Young Mice. Brain Dev. 2012, 34, 181–184. [Google Scholar] [CrossRef]
- Kim, J.M. Ketogenic Diet: Old Treatment, New Beginning. Clin. Neurophysiol. Pract. 2017, 2, 161–162. [Google Scholar] [CrossRef]
- Guelpa, G.; Marie, A. A lutte contre l’epilepsie par la desintoxication et par la reduction altimentaire. Rev. Ther. Med. Chir. 1911, 78, 8–13. [Google Scholar]
- Hartman, A.L.; Rubenstein, J.E.; Kossoff, E.H. Intermittent Fasting: A ‘New’ Historical Strategy for Controlling Seizures? Epilepsy Res. 2013, 104, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Ketonemia and Seizures: Metabolic and Anticonvulsant Effects of Two Ketogenic Diets in Childhood Epilepsy. Pediatr. Res. 1976, 10, 536–540. [Google Scholar] [CrossRef] [PubMed]
- van Delft, R.; Lambrechts, D.; Verschuure, P.; Hulsman, J.; Majoie, M. Blood Beta-Hydroxybutyrate Correlates Better with Seizure Reduction Due to Ketogenic Diet than Do Ketones in the Urine. Seizure 2010, 19, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple Sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Steinman, L.; Zamvil, S.S. Virtues and Pitfalls of EAE for the Development of Therapies for Multiple Sclerosis. Trends Immunol. 2005, 26, 565–571. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235. [Google Scholar] [CrossRef]
- Choi, I.Y.; Piccio, L.; Childress, P.; Bollman, B.; Ghosh, A.; Brandhorst, S.; Suarez, J.; Michalsen, A.; Cross, A.H.; Morgan, T.E.; et al. Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 2016, 15, 2136–2146. [Google Scholar] [CrossRef]
- Müller, H.; Wilhelmi de Toledo, K. Fasting Followed by Vegetarian Diet in Patients with Rheumatoid Arthritis: A Systematic Review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.; Dixit, V.D.; Pearson, M.; Nassar, M.; Maudsley, S.; Carlson, O.; et al. Alternate Day Calorie Restriction Improves Clinical Findings and Reduces Markers of Oxidative Stress and Inflammation in Overweight Adults with Moderate Asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Huen, S.C.; Luan, H.H.; Yu, S.; Zhang, C.; Gallezot, J.; Booth, C.J.; Medzhitov, R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell 2016, 166, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent Fasting in Type 2 Diabetes Mellitus and the Risk of Hypoglycaemia: A Randomized Controlled Trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Finnell, J.S.; Saul, B.C.; Goldhamer, A.C.; Myers, T.R. Is Fasting Safe? A Chart Review of Adverse Events during Medically Supervised, Water-Only Fasting. BMC Complement. Altern. Med. 2018, 18, 67. [Google Scholar] [CrossRef]
- Sigler, M.H. The Mechanism of the Natriuresis of Fasting. J. Clin. Investig. 1975, 55, 377–387. [Google Scholar] [CrossRef]
- Spencer, I.O.B. Death during Therapeutic Starvation for Obesity. Lancet 1968, 291, 1288–1290. [Google Scholar] [CrossRef]
- Cubberley, P.T.; Polster, S.A.; Schulman, C.L. Lactic Acidosis and Death after the Treatment of Obesity by Fasting. N. Engl. J. Med. 1965, 272, 628–630. [Google Scholar] [CrossRef]
- Duncan, G.G.; Jenson, W.K.; Cristofori, F.C.; Schless, G.L. Intermittent fasts in the correction and control of intractable obesity. Am. J. Med. Sci. 1963, 245, 515–520. [Google Scholar] [CrossRef]
- Keys, A.; Brozek, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L. The Biology of Human Starvation; University of Minnesota Press: Minneapolis, MN, USA, 1950. [Google Scholar]
- Benedict, F.G.; Miles, W.R.; Roth, P.; Smith, H.M. Human Vitality and Efficiency under a Prolonged Restricted Diet; Carnegie Institute: Washington DC, USA, 1919. [Google Scholar]
- Schneeweiss, B.; Schoder, M.; Graninger, W.; Roth, E.; Fischer, M.; Lenz, K. Increased Energy Expenditure and Protein Catabolic Rate in Early Starvation. Clin. Nutr. 1991, 10, 8. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; Maclean, P.S.; Melanson, E.L.; Troy Donahoo, W. A Randomized Pilot Study Comparing Zero-Calorie Alternate-Day Fasting to Daily Caloric Restriction in Adults with Obesity: Alternate-Day Fasting Versus Caloric Restriction. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef]
- Zauner, C.; Schneeweiss, B.; Kranz, A.; Madl, C.; Ratheiser, K.; Kramer, L.; Roth, E.; Schneider, B.; Lenz, K. Resting Energy Expenditure in Short-Term Starvation Is Increased as a Result of an Increase in Serum Norepinephrine. Am. J. Clin. Nutr. 2000, 71, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Effect of Starvation and Very Low Calorie Diets on Protein-Energy Interrelationships in Lean and Obese Subjects. Available online: http://archive.unu.edu/unupress/food2/UID07E/UID07E11.HTM (accessed on 12 September 2019).
- Elia, M.; Lammert, O.; Zed, C.; Neale, G. Energy Metabolism during Exercise in Normal Subjects Undergoing Total Starvation. Hum. Nutr. Clin. Nutr. 1984, 38, 355–362. [Google Scholar] [PubMed]
- Nair, K.S.; Woolf, P.D.; Welle, S.L.; Matthews, D.E. Leucine, Glucose, and Energy Metabolism after 3 Days of Fasting in Healthy Human Subjects. Am. J. Clin. Nutr. 1987, 46, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Faber, P.; Lara, J.; Gibney, E.R.; Milne, E.; Ritz, P.; Lobley, G.E.; Elia, M.; Stubbs, R.J.; Johnstone, A.M. Imposed Rate and Extent of Weight Loss in Obese Men and Adaptive Changes in Resting and Total Energy Expenditure. Metabolism 2015, 64, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Højlund, K.; Wildner-Christensen, M.; Eshøj, O.; Skjærbæk, C.; Holst, J.J.; Koldkjær, O.; Jensen, D.M.; Beck-Nielsen, H. Reference Intervals for Glucose, β-Cell Polypeptides, and Counterregulatory Factors during Prolonged Fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 280, e5–e58. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J. Neuroendocrine responses to starvation and weight loss. N. Engl. J. Med. 1997, 336, 1802–1811. [Google Scholar] [CrossRef]
- Chaston, T.B.; Dixon, J.B.; O’Brien, P.B. Changes in Fat-Free Mass during Significant Weight Loss: A Systematic Review. Int. J. Obes. 2007, 31, 743–750. [Google Scholar] [CrossRef]
- Soenen, S.; Martens, E.A.; Hochstenbach-Waelen, A.; Lemmens, S.G.; Westerterp-Plantenga, M.S. Normal Protein Intake Is Required for Body Weight Loss and Weight Maintenance, and Elevated Protein Intake for Additional Preservation of Resting Energy Expenditure and Fat Free Mass. J. Nutr. 2013, 143, 591–596. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Varady, K.A. Alternate Day Fasting and Endurance Exercise Combine to Reduce Body Weight and Favorably Alter Plasma Lipids in Obese Humans: Alternate Day Fasting and Exercise for Weight Loss. Obesity 2013, 21, 1370–1379. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-Restricted Feeding in Young Men Performing Resistance Training: A Randomized Controlled Trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hoeks, J.; van Herpen, N.A.; Mensink, M.; Moonen-Kornips, E.; van Beurden, D.; Hesselink, M.K.; Schrauwen, P. Prolonged Fasting Identifies Skeletal Muscle Mitochondrial Dysfunction as Consequence Rather Than Cause of Human Insulin Resistance. Diabetes 2010, 59, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Jacquet, J.; Girardier, L. Poststarvation Hyperphagia and Body Fat Overshooting in Humans: A Role for Feedback Signals from Lean and Fat Tissues. Am. J. Clin. Nutr. 1997, 65, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Doucet, É.; Cameron, J. Appetite Control after Weight Loss: What Is the Role of Bloodborne Peptides? Appl. Physiol. Nutr. Metab. 2007, 32, 523–532. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Faber, P.; Gibney, E.; Elia, M.; Horgan, G.; Golden, B.; Stubbs, R. Effect of an Acute Fast on Energy Compensation and Feeding Behaviour in Lean Men and Women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1623–1628. [Google Scholar] [CrossRef]
| Intensity of Food and Drink Restriction | Frequency and Duration of Fasting Periods | Common Combinations Used in Human Studies |
|---|---|---|
| “Pure” fasting (no food or drink, often in the context of religious practices) | Time-restricted feeding (daily four-to-twelve hour eating window) | Water/fluid-only time-restricted feeding |
| Water-only fasting (only water is permitted, plus salt and micronutrients) | Alternate-daily fasting (fasting every other day) | Water/fluid-only alternate-daily fasting |
| Fluid-only fasting (water-only fast plus calorie-free fluids, such as tea and black coffee) | Two-days-per-week fasting (fasting two consecutive days per week) | Limited calorie intake two-days-per-week fasting |
| Limited calorie intake fasting (up to 250–500 kcal per day, via vegetable or bone broths) | Periodic fasting (fasting periods two days to three weeks in duration) | Limited calorie intake periodic fasting |
| Disorder | Evidence in Animals | Evidence in Humans |
|---|---|---|
| Metabolic syndrome (a major risk factor for neurological disease) | Mitigates obesity Improves insulin sensitivity Alleviates hypertension | Mitigates obesity Improves insulin sensitivity Alleviates hypertension |
| Cancer (including cancers that involve the brain) | Probably prevents formation of tumours, and possibly treats established tumours Improves tumour responses to chemotherapy | Ameliorates chemotherapy-related adverse effects May protect normal cells from chemotherapy |
| Neurodegeneration | Improves cognition, and stalls age-related cognitive decline Usually slows neurodegeneration | No direct evidence (only indirect evidence of benefit from ketogenic diets) |
| Stroke | Reduces brain damage Enhances functional recovery | No direct evidence |
| Epilepsy | Probably lessens severity and frequency of seizures | Lessens severity and frequency of seizures |
| Multiple sclerosis | Mitigates pathology and symptoms of experimental autoimmune encephalomyelitis | No direct evidence (only indirect preliminary evidence of benefit from fasting-mimicking diets) |
| Potential Contraindications | Common Adverse Effects |
|---|---|
| People of low body weight | Fatigue |
| Breastfeeding or pregnant women | Insomnia |
| Extremes of age (children, the very old) | Nausea |
| People at high risk of malnutrition | Headache |
| Viral infections | Presyncope |
| Type 1 diabetes | Dyspepsia |
| Renal stones | Back pain |
| Gout | Pain in extremity |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, M.C.L. Fasting as a Therapy in Neurological Disease. Nutrients 2019, 11, 2501. https://doi.org/10.3390/nu11102501
Phillips MCL. Fasting as a Therapy in Neurological Disease. Nutrients. 2019; 11(10):2501. https://doi.org/10.3390/nu11102501
Chicago/Turabian StylePhillips, Matthew C.L. 2019. "Fasting as a Therapy in Neurological Disease" Nutrients 11, no. 10: 2501. https://doi.org/10.3390/nu11102501
APA StylePhillips, M. C. L. (2019). Fasting as a Therapy in Neurological Disease. Nutrients, 11(10), 2501. https://doi.org/10.3390/nu11102501




