Food–Drug Interaction between the Adlay Bran Oil and Drugs in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Adlay Bran Oil
2.3. Animals and Treatment
2.4. Sample Preparation
2.5. HPLC/MS Analysis
2.6. Microsomes Preparation
2.7. CYP Enzyme Activity Assays
2.8. Statistical Analysis
3. Results
3.1. HPLC/MS Chromatograms of the Five CYP Probe Drugs in Rat Plasma
3.2. Single Oral Dose of ABO on the Pharmacokinetic Parameters of the Five Drugs after Intravenous Drug Cocktail Administration in Rats
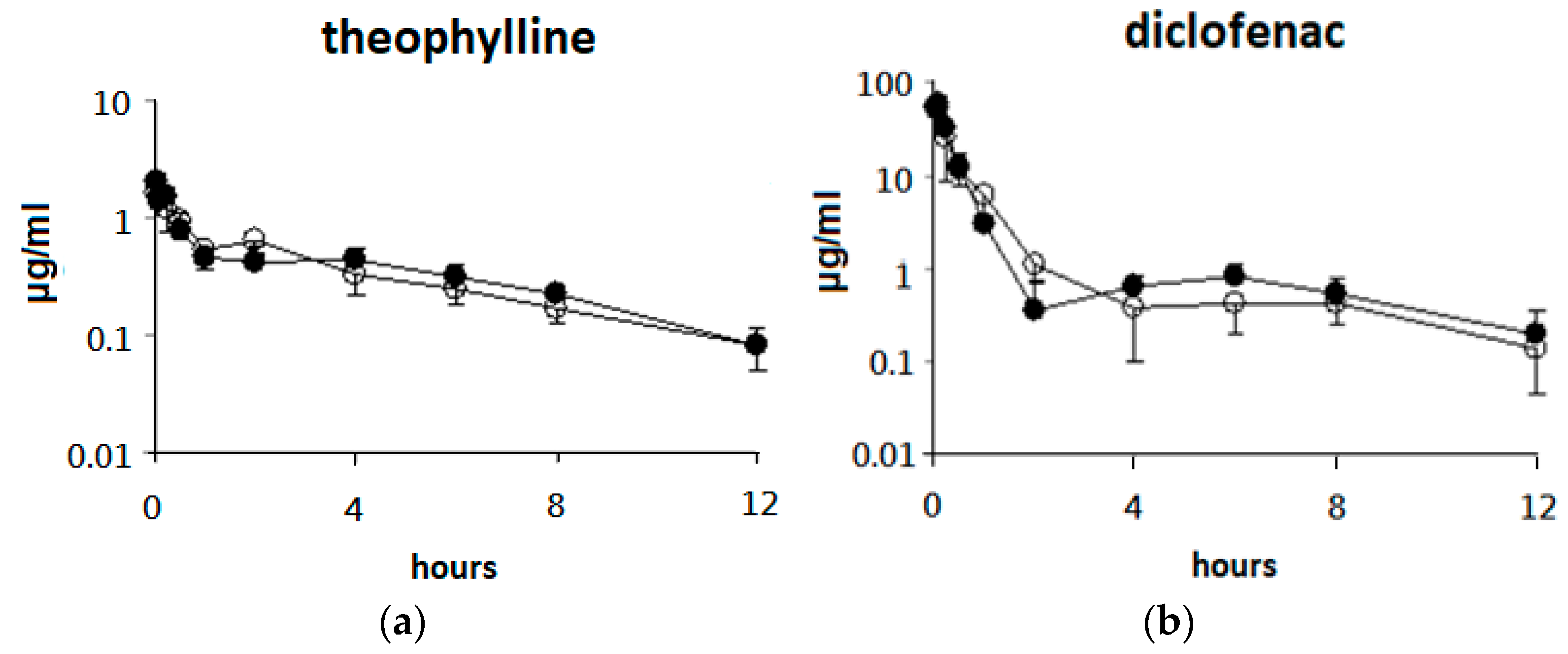
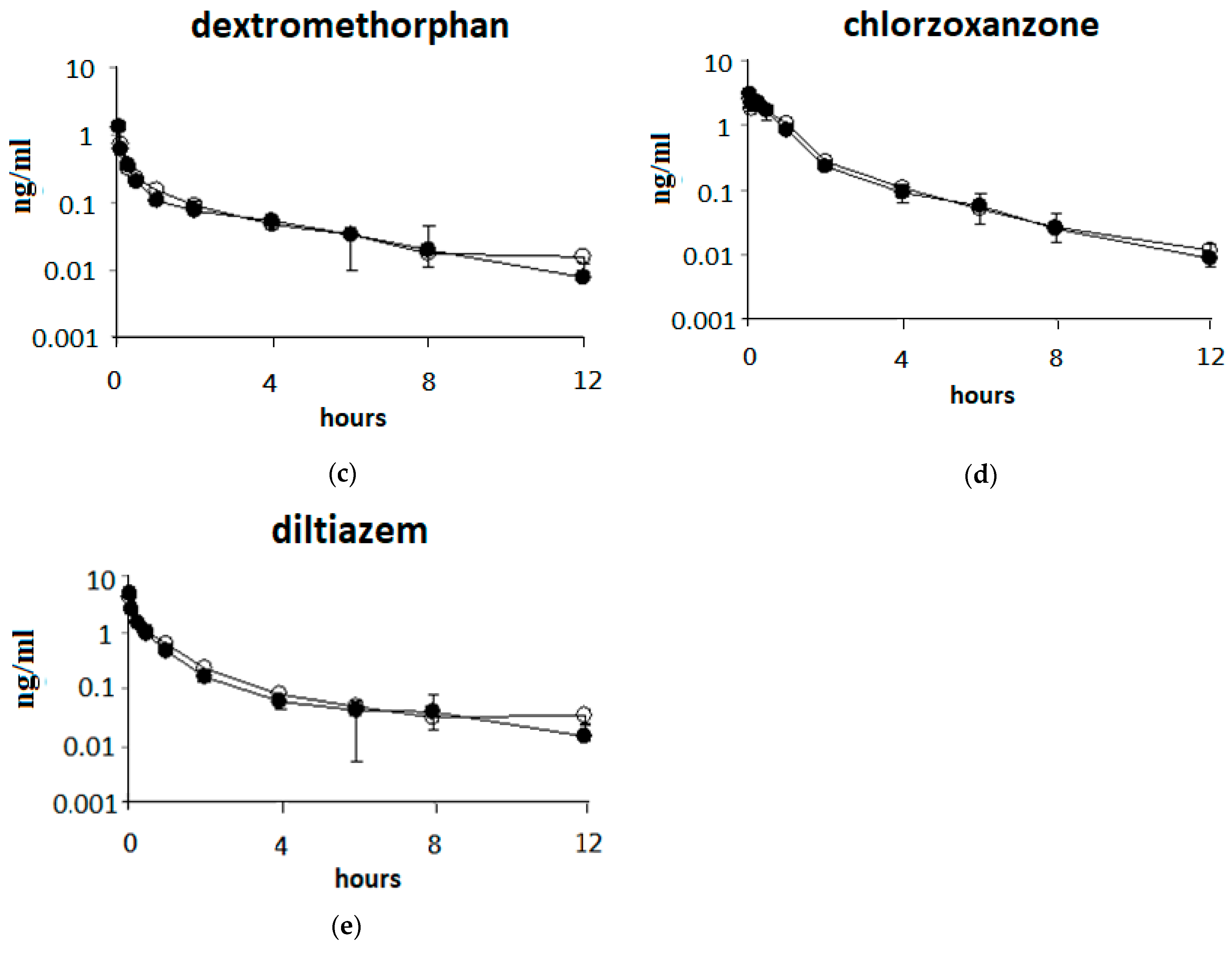
3.3. Single Oral Dose of ABO on the Pharmacokinetic Parameters of Five Drugs after Oral Drug Cocktail Administration in Rats
3.4. Effects of 7 Days of ABO Feeding on the Pharmacokinetic Parameters of Five Drugs after Oral Drug Cocktail Administration in Rats
3.5. Effects of 7 Days of ABO Feeding on Major CYP Enzyme Activities in the Liver and Intestine in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frye, R.F. Probing the world of cytochrome P450 enzymes. Mol. Interv. 2004, 4, 157–162. [Google Scholar] [PubMed]
- Streetman, D.S.; Bertino, J.S., Jr.; Nafziger, A.N. Phenotyping of drug-metabolizing enzymes in adults: A review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics 2000, 10, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-Y.; Ma, J.-S.; Xu, R.-A.; Hu, L.-F.; Wang, Z.; Wang, X.-Q. Effects of Ougan juice on P450 activities using a cocktail method. Die Pharm. 2012, 67, 242–246. [Google Scholar]
- Zadoyan, G.; Rokitta, D.; Klement, S.; Dienel, A.; Hoerr, R.; Gramatte, T.; Fuhr, U. Effect of Ginkgo biloba special extract EGb 761(R) on human cytochrome P450 activity: A cocktail interaction study in healthy volunteers. Eur. J. Clin. Pharmacol. 2012, 68, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-L.; Li, D.; Ren, B.; Jing, G.-P.; Meng, X.-L.; Zhou, Z.-Y.; Yu, Q.; Li, Y.; Wan, L.-L.; Guo, C. Evaluation of impact of Herba Erigerontis injection, a Chinese herbal prescription, on rat hepatic cytochrome P450 enzymes by cocktail probe drugs. J. Ethnopharmacol. 2012, 139, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.J.; Jo, J.H.; Kim, S.; Lee, J.-M.; Lee, S. Development of a simultaneous LC-MS/MS method to predict in vivo drug-drug interaction in mice. Arch. Pharmacal Res. 2018, 41, 450–458. [Google Scholar] [CrossRef]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef]
- Amadi, C.N.; Mgbahurike, A.A. Selected food/herb-drug interactions: Mechanisms and clinical relevance. Am. J. Ther. 2018, 25, e423–e433. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Bibi, Z. Grape fruit juice-drug interactions. Pak. J. Pharm. Sci. 2005, 18, 45–57. [Google Scholar]
- Xie, F.; Ding, X.; Zhang, Q.-Y. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B 2016, 6, 374–383. [Google Scholar] [CrossRef]
- Bibi, Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. 2008, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.A.; Ameer, B. Drug interactions with grapefruit juice. Clin. Pharmacokinet. 1997, 33, 103–121. [Google Scholar]
- Kuo, C.C.; Chen, H.H.; Chiang, W. Adlay (yi yi; “soft-shelled job’s tears”; the seeds of Coix lachryma-jobi L. var. ma-yuen Stapf) is a potential cancer chemopreventive agent toward multistage carcinogenesis processes. J. Tradit. Complement. Med. 2012, 2, 267–275. [Google Scholar] [CrossRef]
- Chen, H.-J.; Lo, Y.-C.; Chiang, W. Inhibitory effects of adlay bran (Coix lachryma-jobi L. var. ma-yuen Stapf) on chemical mediator release and cytokine production in rat basophilic leukemia cells. J. Ethnopharmacol. 2012, 141, 119–127. [Google Scholar] [CrossRef]
- Li, B.; Qiao, L.; Li, L.; Zhang, Y.; Li, K.; Wang, L.; Qiao, Y. A novel antihypertensive derived from adlay (Coix larchryma-jobi L. var. ma-yuen Stapf) glutelin. Molecules 2017, 22, 123. [Google Scholar] [CrossRef]
- Zhao, M.; Zhu, D.; Sun-Waterhouse, D.; Su, G.; Lin, L.; Wang, X.; Dong, Y. In vitro and in vivo studies on adlay-derived seed extracts: Phenolic profiles, antioxidant activities, serum uric acid suppression, and xanthine oxidase inhibitory effects. J. Agric. Food Chem. 2014, 62, 7771–7778. [Google Scholar] [CrossRef]
- Chung, C.-P.; Hsu, H.-Y.; Huang, D.-W.; Hsu, H.-H.; Lin, J.-T.; Shih, C.-K.; Chiang, W. Ethyl acetate fraction of adlay bran ethanolic extract inhibits oncogene expression and suppresses DMH-Induced preneoplastic lesions of the colon in F344 Rats through an anti-inflammatory pathway. J. Agric. Food Chem. 2010, 58, 7616–7623. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Chang, C.-W.; Chiang, W.; Hsieh, S.-C. Adlay bran oil suppresses hepatic gluconeogenesis and attenuates hyperlipidemia in Type 2 diabetes rats. J. Med. Food 2019, 22, 22–28. [Google Scholar] [CrossRef]
- Huang, S.L.; Chen, Y.F.; Chiang, W. Amino acids, fatty acids and proximate composition of the seed of adlay. Food Sci. 1994, 21, 67–74. [Google Scholar]
- Huang, B.-W.; Chiang, M.-T.; Yao, H.-T.; Chiang, W. The effect of adlay oil on plasma lipids, insulin and leptin in rat. Phytomedicine 2005, 12, 433–439. [Google Scholar] [CrossRef]
- Yao, H.-T.; Lin, J.-H.; Chiang, M.-T.; Chiang, W.; Luo, M.-N.; Lii, C.-K. Suppressive effect of the ethanolic extract of adlay bran on cytochrome P-450 enzymes in rat liver and lungs. J. Agric. Food Chem. 2011, 59, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Chung, C.-P.; Chiang, W.; Lin, Y.-L. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Hou, M.F.; Kan, J.Y.; Juan, C.H.; Yuan, S.S.; Luo, K.H.; Chuang, H.Y.; Hu, S.C. Prophylactic treatment with adlay bran extract reduces the risk of severe acute radiation dermatitis: A prospective, randomized, double-blind study. Evid. Based Complement. Alternat. Med. 2015, 312072. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Miao, M.-X.; Zhong, Z.-Y.; Xu, P.; Chen, Y.; Liu, X.-D. Chronic administration of caderofloxacin, a new fluoroquinolone, increases hepatic CYP2E1 expression and activity in rats. Acta Pharmacol. Sin. 2016, 37, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Li, X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int. J. Pharm. 2005, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bobroff, L.B.; Lentz, A.; Turner, R.E. Food/drug and drug/nutrient interactions: What you should know about your medications. Drugs 2009, FCS8092, 1–10. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Yao, H.-T.; Chang, Y.-W.; Lan, S.-J.; Yeh, T.-K. The inhibitory effect of tannic acid on cytochrome P450 enzymes and NADPH-CYP reductase in rat and human liver microsomes. Food Chem. Toxicol. 2008, 46, 645–653. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Chen, W.-C.; Lin, K.-T.; Chang, L.; Yao, H.-T.; Hsieh, H.-P.; Lan, S.-J.; Chen, C.-T.; Chao, Y.-S.; Yeh, T.-K. Development and validation of a liquid chromatography–tandem mass spectrometry for the determination of BPR0L075, a novel antimicrotuble agent, in rat plasma: Application to a pharmacokinetic study. J. Chromatogr. B 2007, 846, 162–168. [Google Scholar] [CrossRef]
- Nosaka, H.; Takagi, K.; Hasegawa, T.; Ogura, Y.; Mizukami, Y.; Satake, T. Pharmacokinetics of theophylline in beagle dogs and asthmatic patients after multiple oral doses of sustained-release theophylline tablet formulation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 528–535. [Google Scholar]
- Lin, L.; Yang, Q.; Zhao, K.; Zhao, M. Identification of the free phenolic profile of Adlay bran by UPLC-QTOF-MS/MS and inhibitory mechanisms of phenolic acids against xanthine oxidase. Food Chem. 2018, 253, 108–118. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. Vitr. 2006, 20, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, T.; Jaroszewski, J.J.; Borucka, B.; Ziolkowski, H. C (max) and t (max) verification using fibonacci sequence and absorption rate. Eur. J. Drug Metab. Pharm. 2013, 38, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Athukuri, B.L.; Neerati, P. Enhanced oral bioavailability of diltiazem by the influence of gallic acid and ellagic acid in male Wistar rats: Involvement of CYP3A and P-gp inhibition. Phytother. Res. 2017, 31, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Kusuhara, M.; Yoshioka, M.; Kuroda, K.; Soga, A.; Nishikawa, F.; Koishi, T.; Nakagawa, M.; Hori, S.; Matsumoto, T.; et al. Studies on interactions between functional foods or dietary supplements and medicines. I. Effects of Ginkgo biloba leaf extract on the pharmacokinetics of diltiazem in rats. Biol. Pharm. Bull. 2003, 26, 1315–1320. [Google Scholar] [CrossRef][Green Version]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D.C. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef]
- Kim, J.K.; Strapazzon, N.; Gallaher, C.M.; Stoll, D.R.; Thomas, W.; Gallaher, D.D.; Trudo, S.P. Comparison of short- and long-term exposure effects of cruciferous and apiaceous vegetables on carcinogen metabolizing enzymes in Wistar rats. Food Chem. Toxicol. 2017, 108, 194–202. [Google Scholar] [CrossRef]
- Rengelshausen, J.; Banfield, M.; Riedel, K.; Burhenne, J.; Weiss, J.; Thomsen, T.; Waltersack, I.; Haefeli, W.E.; Mikus, G. Opposite effects of short-term and long-term St John’s wort intake on voriconazole pharmacokinetics. Clin. Pharmacol. Ther. 2005, 78, 25–33. [Google Scholar] [CrossRef]
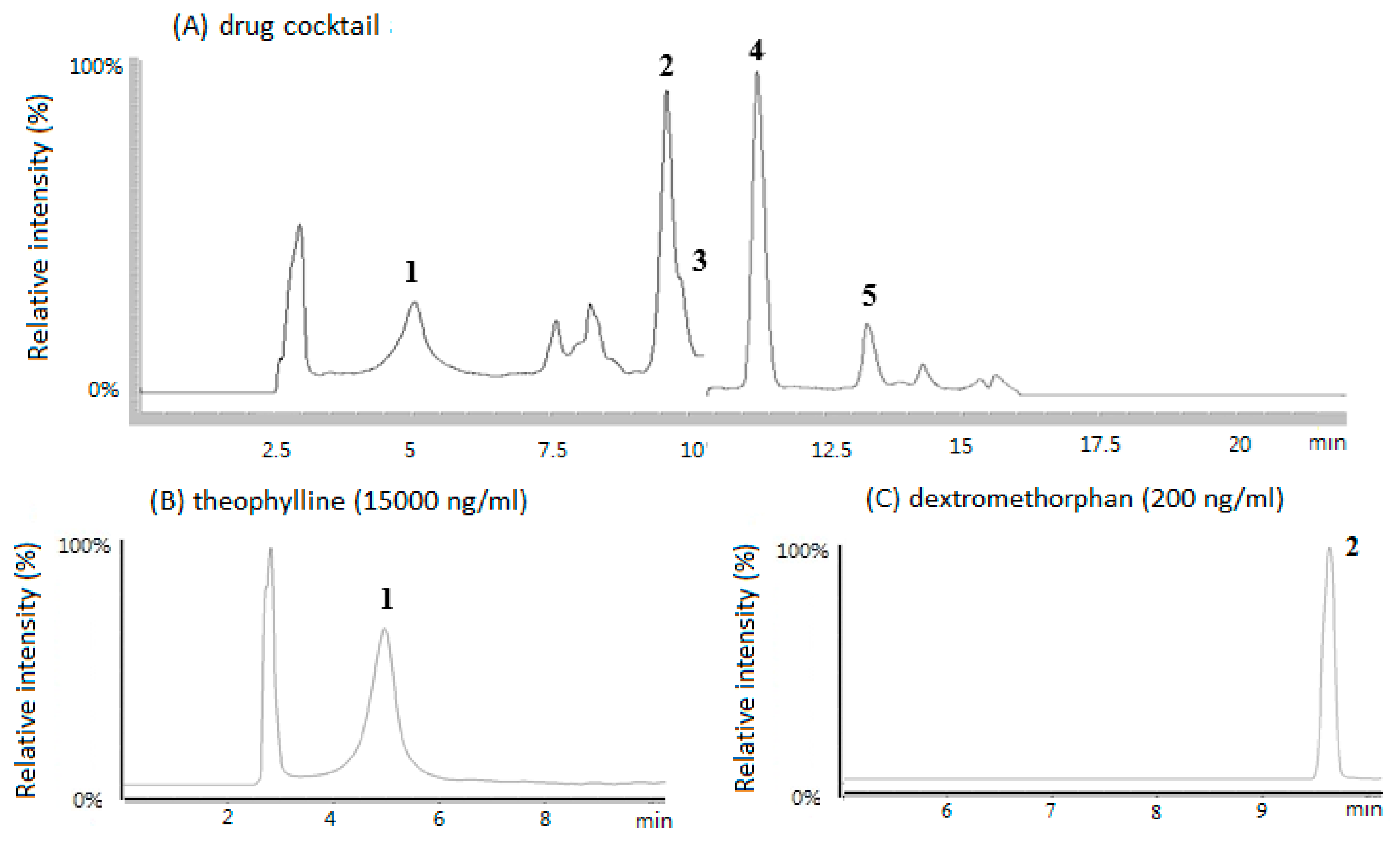
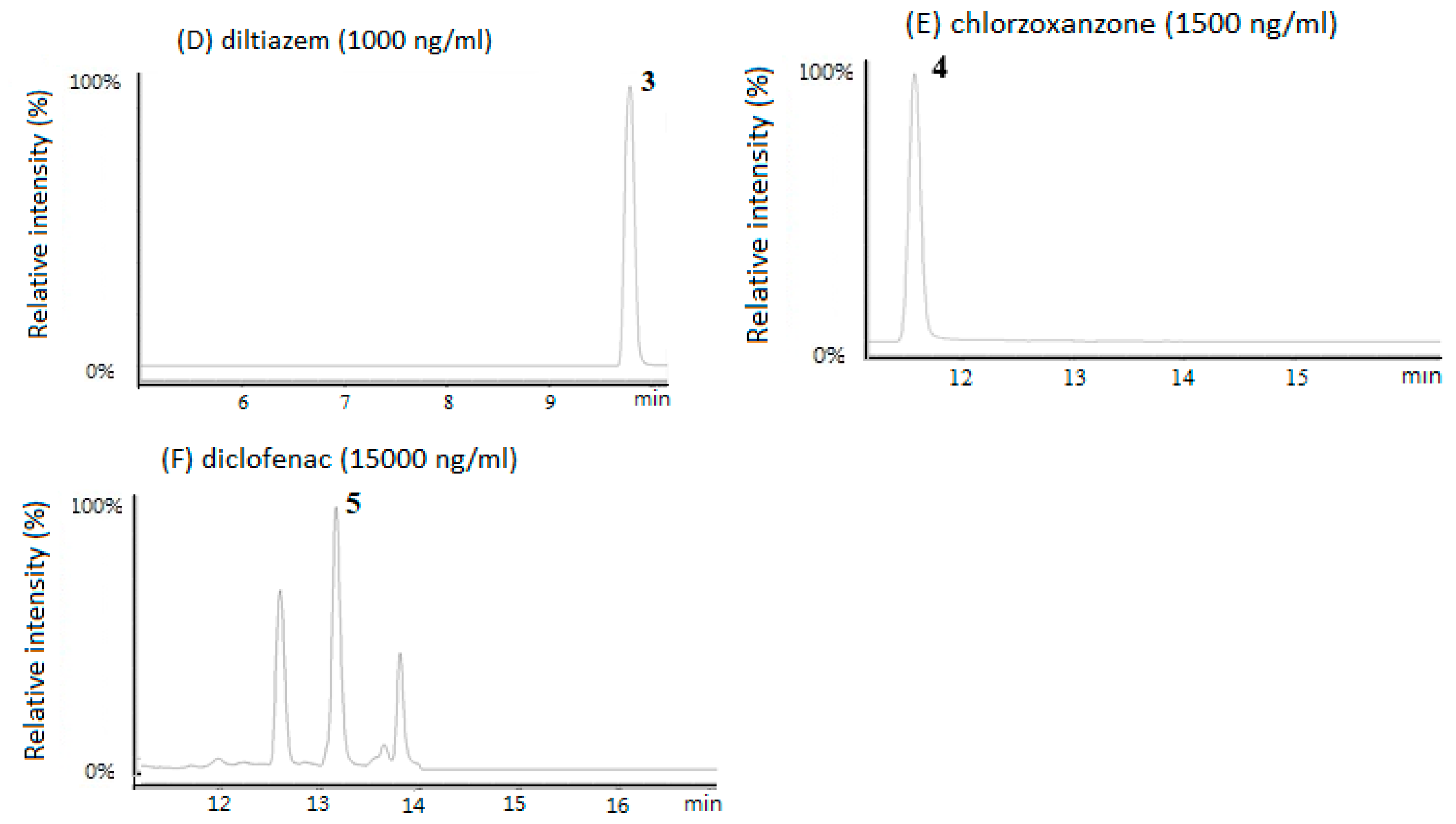
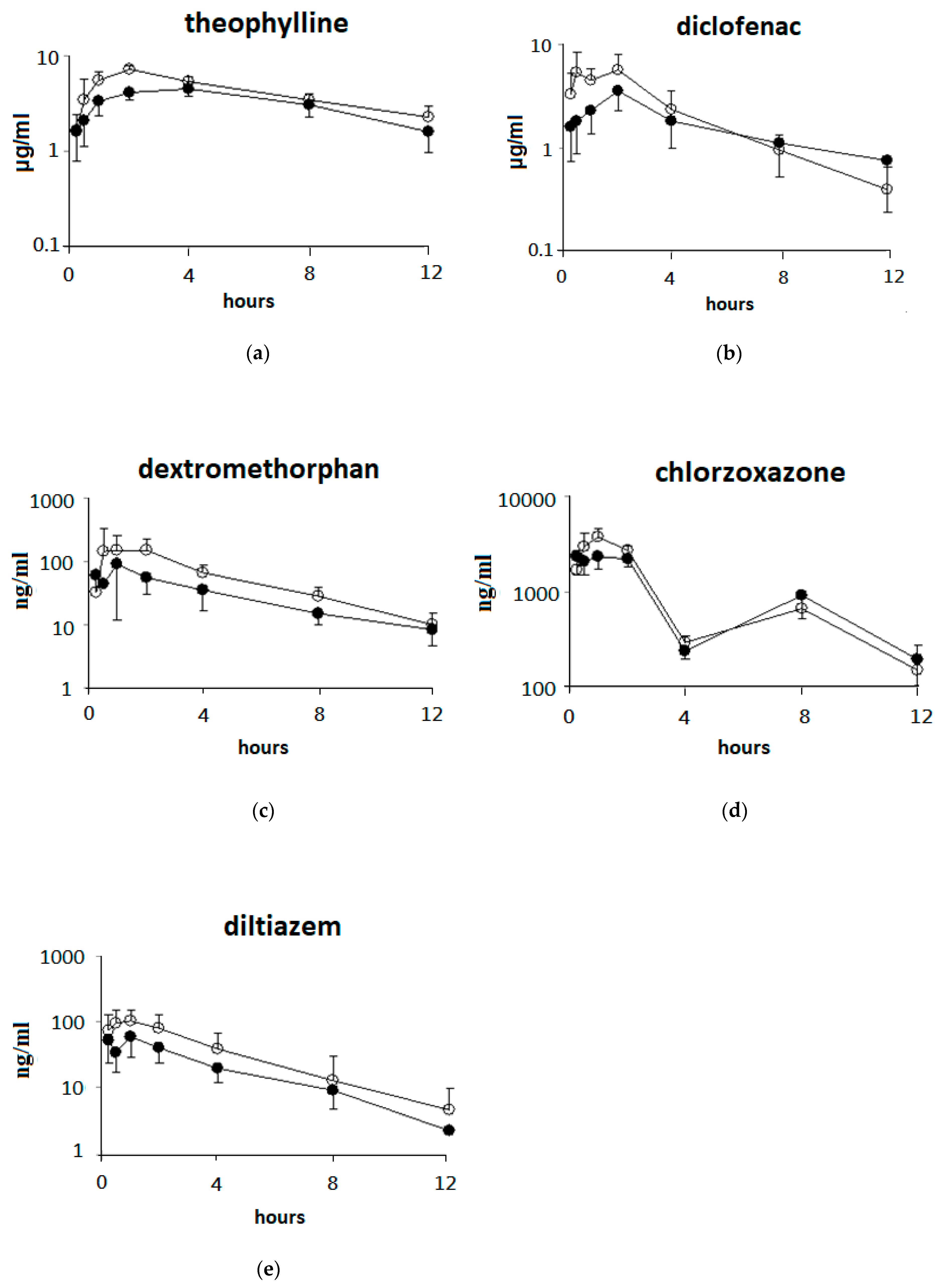
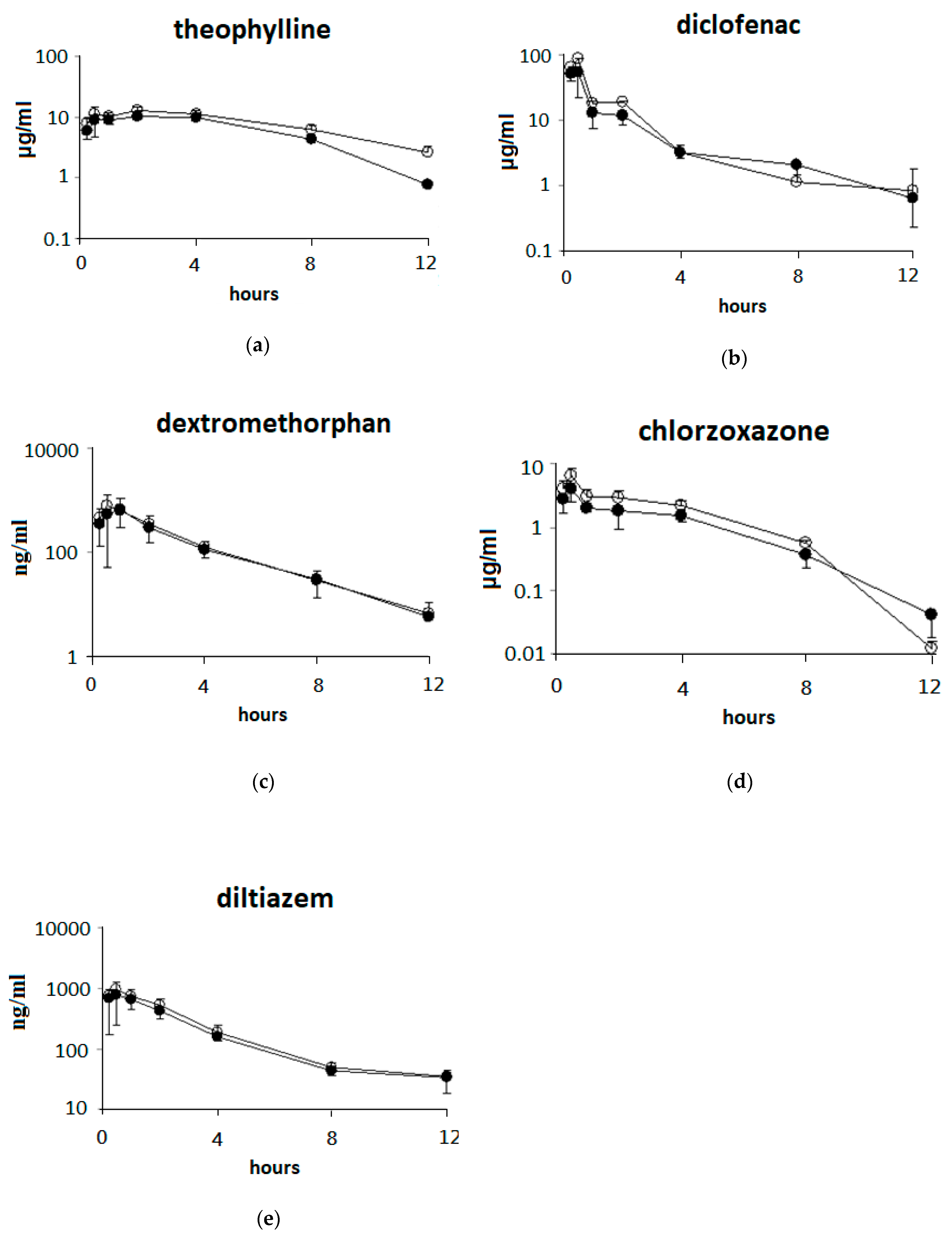
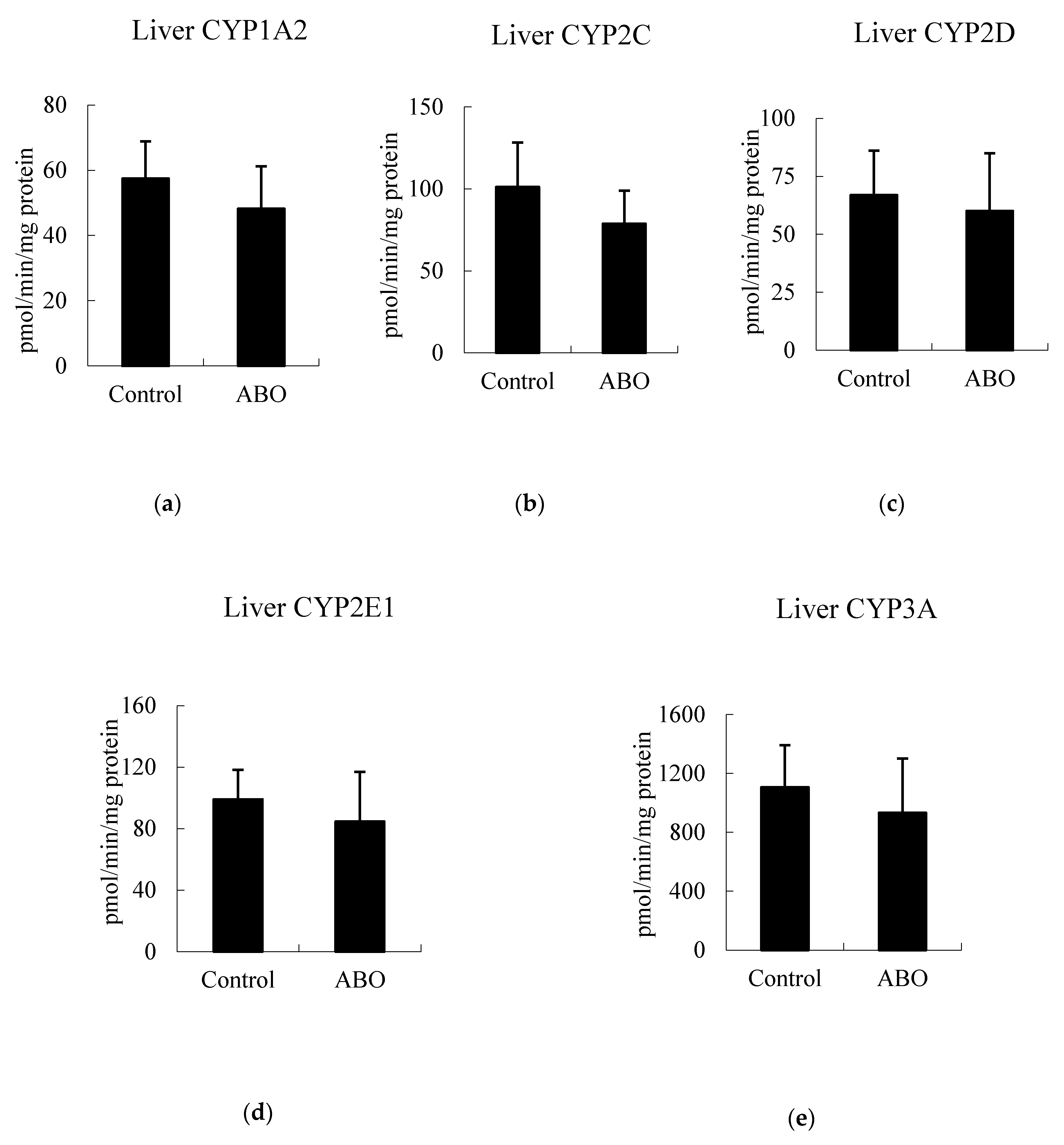
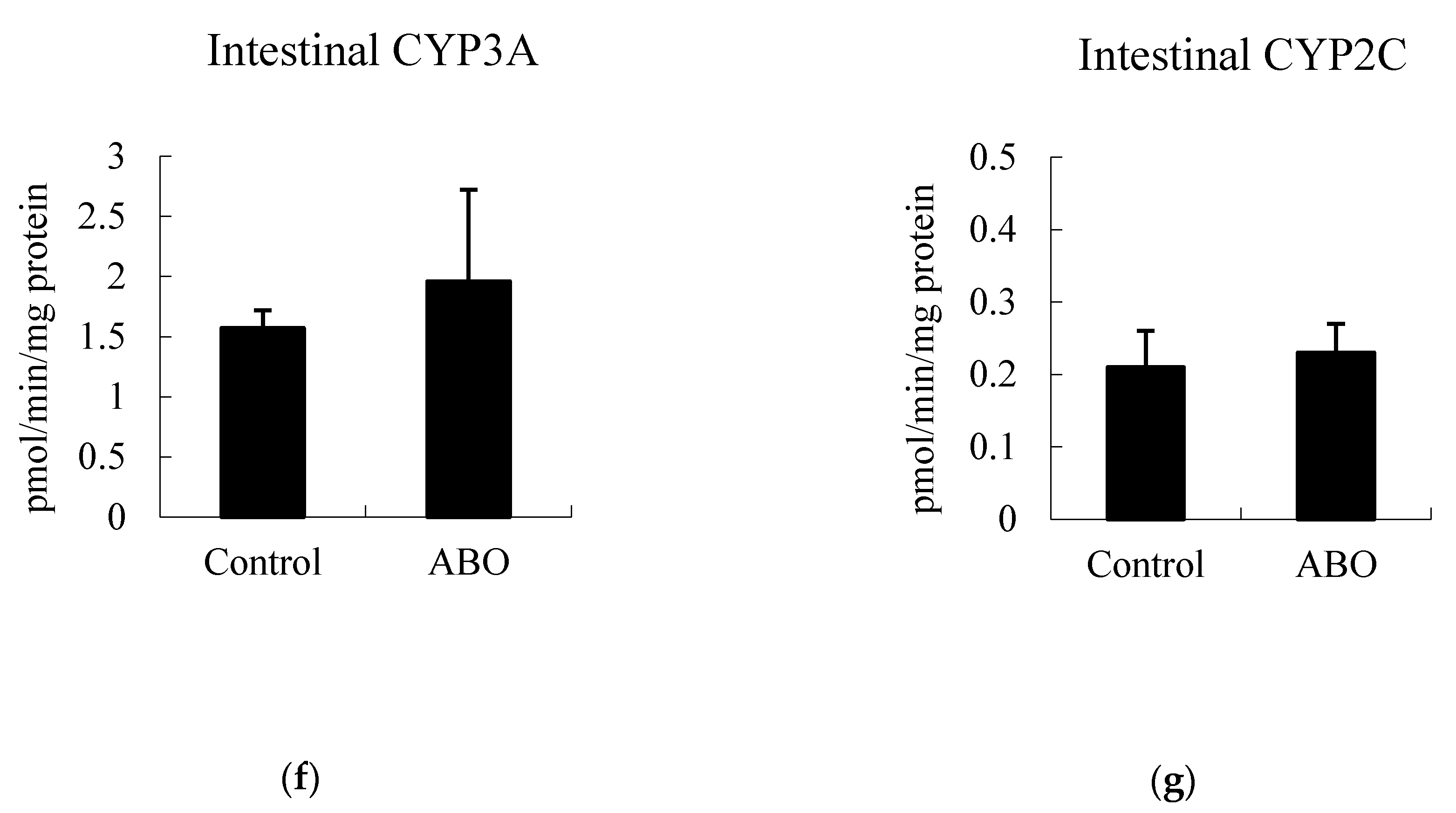
| Drugs | Dose (mg/kg) | AUC (0-t) 2 (μg/mL×h) | T 1/2 (h) | Cl 3 (mL/min/kg) |
|---|---|---|---|---|
| Theophylline (CYP1A2) | ||||
| Control group | 1 | 4.6 ± 0.8 | 3.2 ± 1.1 | 12.7 ± 2.3 |
| ABO group | 4.5 ± 0.7 | 3.7 ± 0.6 | 12.5 ± 1.7 | |
| Diclofenac (CYP2C) | ||||
| Control group | 10 | 30.1 ± 3.2 | 2.9 ± 2.1 | 19.2 ± 2.3 |
| ABO group | 28.7 ± 6.7 | 2.6 ± 2.1 | 20.5 ± 5.3 | |
| Dextromethorphan (CYP2D) | ||||
| Control group | 5 | 0.76 ± 0.17 | 2.4 ± 0.7 | 388.4 ± 65.8 |
| ABO group | 0.89 ± 0.08 | 4.2 ± 3.7 | 318.4 ± 27.9 | |
| Chlorzoxazone (CYP2E1) | ||||
| Control group | 1 | 3.0 ± 0.6 | 1.7 ± 0.6 | 20.1 ± 4.3 |
| ABO group | 3.0 ± 0.7 | 2.4 ± 1.0 | 18.1 ± 2.9 | |
| Diltiazem (CYP3A) | ||||
| Control group | 5 | 5.1 ± 0.7 | 2.2 ± 1.4 | 128.9 ± 16.6 |
| ABO group | 4.3 ± 1.2 | 1.9 ± 0.3 | 115.3 ± 13.9 |
| Drugs | Dose (mg/kg) | AUC (0-t) 2 (μg/mL×h) | Tmax 3 (h) | Cmax 4 (μg/mL) | t1/25 (h) | F% 6 |
|---|---|---|---|---|---|---|
| Theophylline (CYP1A2) | ||||||
| Control group | 10 | 52.9 ± 15.9 | 2.8 ± 1.3 | 4.8 ± 0.7 | 5.6 ± 1.5 | 86.0 ± 15.9 |
| ABO group | 73.9 ± 17.2 * | 2.0 ± 0.0 | 7.1 ± 0.9 * | 6.5 ± 1.9 | 124.8 ± 14.7 * | |
| Diclofenac (CYP2C) | ||||||
| Control group | 20 | 22.2 ± 7.9 | 1.5 ± 0.7 | 4.7 ± 2.2 | 4.1 ± 1.6 | 29.2 ± 8.1 |
| ABO group | 27.1 ± 8.5 | 1.3 ± 0.8 | 6.7 ± 3.0 | 2.5 ± 0.8 | 40.5 ± 12.1 | |
| Dextromethorphan (CYP2D) | ||||||
| Control group | 25 | 0.40 ± 0.15 | 1.2 ± 0.6 | 0.13 ± 0.1 | 3.1 ± 0.5 | 10.0 ± 2.8 |
| ABO group | 0.78 ± 0.23 * | 1.5 ± 0.6 | 0.22 ± 0.2 | 3.1 ± 0.8 | 18.6 ± 6.8 * | |
| Chlorzoxazone (CYP2E1) | ||||||
| Control group | 5 | 12.6 ± 2.7 | 1.3 ± 0.8 | 2.8 ± 0.6 | 4.1 ± 1.9 | 76.2 ± 11.5 |
| ABO group | 13.0 ± 2.3 | 1.3 ± 0.6 | 3.8 ± 0.9 * | 2.7 ± 1.1 | 78.1 ± 11.3 | |
| Diltiazem (CYP3A) | ||||||
| Control group | 40 | 0.24 ± 0.04 | 1.3 ± 0.8 | 0.073 ± 0.028 | 2.0 ± 0.7 | 1.3 ± 0.1 |
| ABO group | 0.45 ± 0.22 # | 1.0 ± 0.8 | 0.131 ± 0.045 * | 2.0 ± 0.8 | 2.2 ± 1.0 |
| Drugs | Dose (mg/kg) | AUC (0-t) 2 (μg/mL×h) | Cmax 3 (μg/mL) | Tmax 4 (h) | t1/25 (h) |
|---|---|---|---|---|---|
| Theophylline (CYP1A2) | |||||
| Control group | 10 | 78.6 ± 8.3 | 11.4 ± 2.9 | 2.1 ± 1.2 | 2.1 ± 1.0 |
| ABO group | 114.3 ± 15.9 * | 13.6 ± 2.6 | 2.1 ± 1.2 | 4.0 ± 0.9 * | |
| Diclofenac (CYP2C) | |||||
| Control group | 20 | 84.8 ± 12.4 | 65.9 ± 35.3 | 0.4 ± 0.1 | 2.5 ± 1.0 |
| ABO group | 106.1 ± 28.3 | 87.0 ± 31.8 | 0.5 ± 0.0 | 1.5 ± 0.3 | |
| Dextromethorphan (CYP2D) | |||||
| Control group | 25 | 1.7 ± 0.8 | 0.76 ± 0.44 | 0.8 ± 0.3 | 1.8 ± 0.5 |
| ABO group | 1.9 ± 1.0 | 0.84 ± 0.49 | 0.8 ± 0.6 | 1.8 ± 0.3 | |
| Chlorzoxazone (CYP2E1) | |||||
| Control group | 5 | 12.5 ± 0.5 | 4.0 ± 1.6 | 0.8 ± 0.6 | 1.5 ± 0.3 |
| ABO group | 19.2 ± 3.7 * | 6.7 ± 2.4 * | 0.5 ± 0.0 | 1.2 ± 0.2 | |
| Diltiazem (CYP3A) | |||||
| Control group | 40 | 1.0 ± 0.6 | 0.81 ± 0.59 | 0.5 ± 0.3 | 1.2 ± 0.1 |
| ABO group | 1.4 ± 0.9 | 0.94 ± 0.74 | 0.8 ± 0.7 | 1.3 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.-T.; Lin, J.-H.; Liu, Y.-T.; Li, M.-L.; Chiang, W. Food–Drug Interaction between the Adlay Bran Oil and Drugs in Rats. Nutrients 2019, 11, 2473. https://doi.org/10.3390/nu11102473
Yao H-T, Lin J-H, Liu Y-T, Li M-L, Chiang W. Food–Drug Interaction between the Adlay Bran Oil and Drugs in Rats. Nutrients. 2019; 11(10):2473. https://doi.org/10.3390/nu11102473
Chicago/Turabian StyleYao, Hsien-Tsung, Jia-Hsuan Lin, Yun-Ta Liu, Mei-Ling Li, and Wenchang Chiang. 2019. "Food–Drug Interaction between the Adlay Bran Oil and Drugs in Rats" Nutrients 11, no. 10: 2473. https://doi.org/10.3390/nu11102473
APA StyleYao, H.-T., Lin, J.-H., Liu, Y.-T., Li, M.-L., & Chiang, W. (2019). Food–Drug Interaction between the Adlay Bran Oil and Drugs in Rats. Nutrients, 11(10), 2473. https://doi.org/10.3390/nu11102473






