In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Treatment of the LNCaP Cells
2.4. Animals Used to Establish the TP -Induced BPH Model
2.5. Experiments Involving Rats with TP-Induced BPH
2.6. Hematoxylin and Eosin (HE) Staining
2.7. Western Blotting Assay
2.8. Determination of the Serum DHT Levels
2.9. Statistical Analysis
3. Results
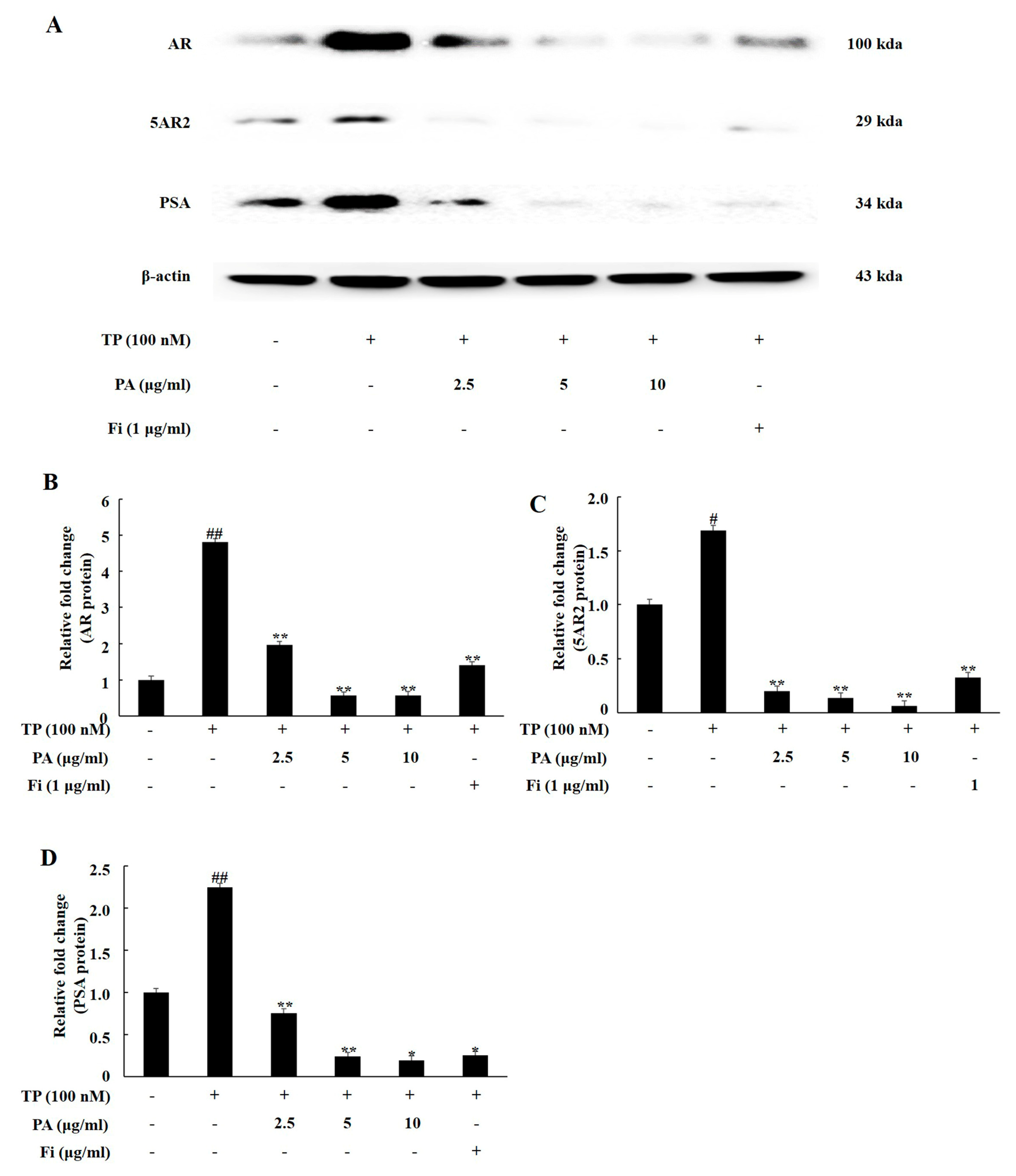
3.1. PA Downregulated the Expression of Androgen Signaling-Related Prosteins in TP-Treated Lncap Cells
3.2. PA Downregulated the Expression of Androgen Signaling-Related Prosteins in DHT-Treated LNCaP Cells
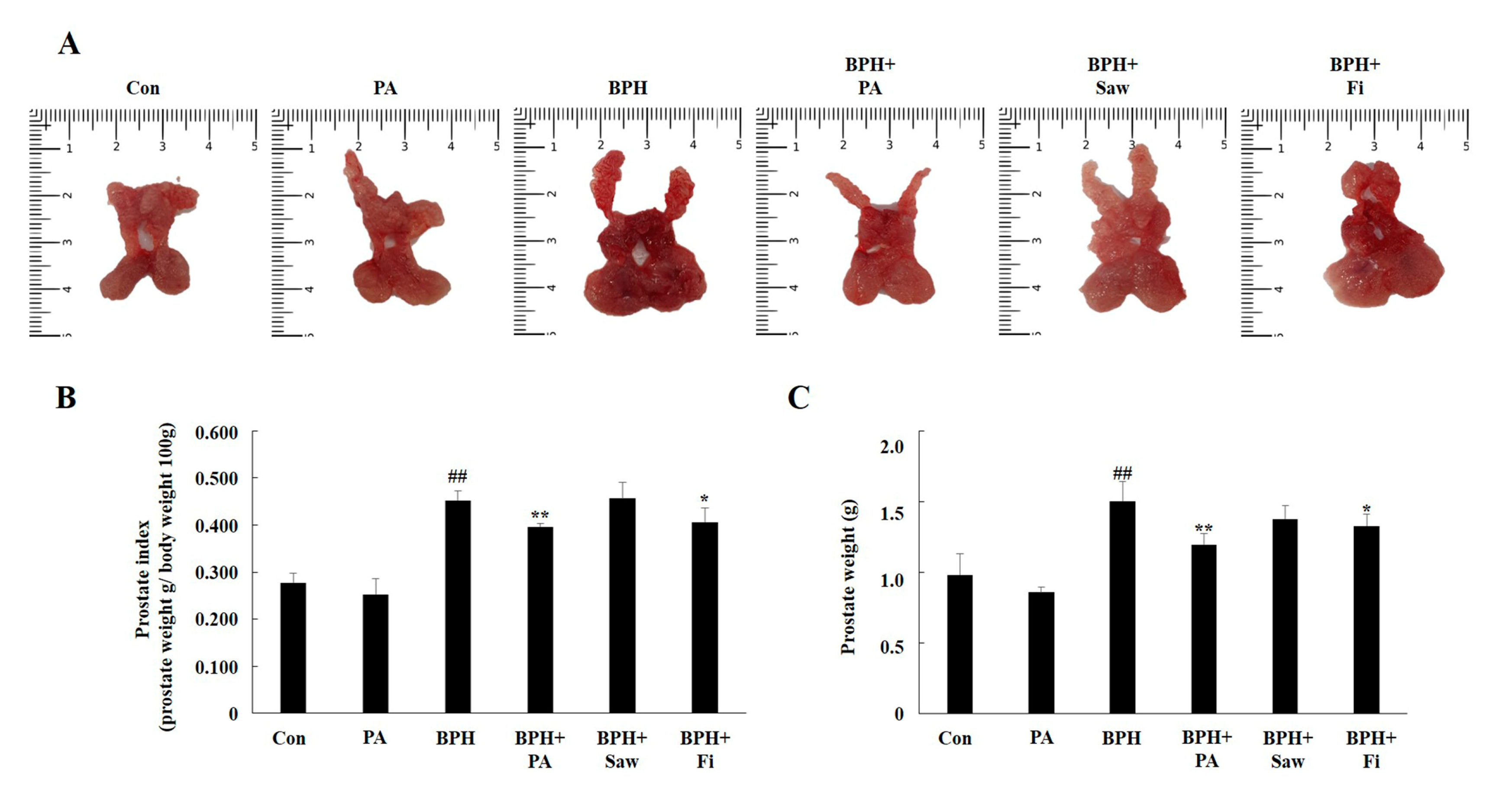
3.3. Prostate Index Changes
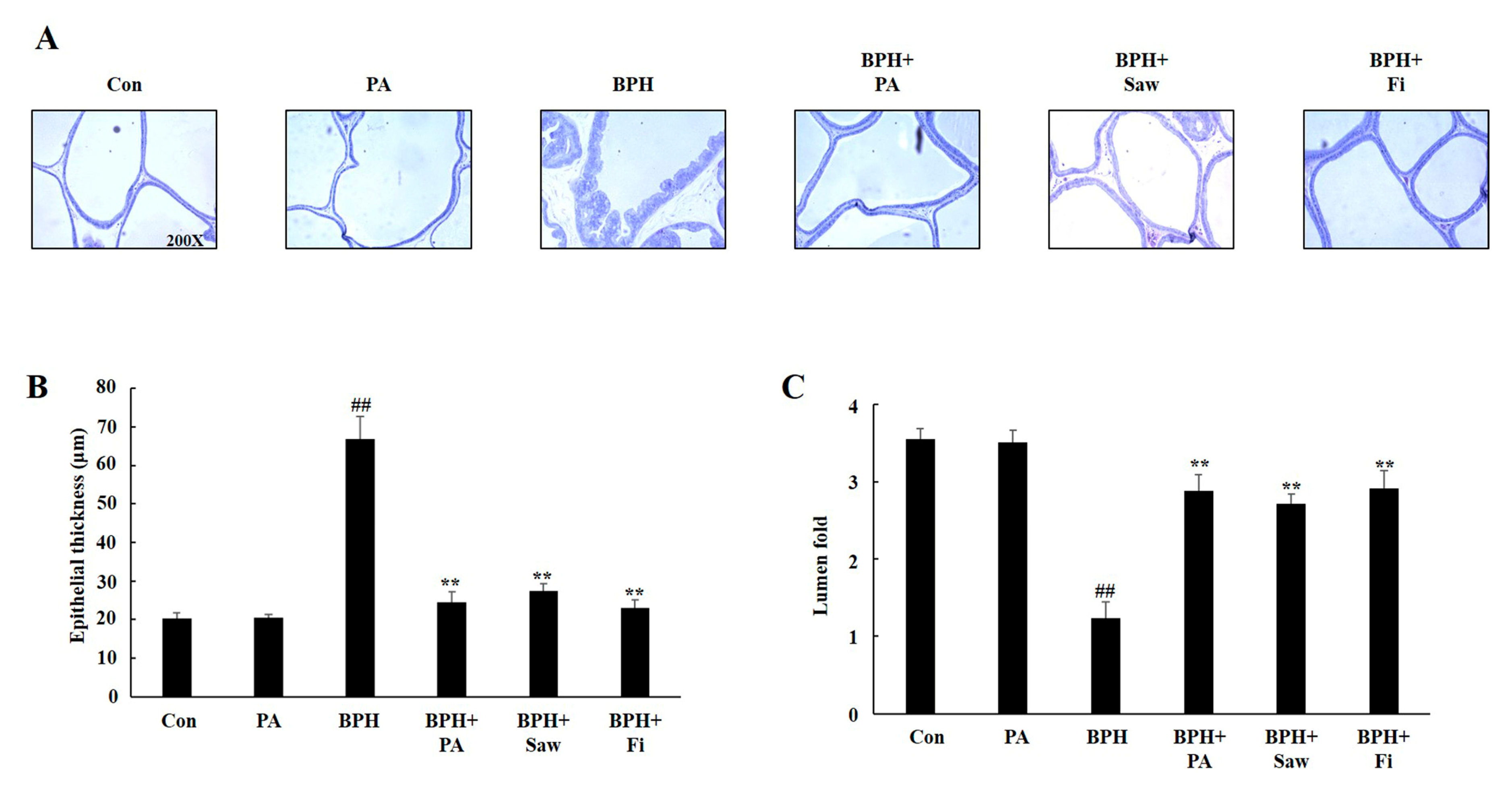
3.4. Histology of the Prostate Tissues of Rats with TP-Induced BPH
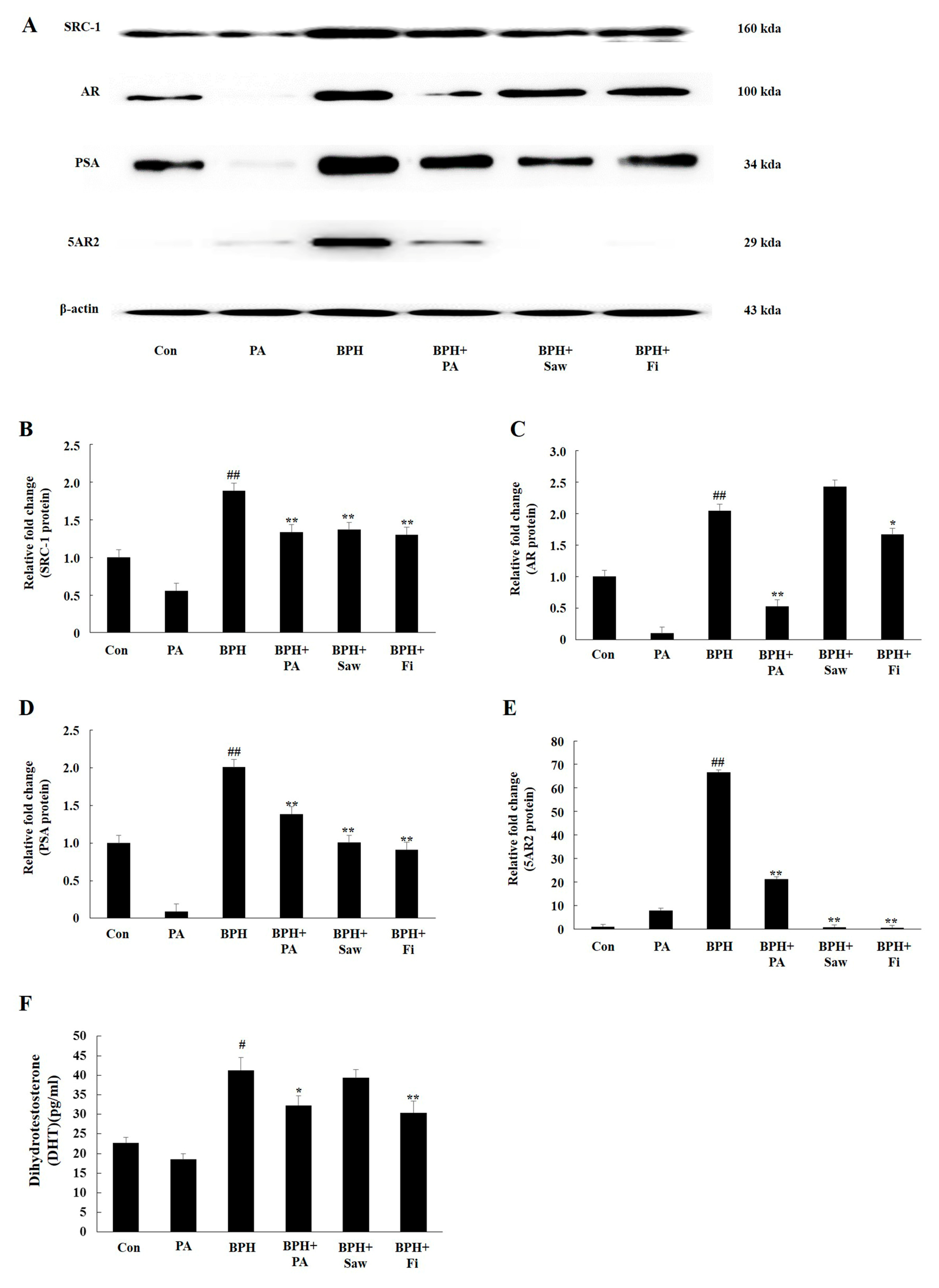
3.5. PA Downregulated the Expression of 5AR2, AR, SRC1, and PSA in Prostate Tissues from Rats with BPH
3.6. PA Decreased the Serum Levels of DHT
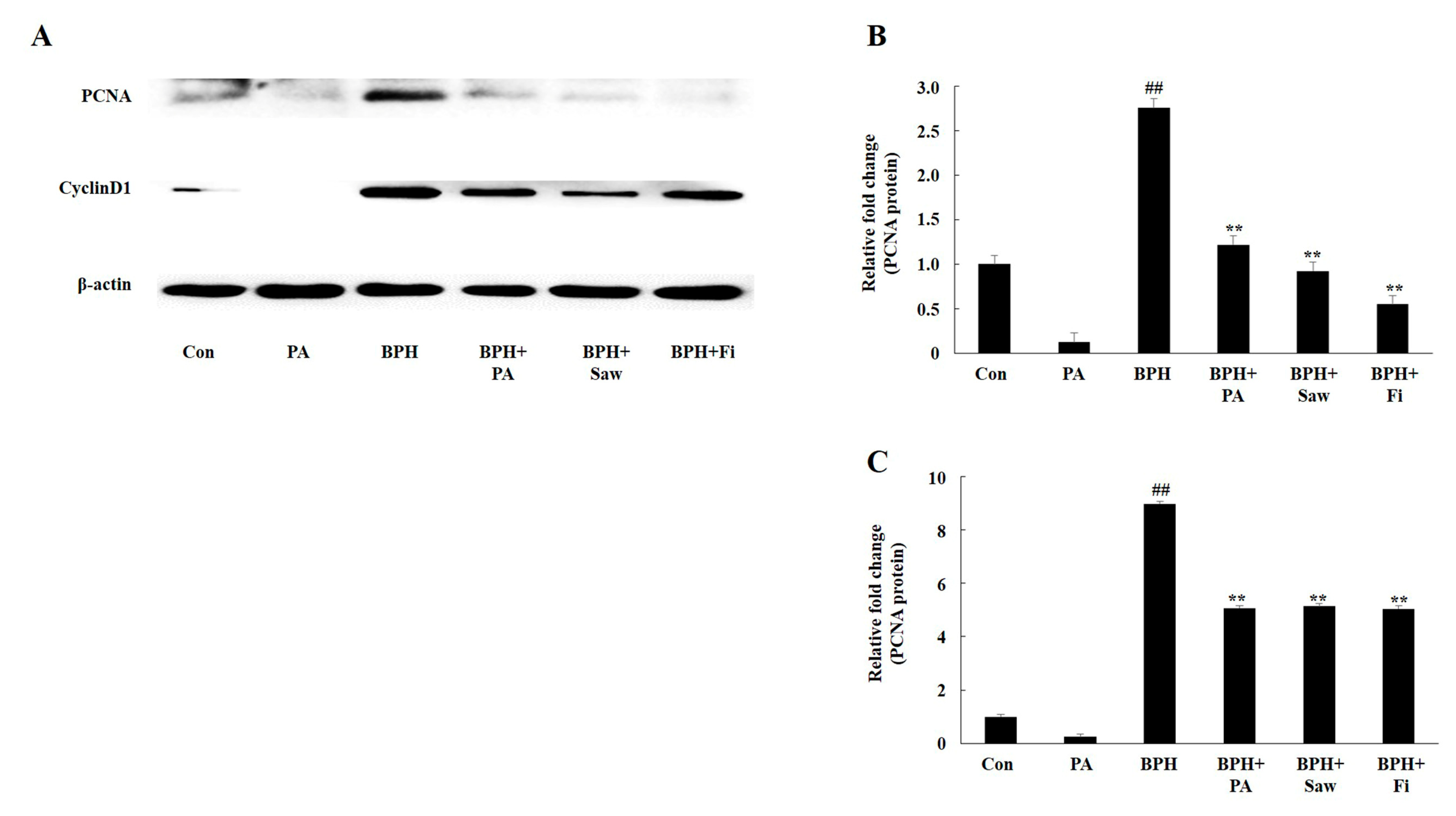
3.7. PA Downregulated the Expression of PCNA and Cyclin D1 in the Prostate Tissues of Rats with BPH
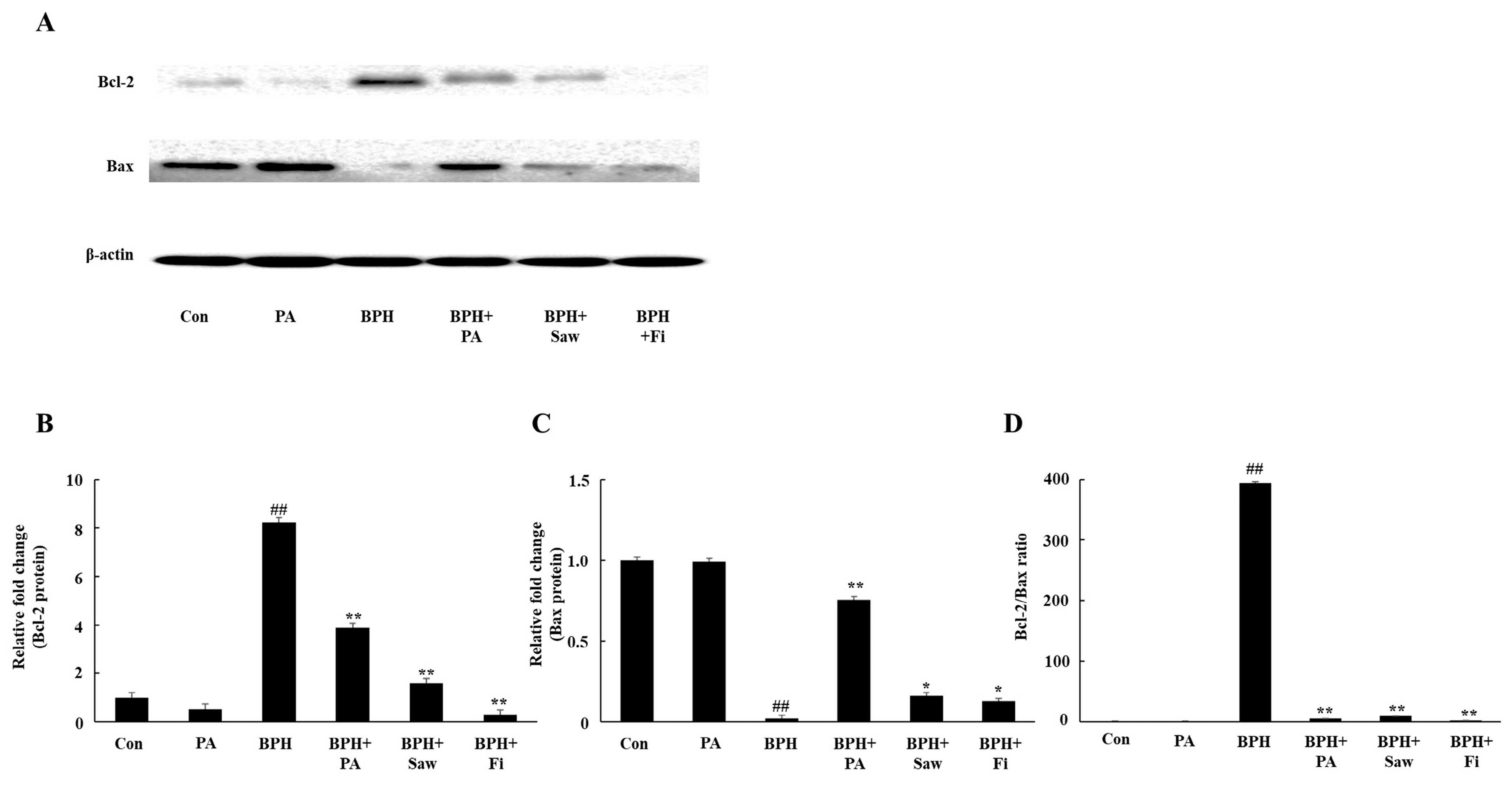
3.8. PA Downregulated the Expression of Bcl-2 and Upregulated the Expression of Bax in the Prostate Tissues of Rats with BPH
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- Sarma, A.V.; Wei, J.T. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N. Engl. J. Med. 2012, 367, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Barkin, J. Benign prostatic hyperplasia and lower urinary tract symptoms: Evidence and approaches for best case management. Can. J. Urol. 2011, 18, 14–19. [Google Scholar] [PubMed]
- Barry, M.J.; Fowler, F.J., Jr.; O’leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American urological association symptom index for benign prostatic hyperplasia. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Kim, S.K.; Seok, H.; Park, H.J.; Jeon, H.S.; Kang, S.W.; Lee, B.C.; Yi, J.; Song, S.Y.; Lee, S.H.; Kim, Y.O.; et al. Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement. Altern. Med. 2015, 15, 380–386. [Google Scholar] [CrossRef]
- Choi, H.M.; Jung, Y.; Park, J.B.; Kim, H.L.; Youn, D.H.; Kang, J.W.; Jeong, M.Y.; Lee, J.H.; Yang, W.M.; Lee, S.G.; et al. Cinnamomi cortex (Cinnamomum verum) suppresses testosterone-induced benign prostatic hyperplasia by regulating 5α-reductase. Sci. Rep. 2016, 6, 31906–31917. [Google Scholar] [CrossRef]
- Thigpen, A.E.; Silver, R.I.; Guileyardo, J.M.; Casey, M.L.; McConnell, J.D.; Russell, D.W. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J. Clin. Investig. 1993, 92, 903–910. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Boyle, P.; Nickel, J.C.; Hoefner, K.; Andriole, G. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2012, 60, 434–441. [Google Scholar] [CrossRef]
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef]
- Levitt, J.M.; Slawin, K.M. Prostate-specific antigen and prostate-specific antigen derivatives as predictors of benign prostatic hyperplasia progress. Curr. Urol. Rep. 2007, 8, 269–274. [Google Scholar] [CrossRef]
- Chen, Y.; Robles, A.I.; Martinez, L.A.; Liu, F.; Gimenez-Conti, I.B.; Conti, C.J. Expression of G1 cyclins, cyclin-dependent kinases, and cyclin-dependent kinase inhibitors in androgen-induced prostate proliferation in castrated rats. Cell Growth Differ. 1996, 7, 1571–1578. [Google Scholar] [PubMed]
- Grana, X.; Redy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Zhong, X.Y.; Lin, J.M.; Zhou, J.H.; Xu, W.; Hong, Z.F.; Peng, J. Qianliening capsule treats benign prostatic hyperplasia (BPH) by down-egulating the expression of PCNA, CyclinD1 and CDK4. Afr. J. Biotechnol. 2012, 11, 7731–7737. [Google Scholar]
- Wang, W.; Bergh, A.; Damber, J.E. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 2004, 61, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Berchem, G.; Tarkington, M.A.; Krajewski, S.; Krajewski, M.; Reed, J.C.; Tehan, T.; Ortega, L.; Lage, J.; Gelmann, E.P. Resistance to apoptosis and up regulation of Bcl-2 in benign prostatic hyperplasia after androgen deprivation. J. Urol. 1997, 158, 212–216. [Google Scholar] [CrossRef]
- Park, E.S.; Lee, M.Y.; Jeon, W.W.; Seo, C.S.; You, S.S.; Shin, H.K. Paljung-San, a traditional herbal medicine, attenuates benign prostatic hyperplasia in vitro and in vivo. J. Ethnopharmacol. 2018, 218, 109–115. [Google Scholar] [CrossRef]
- Gomley, G.J.; Stoner, E.; Bruskewitz, R.C.; McGinley, J.I.; Walsh, P.C.; McConnell, J.D.; Andriole, G.L.; Geller, J.; Bracken, B.R.; Tenover, J.S.; et al. The effect of finasteride in men with benign prostatic hyperplasia. Engl. J. Med. 1992, 327, 1185–1191. [Google Scholar] [CrossRef]
- Ma, Y.F.; Guo, N.N.; Chu, J.; Jin, S.; Yang, B.; Li, J.; Zhang, T.; Guo, J.T.; Chen, L.; Liang, C.Y.; et al. Glycyrrhizin treatment inhibits proliferation and invasive potential of lung cancer cells. Int. J. Clin. Exp. Med. 2016, 9, 10592–10596. [Google Scholar]
- Wilt, T.J.; Ishani, A.; Stark, G.; MacDonald, R.; Lau, J.; Mulrow, C. Saw palmetto extracts for treatment of benign prostatic hyperplasia: A systematic review. JAMA 1998, 280, 1604–1609. [Google Scholar] [CrossRef]
- Marks, L.S.; Partin, A.W.; Epstein, J.I.; Tyler, V.E.; Simon, I.; Macairan, M.L.; Chan, T.L.; Dorey, F.J.; Garris, J.B.; Veltri, R.W.; et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J. Urol. 2000, 163, 1451–1456. [Google Scholar] [CrossRef]
- Bent, S.; Kane, C.; Shinohara, K.; Neuhaus, J.; Hudes, E.S.; Goldberg, H.; Avins, A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006, 354, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Cichewicz, R.H.; Chandra, A.; Nair, M.G. Cyclooxygenase inhibitory and antioxidant compounds from crabapple fruits. J. Agric. Food Chem. 2003, 51, 1948–1951. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Tokutake, S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J. Agric. Food Chem. 2002, 50, 4983–4988. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jayaprakasam, B.; Seeram, N.P.; Olson, L.K.; DeWitt, D.; Nair, M.G. Nair Insulin secretion and cyclooxygenase enzyme inhibition by cabernet sauvignon grape skin compounds. J. Agric. Food Chem. 2004, 52, 228–233. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, E.K.; Lee, S.J.; Kim, Y.S.; Moon, S.H.; Jeon, B.T.; Sung, S.H.; Kim, E.T.; Park, P.J. Antioxidant activity and protective effect of anthocyanin oligomers on H2O2-triggered G2/M arrest in retinal cells. J. Agric. Food Chem. 2012, 60, 4282–4288. [Google Scholar] [CrossRef]
- Yi, W.; Fischer, J.; Akoh, C.C. Study of anticancer activities of muscadine grape phenolics in vitro. J. Agric. Food Chem. 2005, 53, 8804–8812. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Tamil, S.; Sakartah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Miguel, M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Jang, H.; Ha, U.S.; Kim, S.J.; Yoon, B.I.; Han, D.S.; Yuk, S.M.; Kim, S.W. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 2010, 58, 12686–12691. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Tang, L.P.; He, R.R.; Xu, Z.; He, Q.Q.; Xiang, F.J.; Su, W.W.; Kurihara, H. Anthocyanins extract from bilberry enhances the therapeutic effect of pollen of Brassica napus L. on stress-provoked benign prostatic hyperplasia in restrained mice. J. Funct. Foods. 2013, 5, 1357–1365. [Google Scholar] [CrossRef]
- Ohtake, A.; Ukai, M.; Saitoh, C.; Sonoda, R.; Noguchi, Y.; Okutsu, H.; Yuyama, H.; Sato, S.; Sasamata, M.; Miyata, K. Effect of tamsulosin on spontaneous bladder contraction in conscious rats with bladder outlet obstruction: Comparison with effect on intraurethral pressure. Eur. J. Pharmacol. 2006, 545, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.; Kim, H.L.; Youn, D.H.; Kang, J.; Lim, S.; Jeong, M.Y.; Sethi, G.; Park, S.J.; Ahn, K.S.; et al. Vanillic acid attenuates testosterone-induced benign prostatic hyperplasia in rats and inhibits proliferation of prostatic epithelial cells. Oncotarget 2017, 8, 87194–87208. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Reda, D.J.; Wasson, J.H.; Anderson, R.J.; Abdellatif, M.; Bruskewitz, R.C. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: A Department of Veterans Affairs cooperative study. J. Urol. 1998, 160, 12–17. [Google Scholar] [CrossRef]
- McConnell, J.D.; Wilson, J.D.; George, F.W.; Geller, J.; Pappas, F.; Stoner, E. Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J. Clin. Endocrinol. Metab. 1992, 74, 505–508. [Google Scholar]
- Lepor, H. Alpha-blockers for the treatment of benign prostatic hyperplasia. Urol. Clin. North. Am. 2016, 43, 311–323. [Google Scholar] [CrossRef]
- Traish, A.M.; Melcangi, R.C.; Bortolato, M.; Garcia-Segura, L.M.; Zitzmann, M. Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Rev. Endocr. Metab. Disord. 2015, 16, 177–198. [Google Scholar] [CrossRef]
- Marks, L.S.; Tyler, V.E. Saw palmetto extract: Newest (and oldest) treatment alternative for men with symptomatic benign prostatic hyperplasia. Urology 1999, 53, 457–461. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-J.; Fan, M.; Tang, Y.; Yang, H.P.; Hwang, J.-Y.; Kim, E.-K. In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia. Nutrients 2019, 11, 2444. https://doi.org/10.3390/nu11102444
Choi Y-J, Fan M, Tang Y, Yang HP, Hwang J-Y, Kim E-K. In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia. Nutrients. 2019; 11(10):2444. https://doi.org/10.3390/nu11102444
Chicago/Turabian StyleChoi, Young-Jin, Meiqi Fan, Yujiao Tang, Hyun Pil Yang, Ji-Young Hwang, and Eun-Kyung Kim. 2019. "In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia" Nutrients 11, no. 10: 2444. https://doi.org/10.3390/nu11102444
APA StyleChoi, Y.-J., Fan, M., Tang, Y., Yang, H. P., Hwang, J.-Y., & Kim, E.-K. (2019). In Vivo Effects of Polymerized Anthocyanin from Grape Skin on Benign Prostatic Hyperplasia. Nutrients, 11(10), 2444. https://doi.org/10.3390/nu11102444




