Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models
Abstract
1. Introduction
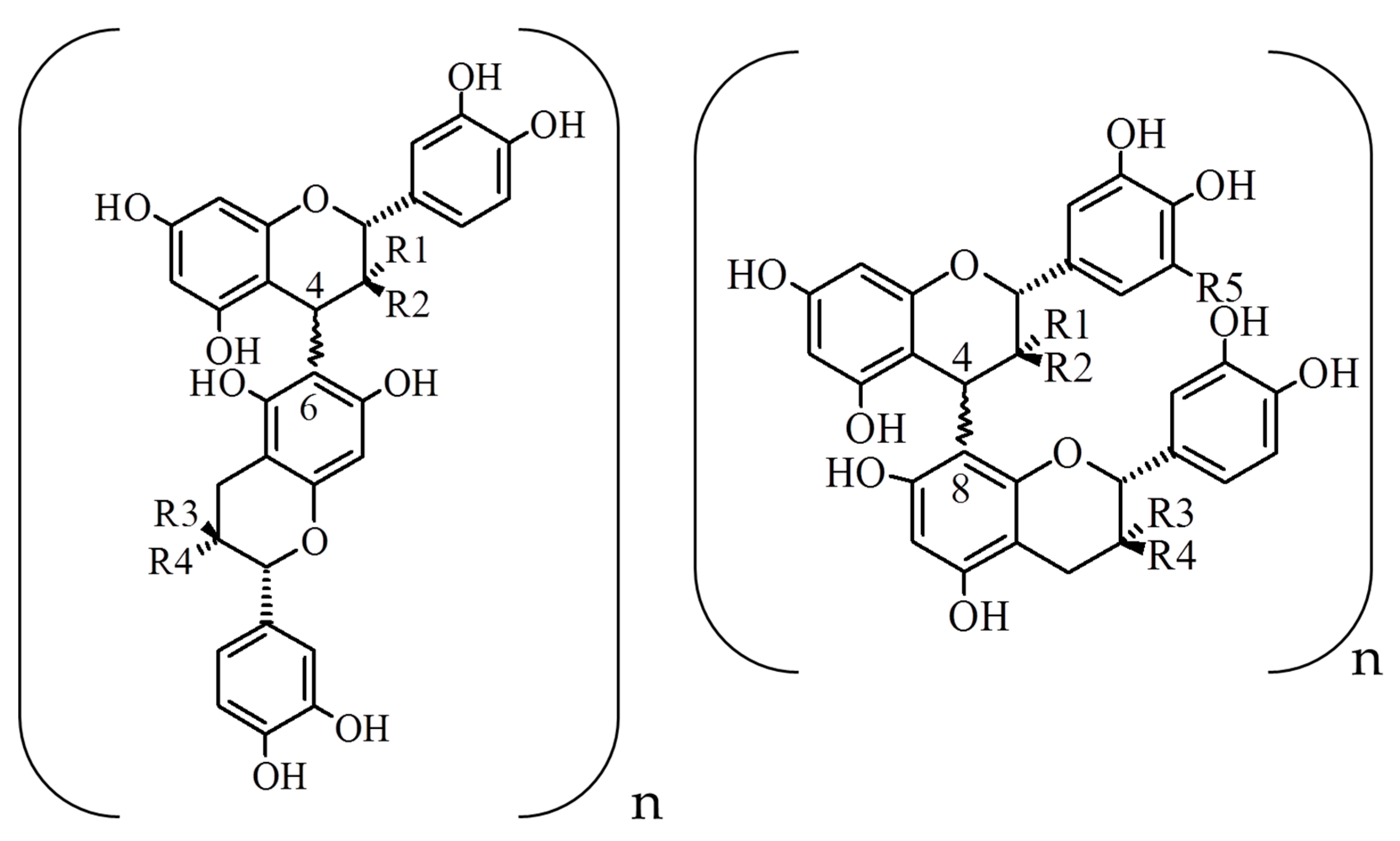
2. Composition and Content of Proanthocyanidins in Grape Seeds and Grape Seed Extracts
3. Biological Activities of Proanthocyanidins from Grape Seeds
3.1. Oxidative Stress
3.2. Inflammation
3.3. Metabolic Syndrome-Related Disorders
3.3.1. Obesity
3.3.2. Diabetes
3.3.3. Cardiovascular Risk Disease
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacoth. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’antona, N. Polyphenolic Profile and Antioxidant Activity of Olive Mill Wastewater from Two Sicilian Olive Cultivars: Cerasuola and Nocellara Etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Neilson, A.P.; O’Keefe, S.F.; Bolling, B.W. High-Molecular-Weight Proanthocyanidins in Foods: Overcoming Analytical Challenges in Pursuit of Novel Dietary Bioactive Components. Annu. Rev. Food Sci. Technol. 2016, 7, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Proanthocyanidins in Cereals and Pseudocereals. Crit. Rev. Food Sci. Nutr. 2019, 59, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Stürzenbaum, S.R. Proanthocyanidins of Natural Origin: Molecular Mechanisms and Implications for Lipid Disorder and Aging-Associated Diseases. Adv. Nutr. 2019, 10, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Sundararajan, A.; Rane, H.S.; Ramaraj, T.; Sena, J.; Howell, A.B.; Bernardo, S.M.; Schilkey, F.D.; Lee, S.A. Cranberry-derived proanthocyanidins induce a differential transcriptomic response within Candida albicans urinary biofilms. PLoS ONE 2018, 13, e0201969. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Uberos, J.; Jiménez-Sánchez, C.; Peña, A.; Segura-Carretero, A. Antibacterial activity of isolated phenolic compounds from cranberry (Vaccinium macrocarpon) against Escherichia coli. Food Funct. 2016, 7, 1564–1573. [Google Scholar] [CrossRef]
- Maki, K.C.; Nieman, K.M.; Schild, A.L.; Kaspar, K.L.; Khoo, C. The Effect of Cranberry Juice Consumption on the Recurrence of Urinary Tract Infection: Relationship to Baseline Risk Factors. J. Am. Coll. Nutr. 2018, 37, 121–126. [Google Scholar] [CrossRef]
- Johnson, M.H.; De Mejia, E.G.; Fan, J.; Lila, M.A.; Yousef, G.G. Anthocyanins and proanthocyanidins from blueberry-blackberry fermented beverages inhibit markers of inflammation in macrophages and carbohydrate-utilizing enzymes in vitro. Mol. Nutr. Food Res. 2013, 57, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antihypertensive and Vasorelaxant Effects of Water-Soluble Proanthocyanidins from Persimmon Leaf Tea in Spontaneously Hypertensive Rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free Radical Scavenging, Antioxidant and Cancer Chemoprevention by Grape Seed Proanthocyanidin: An Overview. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 768, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Pimentel, F.; Costa, A.S.; Alves, R.C.; Oliveira, M.B.P. Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 2016, 57, 31–39. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules 2019, 24, 677. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control. 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Machado, N.F.L.; Domínguez-Perles, R. Addressing Facts and Gaps in the Phenolics Chemistry of Winery By-Products. Molecules 2017, 22, 286. [Google Scholar] [CrossRef]
- Tang, G.-Y.; Zhao, C.-N.; Liu, Q.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Potential of Grape Wastes as a Natural Source of Bioactive Compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Prodanov, M.; Vacas, V.; Hernández, T.; Estrella, I.; Amador, B.; Winterhalter, P. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. J. Food Compos. Anal. 2013, 31, 284–292. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; A Kuszynski, C.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, H. Phytochemical Constituents, Health Benefits, and Industrial Applications of Grape Seeds: A Mini-Review. Antioxidants 2017, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, S. Lipophilized Grape Seed Proanthocyanidin Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2017, 65, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Biosynthesis and Genetic Regulation of Proanthocyanidins in Plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Freitas, V.A.; Glories, Y.; Bourgeois, G.; Vitry, C. Characterisation of oligomeric and polymeric procyanidins from grape seeds by liquid secondary ion mass spectrometry. Phytochemistry 1998, 49, 1435–1441. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of Procyanidins and Anthocyanins in Blueberries and Cranberries (Vaccinium spp.) Using High-Performance Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Travaglia, F.; Bordiga, M.; Locatelli, M.; Coisson, J.D.; Arlorio, M. Polymeric Proanthocyanidins in Skins and Seeds of 37 Vitis vinifera L. Cultivars: A Methodological Comparative Study. J. Food Sci. 2011, 76, 742. [Google Scholar] [CrossRef]
- Escribano-Bailon, T.; Gutierrez-Fernandez, Y.; Rivas-Gonzalo, J.-C.; Santos-Buelga, C. Characterization of procyanidins of Vitis vinifera variety Tinta del Pais grape seeds. J. Agric. Food Chem. 1992, 40, 1794–1799. [Google Scholar] [CrossRef]
- Fuleki, T.; Da Silva, J.M.R. Catechin and Procyanidin Composition of Seeds from Grape Cultivars Grown in Ontario. J. Agric. Food Chem. 1997, 45, 1156–1160. [Google Scholar] [CrossRef]
- Bombai, G.; Pasini, F.; Verardo, V.; Sevindik, O.; Di Foggia, M.; Tessarin, P.; Bregoli, A.M.; Caboni, M.F.; Rombolà, A.D. Monitoring of compositional changes during berry ripening in grape seed extracts of cv. Sangiovese (Vitis vinifera L.). J. Sci. Food Agric. 2017, 97, 3058–3064. [Google Scholar] [CrossRef] [PubMed]
- Genebra, T.; Santos, R.R.; Francisco, R.; Pinto-Marijuan, M.; Brossa, R.; Serra, A.T.; Duarte, C.M.M.; Chaves, M.M.; Zarrouk, O. Proanthocyanidin Accumulation and Biosynthesis Are Modulated by the Irrigation Regime in Tempranillo Seeds. Int. J. Mol. Sci. 2014, 15, 11862–11877. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Herrero, M.; Prodanov, M.; Ibáñez, E.; Cifuentes, A. Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction × reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 4627–4638. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of Alternative Physical Treatments (Ultrasounds, Pulsed Electric Fields, and High-Voltage Electrical Discharges) on Selective Recovery of Bio-compounds from Fermented Grape Pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef]
- Kuhnert, S.; Lehmann, L.; Winterhalter, P. Rapid characterisation of grape seed extracts by a novel HPLC method on a diol stationary phase. J. Funct. Foods 2015, 15, 225–232. [Google Scholar] [CrossRef]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Strazzanti, A.; Gangi, S.; Di Giacomo, C.; Basile, F.; Renis, M. Effects of Physical Activity on Systemic oxidative/DNA Status in Breast Cancer Survivors. Oncol. Lett. 2017, 13, 441–448. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; He, P.; Xu, S.; Ma, R.; Ding, Y.; Mu, L.; Li, S. Fluoride-induced iron overload contributes to hepatic oxidative damage in mouse and the protective role of Grape seed proanthocyanidin extract. J. Toxicol. Sci. 2018, 43, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The molecular and biochemical insight view of grape seed proanthocyanidins in ameliorating cadmium-induced testes-toxicity in rat model: Implication of PI3K/Akt/Nrf-2 signaling. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.H.; Yu, Z.L.; Bu, Y.J.; Xu, P.W.; Xi, J.Y.; Liang, H.Y. Grape seed proanthocyanidin extract alleviates high-fat diet induced testicular toxicity in rats. RSC Adv. 2019, 9, 11842–11850. [Google Scholar] [CrossRef]
- Xianchu, L.; Ming, L.; Xiangbin, L.; Lan, Z. Grape seed proanthocyanidin extract supplementation affects exhaustive exercise-induced fatigue in mice. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Oxidative Stress Induced by Cadmium in the Plasma, Erythrocytes and Lymphocytes of Rats: Attenuation by Grape Seed Proanthocyanidins. Hum. Exp. Toxicol. 2016, 35, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Liu, J.M.; Guo, S.X. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-induced Oxidative Reproductive Toxicity in Male Mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar]
- Lu, J.; Jiang, H.; Liu, B.; Baiyun, R.; Li, S.; Lv, Y.; Li, D.; Qiao, S.; Tan, X.; Zhang, Z. Grape seed procyanidin extract protects against Pb-induced lung toxicity by activating the AMPK/Nrf2/p62 signaling axis. Food Chem. Toxicol. 2018, 116, 59–69. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suárez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Thiruchenduran, M.; Vijayan, N.A.; Sawaminathan, J.K.; Devaraj, S.N. Protective effect of grape seed proanthocyanidins against cholesterol cholic acid diet-induced hypercholesterolemia in rats. Cardiovasc. Pathol. 2011, 20, 361–368. [Google Scholar] [CrossRef]
- Huang, L.-L.; Pan, C.; Wang, L.; Ding, L.; Guo, K.; Wang, H.-Z.; Xu, A.-M.; Gao, S. Protective effects of grape seed proanthocyanidins on cardiovascular remodeling in DOCA-salt hypertension rats. J. Nutr. Biochem. 2015, 26, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Iglesias, A.; Pajuelo, D.; Quesada, H.; Díaz, S.; Bladé, C.; Arola, L.; Salvadó, M.J.; Mulero, M. Grape Seed Proanthocyanidin Extract Improves the Hepatic Glutathione Metabolism in Obese Z Ucker Rats. Mol. Nutr. Food Res 2014, 58, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress-a review. BioMetals 2018, 31, 909–926. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C. Effects of Flavonoids on Immune and Inflammatory Cell Functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Li, W.G.; Zhang, X.Y.; Wu, Y.J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Weseler, A.R.; Bast, A. Masquelier’s Grape Seed Extract: From Basic Flavonoid Research to a Well-Characterized Food Supplement with Health Benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M.; Blay, M.T. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Back cover: Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1770096. [Google Scholar] [CrossRef]
- González-Quilen, C.; Gil-Cardoso, K.; Ginés, I.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Terra, X.; Blay, M.T. Grape-Seed Proanthocyanidins are Able to Reverse Intestinal Dysfunction and Metabolic Endotoxemia Induced by a Cafeteria Diet in Wistar Rats. Nutrients 2019, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Zhao, J.; Yan, J.; Meng, H.; Zhan, H.; Chen, L.; Yuan, L. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic Compounds as Natural and Multifunctional Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Terra, X.; Blay, M.; Pérez-Vendrell, A.M.; Pinent, M.; Ardévol, A. Grape Seed Proanthocyanidins Target the Enteroendocrine System in cafeteria-diet-fed Rats. Mol. Nutr. Food Res. 2019, 63, 1800912. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A Specific Dose of Grape Seed-Derived Proanthocyanidins to Inhibit Body Weight Gain Limits Food Intake and Increases Energy Expenditure in Rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Hayes, M.R.; Skibicka, K.P. GLP-1 and weight loss: Unraveling the diverse neural circuitry. Am. J. Physiol. Integr. Comp. Physiol. 2016, 310, R885–R895. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A.; Vila, Z.P. Lack of Tissue Accumulation of Grape Seed Flavanols after Daily Long-Term Administration in Healthy and Cafeteria-Diet Obese Rats. J. Agric. Food Chem. 2015, 63, 9996–10003. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining Conditions for Optimal Inhibition of Food Intake in Rats by a Grape-Seed Derived Proanthocyanidin Extract. Nutrients 2016, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Montagut, G.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pinent, M.; Ardèvol, A.; Blay, M.T. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance☆. J. Nutr. Biochem. 2010, 21, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; I Bravo, F.; Suárez, M.; Arola, L.; Bladé, C. Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Bladé, C.; Suárez, M.; Arola-Arnal, A. Grape Seed Proanthocyanidins Improve White Adipose Tissue Expansion during Diet-Induced Obesity Development in Rats. Int. J. Mol. Sci. 2018, 19, 2632. [Google Scholar] [CrossRef]
- Caimari, A.; Del Bas, J.; Crescenti, A.; Arola, L. Low Doses of Grape Seed Procyanidins Reduce Adiposity and Improve the Plasma Lipid Profile in Hamsters. Int. J. Obes. 2013, 37, 576. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.; Dai, X.; Jiang, Y.; Bao, L.; Li, Y.; Li, Y. Grape seed proanthocyanidins ameliorate pancreatic beta-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stress. Nutr. Metab. 2013, 10, 51. [Google Scholar] [CrossRef]
- Ginés, I.; Gil-Cardoso, K.; Serrano, J.; Casanova-Martí, À.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients 2018, 10, 315. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Liu, Z.; Gao, Z.; Li, B.; Zhang, D.; Zhang, Z.; Jiang, X.; Liu, Z.; Meng, L. Grape Seed Proanthocyanidin Extract Ameliorates Diabetic Bladder Dysfunction Via the Activation of the Nrf2 Pathway. PLoS ONE. 2015, 10, e0126457. [Google Scholar] [CrossRef]
- Favaretto, F.; Milan, G.; Collin, G.B.; Marshall, J.D.; Stasi, F.; Maffei, P.; Vettor, R.; Naggert, J.K. GLUT4 Defects in Adipose Tissue Are Early Signs of Metabolic Alterations in Alms1GT/GT, a Mouse Model for Obesity and Insulin Resistance. PLoS ONE 2014, 9, e109540. [Google Scholar] [CrossRef]
- Cedó, L.; Castell-Auví, A.; Pallarés, V.; Blay, M.; Ardèvol, A.; Arola, L.; Pinent, M.; Blay, M.T. Grape seed procyanidin extract modulates proliferation and apoptosis of pancreatic beta-cells. Food Chem. 2013, 138, 524–530. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Bhuvaneswari, S.; Sreeja, S.; Anuradha, C.V. Grape Seed Proanthocyanidins and Metformin Act by Different Mechanisms to Promote Insulin Signaling in Rats Fed High Calorie Diet. J. Cell Commun. Signal. 2014, 8, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, L.; Yu, T.; Zhu, J.; Shen, B.; Zhang, Y.; Wang, H.; Gao, S. Effects of Oligomeric Grape Seed Proanthocyanidins on Heart, Aorta, Kidney in DOCA-salt Mice: Role of Oxidative Stress. Phytother. Res. 2013, 27, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
| GSPE Extract Composition | GSPE Purity * | Dose | Study Design | Major Outcomes | Reference |
|---|---|---|---|---|---|
| Oxidative stress | |||||
| NE | 99% | 400 mg/kg BW/day | Fluoride-induced iron overload Kunming male mice; GSPE (5 weeks) | ↓ ALT, AST, MDA, iron content; ↑ GSH-Px, SOD, T-AOC | [42] |
| 54% dimeric, 13% trimeric procyanidins, and 7% tetrameric proanthocyanidins | NE | 100 mg/kg BW | Cd intoxicated-adult male albino Wistar rats; GSPE (4 weeks) | ↓ ROS, TBARS, LOOH, PC, CD, and NO, ↑ GSH, TSH, Vitamin C and E | [43] |
| Dimer (56%), trimer (12%), tetramer (6.6%), monomers and other high-molecular mass oligomers (20.4%) | >95% | 100 and 300 mg/kg BW | HFD fed male Sprague Dawley rats; GSPE (13 weeks) | ↓ MDA levels and ↑ GSH, GSH-Px and SOD activities of the testes tissue | [44] |
| NE | ≥95% | 1, 50 and 100 mg/kg BW/day | Male ICR mice; GSPE (28 days) | ↑ T-AOC, SOD, CAT and ↓ MDA activities in plasma and in skeletal muscle (50 and 100 mg/kg BW day of GSPE) | [45] |
| 54% dimeric, 13% trimeric and 7% tetrameric proanthocyanidins | NE | 100 mg/kg BW/day | Cd-intoxicated male albino Wistar rats; GSPE (4 weeks) | ↓ AST, ALT, ACP, LDH and ΥGT and ↑ ALP; ↓ TBARS, LH, NO plasma levels and ↑ vitamins C and E and GSH plasma concentration | [46] |
| NE | >95% | High dose 400 mg/kg BW/day, 100, 200, 400 mg/kg BW/day | As-induced Oxidative Reproductive Toxicity male Kunming mice; GSPE (5 weeks) | GSPE (400 mg/kg/BW) ↓ MDA and 8-OHdG levels and ↑ T-AOC, GSH and SOD activities | [47] |
| 56% dimeric, 12% trimeric procyanidins, and 6.6% tetrameric PACs | NE | 200 mg/kg BW/day | Pb-induced lung toxicity male Wistar rats, GSPE (5 weeks) | ↓ MDA levels and total Pb accumulation in the lung. ↑ GSH, SOD, γ-GCS activities and Nrf2 levels in lung tissue | [48] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 375 mg/kg BW/day | Male, spontaneously hypertensive rats, GSPE (2 days) | No changes in MDA liver tissue and plasma ACE, ↑ GSH | [49] |
| NE | 37% | 100 mg/kg BW/day | Hypercholesterolemic induced-male Wistar rats; GSPE (30 days) | ↑ SOD, CAT, GSH, ascorbate and α -tocopherol in cardiac tissue | [50] |
| NE | NE | 150, 240, 384 mg/kg BW/day | DOCA hypertension-induced SD rats; GSPE (4 weeks) | ↑ SOD activities, inhibition of the increase of serum and cardiac tissue MDA, inhibition of p-JNK1/2 and p-p38MAPK | [51] |
| Catechin (58 mol/g), epicatechin (52 mol/g), epigallocatechin (5.50 mol/g), epicatechin gallate (89 mol/g), epigallocatechin gallate (1.40 mol/g), dimeric procyanidins (250 mol/g), trimeric procyanidins (15.68 mol/g), tetrameric procyanidins (8.8 mol/g), pentameric procyanidins (0.73 mol/g), and hexameric procyanidins (0.38mol/g) | >75% | 35 mg/kg BW/day | Obese Zucker rats; GSPE (10 weeks) | ↑ GSH/GSSG ratio and ORAC, ↓ GSSG content | [52] |
| GSPE Extract Composition | GSPE Purity* | Dose | Study Design | Major Outcomes | Reference |
|---|---|---|---|---|---|
| Inflammation | |||||
| NE | >95% | 300 mg/kg BW/day | Specific-pathogen free male C57BL/6 mice fed with HFD; GSPE (7 weeks) | ↓ Plasma levels of TNF-α, IL-6 and MCP-1 | [61] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 345 mg/kg BW/day | HFD fed male Zucker rats; GSPE (19 weeks) | ↓ CRP, ↑ adiponectin plasma levels, no differences in IL-6 plasma levels | [59] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 30 mg/kg BW/day | HFD fed female Wistar rats; GSPE (15 weeks) - Preventive treatment | ↓ CRP and TNF-α plasma and adipose tissue levels, ↓ IL-6, Emr1 and ↑ adiponectin in adipose tissue | [60] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 10 or 20 mg/animal/day | Diet-induced obese female Wistar rats; GSPE (10 days or 30 days) | ↓ CRP and TNF-α plasma levels after 10 days (20 mg/animal/day) | [60] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 100 and 500 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE (2 weeks) | ↓ TNF-α secretions, ↓ transepithelial electrical resistance in small and large intestine, ↓ plasma LPS to basal levels | [62] |
| 56% dimeric, 12% trimeric procyanidins, and 6.6% tetrameric PACs | NE | 200 mg/kg BW/day | Pb-induced lung toxicity male Wistar rats, GSPE (5 weeks) | ↓ inflammatory cells in the lung tissue, ↓ TNF-α in lung tissue | [48] |
| NE | ≥95% | 1, 50 and 100 mg/kg BW/day | Male ICR mice; GSPE (28 days) | ↓ TNF-α and IL-1β activities in plasma and in skeletal muscle (50 and 100 mg/kg BW day of GSPE) | [45] |
| NE | 99.5 g GSP/mL | 10 mL/kg BW/day | Monocrotaline-induced PAH male Sprague–Dawley rats; GSPE (3 weeks) | Down regulation of myeloperoxidase, IL-1β, IL-6 and TNF-α in lung tissue | [63] |
| GSPE Extract Composition | GSPE Purity * | Dose | Study Design | Major Outcomes | Reference |
|---|---|---|---|---|---|
| Obesity | |||||
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 500 mg/kg BW/day | Aged male Wistar rats; GSPE (8 days) | ↓ Food intake, ↑ energy expenditure, ↓ BW | [71] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | Acute treatment 1000 mg/kg; Chronic treatment: 500 and 1000 mg/kg BW/day | Female Wistar rats; GSPE (8 days) | Acute treatment: ↑ GLP-1 plasma levels, CART mRNA expression; chronic treatment: no differences in leptin levels; no clear effects on the hypothalamic mRNA levels | [68] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 100 and 500 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE (2 weeks) | No changes in adiposity, ↓ BW gain at 500 mg/kg/BW | [62] |
| Catechin (121 ± 3 mg/g), epicatechin (93 ± 4 mg/g), PAC dimer B1 (89 ± 3 mg/g), PAC dimer B3 (46 ± 2 mg/g) | NE | 25 mg/kg BW/day | Diet-induced obese male Wistar rats; GSPE (3 weeks) | ↓ adipocyte size, no reduction of BW gain, no reversion of adiposity in WAT | [73] |
| Phenolic acids (1.63%), as well as monomeric (20.9%), dimeric (20.7%), trimeric (17.3%) and oligomeric (39.41%) procyanidins. | NE | 25 mg/kg BW/day | Male Golden Syrian hamsters fed with HFD; GSPE (15 days) | ↓ Adiposity index, the weight of the WAT depots and the BW gain | [75] |
| Dimer (56%), trimer (12%), tetramer (6.6%), monomers and other high-molecular mass oligomers (20.4%) | >95% | 300 mg/kg BW | HFD fed male Sprague Dawley rats; GSPE (13 weeks) | ↓ Relative weight of WAT | [44] |
| NE | >95% | 300 mg/kg BW/day | Specific-pathogen free male C57BL/6 mice fed with an HFD; GSPE (7 weeks) | ↓ Epidydimal fat mass, no changes in BW | [61] |
| NE | 37% | 100 mg/kg BW/day | Hypercholesterolemic induced-male Wistar rats; GSPE (30 days) | ↓ BW | [50] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 25 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE (10 days and 30 days) | ↓ the total amount of visceral adipose tissue, no reduction of BW gain, no changes in plasma leptin levels after 30 days intervention | [72] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 345 mg/kg BW/day | HFD fed Male Zucker rats; GSPE (19 weeks) | No changes in adiposity index or in BW | [59] |
| Monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%) and oligomeric (5–13 units) (31.7%) procyanidins and phenolic acids (4.7 %) | >75% | 30 mg/kg BW/day | HFD fed female Wistar rats; GSPE (15 weeks) - Preventive treatment | ↓ BW gain, no changes in adiposity or the weight of fat depots | [60] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 10 or 20 mg/animal/day | Diet-induced obese female Wistar rats; GSPE (10 days or 30 days) | No significant ↓in adiposity index or in BW | [60] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 25 mg/kg BW | Diet-induced obese male Wistar rats; GSPE (12 weeks) | No significant reduction in weight gain or reverse or adiposity, ↓ adipocyte size | [73] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 25, 100 and 200 mg/kg BW | Diet-induced obese male Wistar rats; GSPE (3 weeks) | Not improvement of adiposity index, prevention in the increase of the area and volume of the WAT, no change in leptin plasma levels, BW and upregulation PPARΥ (200 mg/kg BW) | [74] |
| Monomers of flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 500 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE intermittently | ↓ BW, total WAT, BAT, % visceral adiposity and % total adiposity | [77] |
| Diabetes | |||||
| 6.1% catechin, 6.78% epicatechin, 55.59% dimeric forms, 11.91% trimeric forms, 6.55% tetrameric forms and small amounts of other polymeric forms | 96.64% | 500 mg/kg BW/day | STZ-induced diabetic male Sprague–Dawley rats with basal diet; GSPE (16 weeks) | The score of beta-cell function and the abnormal oral glucose tolerance partially reversed, ↑ normal insulin content | [76] |
| Monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%) and oligomeric (5–13 units; 31.7%) | >75% | 25 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE (10 days and 30 days) | ↓ fasting plasma insulin levels after 10 and 30 days | [72] |
| Monomeric (21.3%), dimeric (17.4%), trimeric (16.3%), tetrameric (13.3%) and oligomeric (5–13 units) (31.7%) procyanidins and phenolic acids (4.7%) | NE | 345 mg/kg BW/day | HFD fed Male Zucker rats; GSPE (19 weeks) | ↓ glucose levels | [59] |
| 89 % PAC, 6 % monomers, and 5 % other materials | NE | 100 mg/kg BW/day | High fat-fructose diet fed male Wistar rats; GSPE (45 days) | ↓ glucose and insulin levels, ↑ insulin sensitivity, restoration of the activities of glycolytic enzymes in the liver | [81] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PAs | >75% | 25, 100 and 200 mg/kg BW | Diet-induced obese male Wistar rats; GSPE (12 weeks) | No significant changes in glucose, insulin or HOMA-IR plasma levels | [74] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PAs | >75% | 25 mg/kg BW/day | Diet-induced obese male Wistar rats; GSPE (3 weeks) | ↓ plasma glucose and insulin levels | [73] |
| Cardiovascular risk disease | |||||
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of procyanidins. | >75% | 375 mg/kg BW | Male, spontaneously hypertensive rats; GSPE (2 days) | ↓ DBP and SBP | [49] |
| NE | 37% | 100 mg/kg BW | Hypercholesterolemic induced-male Wistar rats; GSPE (30 days) | ↓ Tissue and serum cholesterol levels, LDL, serum free fatty acids, serum TAGs and ↑ (p < 0.01) serum phospholipids and HDL. | [50] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PAs | >75% | 25 mg/kg BW/day | Diet-induced obese male Wistar rats; GSPE (3 weeks) | ↓ TC | [73] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 345 mg/kg BW/day | HFD fed Male Zucker rats; GSPE (19 weeks) | No changes in total plasma cholesterol | [59] |
| NE | NE | 150, 240, 384 mg/kg BW/day | DOCA hypertension-induced SD rats; GSPE (4 weeks) | ↓ SBP, reversion of morphological hypertrophy of heart, ↑ in aortic rings vasodilatation | [51] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 25, 100 and 200 mg/kg BW | Diet-induced obese male Wistar rats; GSPE (12 weeks) | No significant changes in plasma TC, TAGs, HDL | [74] |
| Flavan-3-ols (21.3%), dimers (17.4%), trimers (16.3%), tetramers (13.3%) and oligomers (5–13 units; 31.7%) of PACs | >75% | 500 mg/kg BW/day | Diet-induced obese female Wistar rats; GSPE intermittently | No significant changes in plasma TAGs, fatty acids and cholesterol levels | [77] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. https://doi.org/10.3390/nu11102435
Rodríguez-Pérez C, García-Villanova B, Guerra-Hernández E, Verardo V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients. 2019; 11(10):2435. https://doi.org/10.3390/nu11102435
Chicago/Turabian StyleRodríguez-Pérez, Celia, Belén García-Villanova, Eduardo Guerra-Hernández, and Vito Verardo. 2019. "Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models" Nutrients 11, no. 10: 2435. https://doi.org/10.3390/nu11102435
APA StyleRodríguez-Pérez, C., García-Villanova, B., Guerra-Hernández, E., & Verardo, V. (2019). Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients, 11(10), 2435. https://doi.org/10.3390/nu11102435








