Identification of Dietary Patterns Related to Metabolic Diseases and Their Association with Cardiovascular Disease: From the Korean Genome and Epidemiology Study
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Subjects
2.2. Food Intake Data and DP Assessment
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
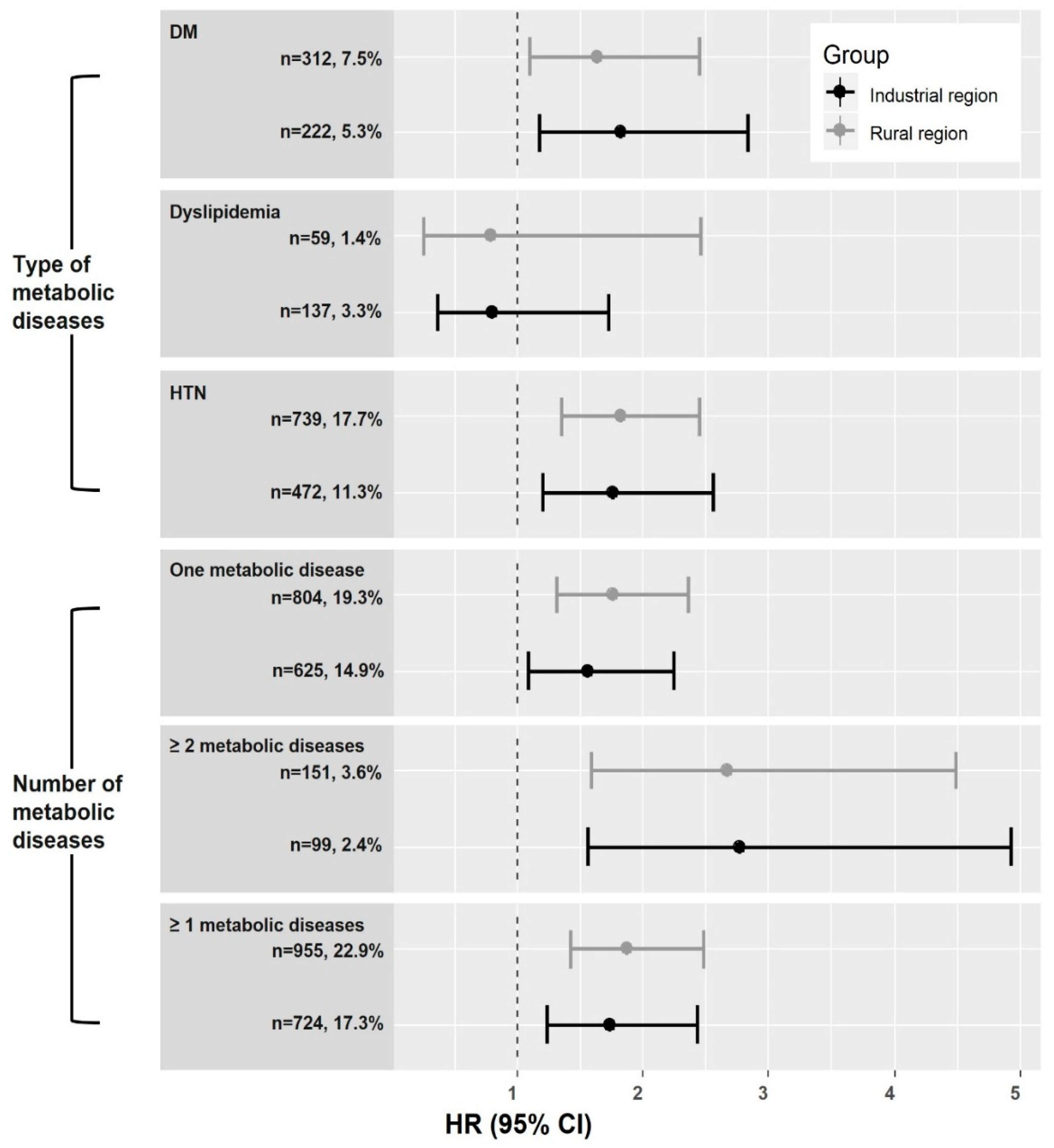
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk; World Health Organization: Geneva, Switzerland, 2007; Available online: http://www.who.int/iris/handle/10665/43685 (accessed on 25 January 2019).
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 132, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea. Cause of Death Statistics in 2017. Available online: http://www.kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=read&bSeq=&aSeq=370710&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt= (accessed on 12 February 2019).
- Shin, J.; Park, J.B.; Kim, K.I.; Kim, J.H.; Yang, D.H.; Pyun, W.B.; Kim, Y.G.; Kim, G.H.; Chae, S.C. Guideline Committee of the Korean Society of Hypertension. 2013 Korean Society of Hypertension guidelines for the management of hypertension: Part I-epidemiology and diagnosis of hypertension. Clin. Hypertens. 2015, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Korean Diabetes Association. 2015 Treatment Guidelines for Diabetes, 5th ed.; Available online: http://www.diabetes.or.kr/pro/publish/guide.php?code=guide&number=638&mode=view (accessed on 25 January 2019).
- Mertens, E.; Markey, O.; Geleijnse, J.M.; Givens, D.I.; Lovegrove, J.A. DPs in Relation to Cardiovascular Disease Incidence and Risk Markers in a Middle-Aged British Male Population: Data from the Caerphilly Prospective Study. Nutrients 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. DPs and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef]
- Hu, F.B.; Rimm, E.B.; Stampfer, M.J.; Ascherio, A.; Spiegelman, D.; Willett, W.C. Prospective study of major DPs and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000, 72, 912–921. [Google Scholar] [CrossRef]
- Hou, L.; Li, F.; Wang, Y.; Ou, Z.; Xu, D.; Tan, W.; Dai, M. Association between DPs and coronary heart disease: A meta-analysis of prospective cohort studies. Int. J. Clin. Exp. Med. 2015, 8, 781–790. [Google Scholar]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Lee, H.A.; Son, N.; Lee, W.K.; Park, H. A Diabetes-Related DP Is Associated with Incident Diabetes in Obese Men in the Korean Genome Epidemiology Study. J. Nutr. 2019, 149, 323–329. [Google Scholar] [CrossRef]
- Baik, I.; Cho, N.H.; Kim, S.H.; Shin, C. Dietary information improves cardiovascular disease risk prediction models. Eur. J. Clin. Nutr. 2013, 67, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.V.; Forouhi, N.G.; Khaw, K.T.; Wareham, N.J.; Monsivais, P. Accordance to the Dietary Approaches to Stop Hypertension diet pattern and cardiovascular disease in a British, population-based cohort. Eur. J. Epidemiol. 2018, 33, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, J.Y. Development of the Korean Version of Global Physical Activity Questionnaire and Assessment of Reliability and Validity Internet; Korea Centers for Disease Control and Prevention: Cheongju, Korea, 2013. Available online: http://www.cdc.go.kr (accessed on 16 July 2018).
- Osonoi, Y.; Mita, T.; Osonoi, T.; Saito, M.; Tamasawa, A.; Nakayama, S.; Someya, Y.; Ishida, H.; Kanazawa, A.; Gosho, M.; et al. Relationship between dietary patterns and risk factors for cardiovascular disease in patients with type 2 diabetes mellitus: A cross-sectional study. Nutr. J. 2016, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, Y.S.; Chang, H.C.; Moon, M.K.; Song, Y. Association between dietary patterns and blood lipid profiles in Korean adults with type 2 diabetes. J. Korean Med. Sci. 2011, 26, 1201–1208. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Koh, W.P.; Yuan, J.M.; Gross, M.D.; Pereira, M.A. Dietary patterns and mortality in a Chinese population. Am. J. Clin. Nutr. 2014, 100, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Woo, H.D.; Shin, A.; Kim, J. Dietary Patterns of Korean Adults and the Prevalence of Metabolic Syndrome: A Cross-Sectional Study. PLoS ONE 2014, 9, e111593. [Google Scholar] [CrossRef]
- Park, K.; Son, J.; Jang, J.; Kang, R.; Chung, H.K.; Lee, K.W.; Lee, S.M.; Lim, H.; Shin, M.J. Unprocessed Meat Consumption and Incident Cardiovascular Diseases in Korean Adults: The Korean Genome and Epidemiology Study (KoGES). Nutrients 2017, 9, 498. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G.; Pan, A.; Hu, F.B. Red and processed meat consumption and mortality: Dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016, 19, 893–905. [Google Scholar] [CrossRef]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospectivecohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development, Meat Consumption (Indicator). Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 25 January 2019). [CrossRef]
- Khaing, W.; Vallibhakara, S.A.; Attia, J.; McEvoy, M.; Thakkinstian, A. Effects of education and income on cardiovascular outcomes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2017, 24, 1032–1042. [Google Scholar] [CrossRef]
- Darmon, N.; Drewnowski, A. Does social class predict diet quality? Am. J. Clin. Nutr. 2008, 87, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haring, B.; Gronroos, N.; Nettleton, J.A.; von Ballmoos, M.C.; Selvin, E.; Alonso, A. Dietary protein intake and coronary heart disease in a large community based cohort: Results from the Atherosclerosis Risk in Communities (ARIC) study. PLoS ONE 2014, 9, e109552. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Huma Seurvices (USDHHS), National Institutes of Health (NIH), Office of Dietary Supplements. Niacin Fact Sheet for Health Professionals. 2019. Available online: https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/ (accessed on 25 January 2019).
- Rebholz, C.M.; Friedman, E.E.; Powers, L.J.; Arroyave, W.D.; He, J.; Kelly, T.N. Dietary Protein Intake and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Epidemiol. 2012, 176 (Suppl. S7), S27–S43. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, H. Diet-Related Risk Factors for Incident Hypertension During an 11-Year Follow-Up: The Korean Genome Epidemiology Study. Nutrients 2018, 10, 1077. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total (n = 8352) | Subjects with Metabolic Diseases (n = 1679, 20.1%) | Subjects without Metabolic Diseases (n = 6673, 79.9%) | p |
|---|---|---|---|---|
| Age (years) | 52.02 ± 8.84 | 55.68 ± 8.54 | 51.10 ± 8.67 | <0.0001 |
| Male | 3987 (47.74) | 774 (46.10) | 3213 (48.15) | 0.14 |
| Rural region | 4169 (49.92) | 955 (56.88) | 3214 (48.16) | <0.0001 |
| Education level | ||||
| Under high school | 4613 (55.54) | 1012 (60.74) | 3601 (54.24) | <0.0001 |
| Graduated high school | 2555 (30.76) | 414 (24.85) | 2141 (32.25) | |
| Some college or higher | 1137 (13.69) | 240 (14.41) | 897 (13.51) | |
| Monthly income (KRW), % | ||||
| <1,000,000 | 2821 (34.26) | 699 (42.13) | 2122 (32.27) | <0.0001 |
| 1,000,000 ≤ 1,999,999 | 2428 (29.48) | 461 (27.79) | 1967 (29.91) | |
| ≥2,000,000 | 2986 (36.26) | 499 (30.08) | 2487 (37.82) | |
| BMI (kg/m2) | 24.59 ± 3.13 | 25.68 ± 3.13 | 24.31 ± 3.08 | <0.0001 |
| Normal (<23 kg/m2) | 2594 (31.07) | 325 (19.39) | 2269 (34.01) | <0.0001 |
| Overweight (23–24.9 kg/m2) | 2192 (26.26) | 387 (23.09) | 1805 (27.05) | |
| Obese (≥25 kg/m2) | 3562 (42.67) | 964 (57.52) | 2598 (38.94) | |
| Current smoking | 2102 (25.38) | 354 (21.24) | 1748 (26.42) | <0.0001 |
| Alcohol intake (g/day) | ||||
| Non-intake | 4321 (52.99) | 960 (58.32) | 3361 (51.64) | <0.0001 |
| <15.0 g/day | 2258 (27.69) | 368 (22.36) | 1890 (29.04) | |
| 15.0–24.9g/day | 567 (6.95) | 118 (7.17) | 449 (6.90) | |
| ≥25.0 g/day | 1009 (12.37) | 200 (12.15) | 809 (12.43) | |
| Physical activity (MET-hours/week) | ||||
| Q1 (<25th) | 1885 (22.57) | 381 (22.69) | 1504 (22.54) | 0.94 |
| Q2 (25–49th) | 2289 (27.41) | 466 (27.75) | 1823 (27.32) | |
| Q3 (50–74th) | 2089 (25.01) | 410 (24.42) | 1679 (25.16) | |
| Q4 (≥75th) | 2089 (25.01) | 422 (25.13) | 1667 (24.98) | |
| Parental history of CVD | 306 (3.66) | 58 (3.45) | 248 (3.72) | 0.66 |
| Total energy (kcal) | 1943.78 ± 622.2 | 1924.1 ± 643.3 | 1948.7 ± 616.7 | 0.16 |
| Carbohydrate (g/day) | 342.89 ± 36.34 | 346.6 ± 36.62 | 342.0 ± 36.21 | <0.0001 |
| Protein (g/day) | 65.96 ± 12.32 | 65.84 ± 13.19 | 66.00 ± 12.09 | 0.66 |
| Fat (g/day) | 32.11 ± 12.18 | 30.45 ± 12.14 | 32.52 ± 12.16 | <0.0001 |
| Food Group | Subjects with Metabolic Diseases (n = 1679) | ||
|---|---|---|---|
| DP 1 | DP 2 | DP 3 | |
| Pork | 0.58 | ||
| Shellfish | 0.46 | 0.24 | |
| Beef | 0.46 | ||
| Fish | 0.45 | 0.34 | |
| Chicken | 0.43 | ||
| Mushrooms | 0.39 | 0.34 | |
| Other meat | 0.38 | ||
| Other drinks | 0.21 | ||
| Fruit | 0.54 | ||
| Vegetables | 0.20 | 0.51 | |
| Seaweeds | 0.38 | ||
| Potatoes | 0.24 | ||
| Soybean | 0.24 | ||
| Eggs | 0.21 | ||
| Milk | |||
| Sugar | 0.54 | ||
| Bread | 0.44 | ||
| Coffee | 0.34 | ||
| Noodles | −0.24 | 0.32 | |
| Carbonated drink | 0.26 | ||
| Dairy products | 0.22 | 0.24 | |
| Processed meat | 0.22 | 0.23 | |
| Oil and fat | 0.22 | ||
| Nuts and seeds | |||
| Kimchi | |||
| Rice | −0.48 | −0.54 | −0.59 |
| Variance explained (%) | 38.4 | 17.6 | 12.7 |
| DP1 Score | DP2 Score | DP3 Score | ||||
|---|---|---|---|---|---|---|
| Means | SD | Means | SD | Means | SD | |
| At baseline | ||||||
| with metabolic diseases (n = 1679) | 0.00 | 0.85 | 0.00 | 0.90 | 0.00 | 0.90 |
| without metabolic diseases (n = 6673) | 0.10 | 0.83 | −0.06 | 0.85 | 0.16 | 0.93 |
| p | <0.0001 | 0.01 | <0.0001 | |||
| with DM (n = 534) | 0.05 | 0.93 | 0.04 | 1.03 | −0.01 | 0.96 |
| without DM (n = 7818) | 0.08 | 0.82 | −0.06 | 0.84 | 0.14 | 0.92 |
| p | 0.40 | 0.03 | <0.001 | |||
| with dyslipidemia (n = 196) | 0.16 | 0.77 | −0.07 | 0.90 | 0.24 | 0.85 |
| without dyslipidemia (n = 8156) | 0.08 | 0.83 | −0.05 | 0.86 | 0.12 | 0.93 |
| p | 0.17 | 0.76 | 0.09 | |||
| with HTN (n = 1211) | −0.03 | 0.85 | 0.02 | 0.87 | −0.03 | 0.90 |
| without HTN (n = 7141) | 0.10 | 0.83 | −0.06 | 0.85 | 0.15 | 0.93 |
| p | <0.0001 | <0.01 | <0.0001 | |||
| During the follow-up period | ||||||
| with incident CVD (n = 431) | −0.06 | 0.85 | −0.09 | 0.80 | 0.04 | 0.88 |
| without incident CVD (n = 7921) | 0.09 | 0.83 | −0.05 | 0.86 | 0.13 | 0.93 |
| p | <0.001 | 0.24 | 0.06 | |||
| Model | Quintiles (Q) of Animal-Based DP Score | p for Trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Rural region | Incident case/person-years | 58/7961.9 | 46/7697.4 | 58/7745.1 | 50/7936.4 | 44/7767.6 | |
| Univariate 1 | Ref | 0.79 (0.53–1.17) | 1.01 (0.7–1.46) | 0.85 (0.58–1.25) | 0.77 (0.52–1.14) | 0.31 | |
| Model 1 1 | Ref | 0.78 (0.53–1.16) | 1.08 (0.75–1.56) | 1.01 (0.69–1.48) | 1.00 (0.67–1.50) | 0.63 | |
| Model 2 1 | Ref | 0.79 (0.53–1.18) | 1.13 (0.78–1.65) | 0.98 (0.66–1.46) | 0.96 (0.62–1.47) | 0.79 | |
| Model 3 1 | Ref | 0.79 (0.53–1.19) | 1.14 (0.79–1.66) | 0.99 (0.67–1.46) | 0.96 (0.62–1.47) | 0.79 | |
| Industrial region | Incident case/person-years | 42/8128.4 | 46/8160.4 | 36/8364.3 | 33/8199.7 | 18/8223.2 | |
| Univariate 1 | Ref | 1.07 (0.70–1.62) | 0.8 (0.51–1.25) | 0.76 (0.48–1.20) | 0.42 (0.24–0.72) | <0.001 | |
| Model 1 1 | Ref | 1.05 (0.69–1.59) | 0.78 (0.50–1.23) | 0.81 (0.51–1.28) | 0.43 (0.24–0.75) | 0.002 | |
| Model 2 1 | Ref | 1.05 (0.68–1.60) | 0.81 (0.52–1.28) | 0.82 (0.51–1.30) | 0.42 (0.24–0.74) | 0.002 | |
| Model 3 1 | Ref | 1.03 (0.67–1.58) | 0.79 (0.50–1.25) | 0.82 (0.51–1.30) | 0.41 (0.23–0.72) | 0.002 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.A.; An, H.; Park, H. Identification of Dietary Patterns Related to Metabolic Diseases and Their Association with Cardiovascular Disease: From the Korean Genome and Epidemiology Study. Nutrients 2019, 11, 2434. https://doi.org/10.3390/nu11102434
Lee HA, An H, Park H. Identification of Dietary Patterns Related to Metabolic Diseases and Their Association with Cardiovascular Disease: From the Korean Genome and Epidemiology Study. Nutrients. 2019; 11(10):2434. https://doi.org/10.3390/nu11102434
Chicago/Turabian StyleLee, Hye Ah, Hyoin An, and Hyesook Park. 2019. "Identification of Dietary Patterns Related to Metabolic Diseases and Their Association with Cardiovascular Disease: From the Korean Genome and Epidemiology Study" Nutrients 11, no. 10: 2434. https://doi.org/10.3390/nu11102434
APA StyleLee, H. A., An, H., & Park, H. (2019). Identification of Dietary Patterns Related to Metabolic Diseases and Their Association with Cardiovascular Disease: From the Korean Genome and Epidemiology Study. Nutrients, 11(10), 2434. https://doi.org/10.3390/nu11102434





