Impact of Bacterial Translocation on Sarcopenia in Patients with Decompensated Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Translocation Measurements
2.2. Inflammation Assessment
2.3. Nutritional Assessment
2.4. Metabolic Assessment
2.5. Outcome Variables
2.6. Statistical Analysis
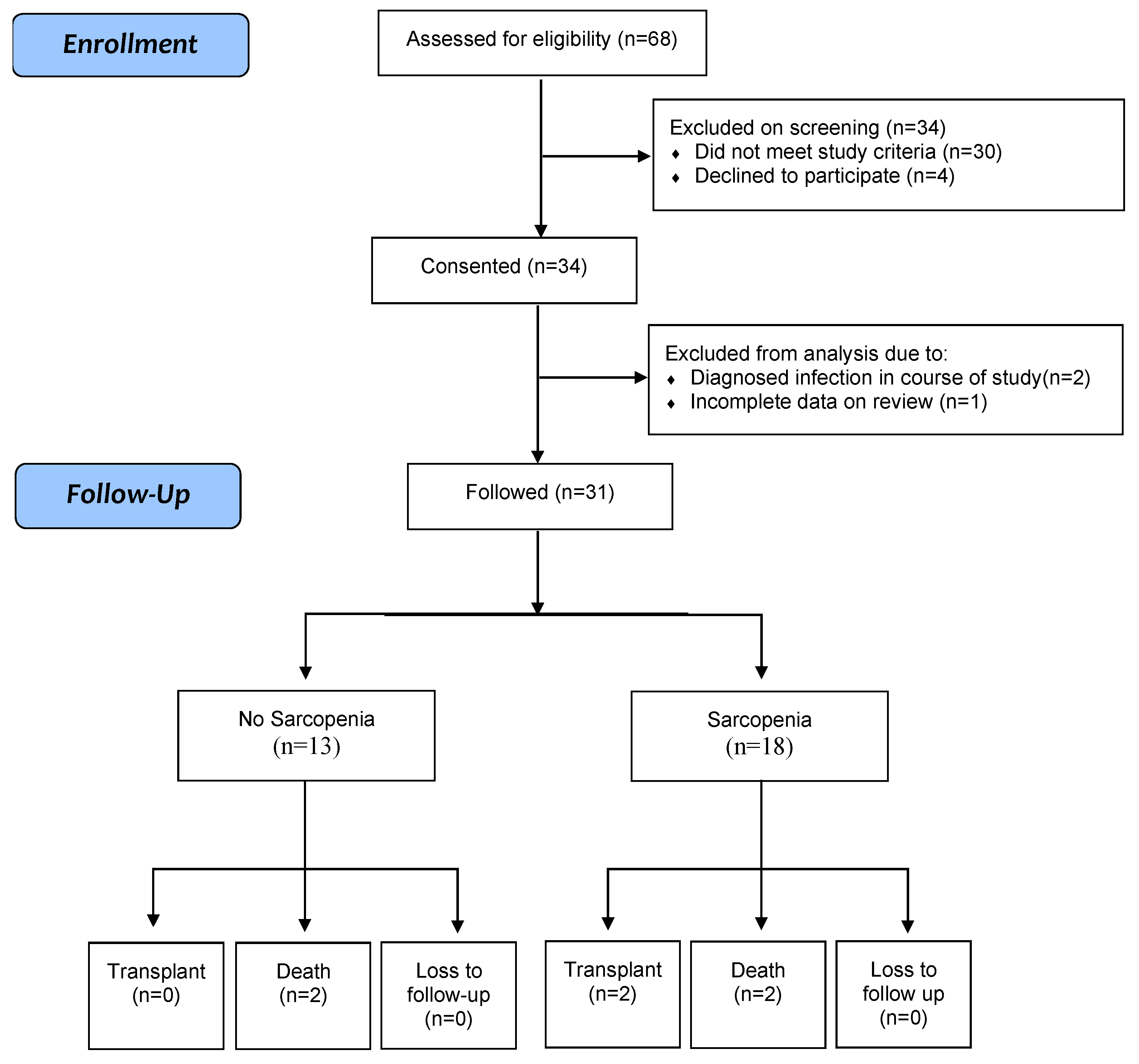
3. Results
3.1. Patient Demographics
3.2. Demographic, Nutritional and Metabolic Characteristics of Sarcopenic Patients
3.3. Possible Risk Factors and Sarcopenia
3.4. Bacterial DNA (bactDNA)
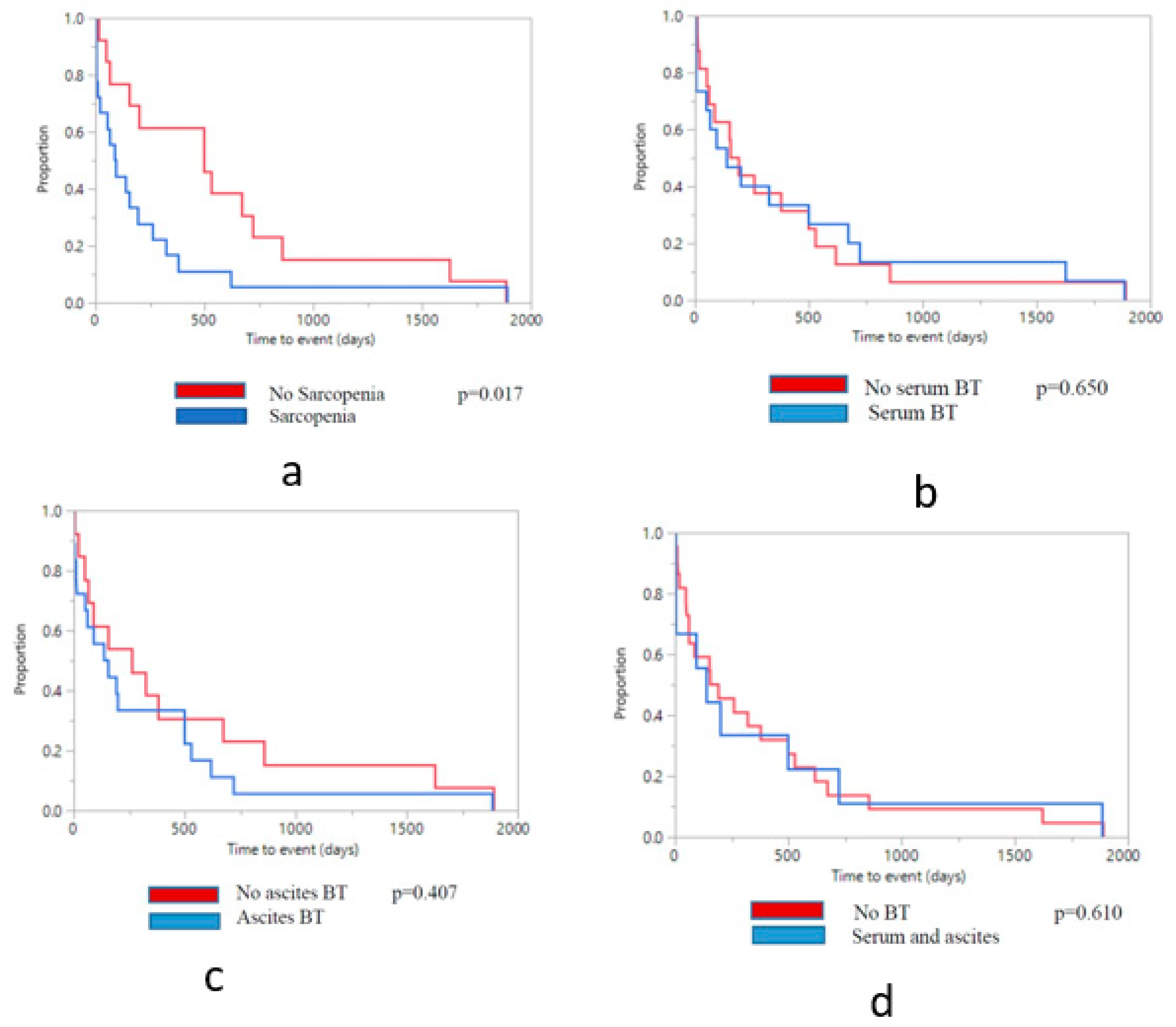
3.5. Clinical Outcomes by Sarcopenia Status
3.6. Clinical Outcomes by Bacterial Translocation Status
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.; Gramlich, L.; Bain, V.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl. 2012, 18, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Giusto, M.; Lucidi, C.; Giannelli, V.; Pentassuglio, I.; Di Gregorio, V.; Lattanzi, B.; Riggio, O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: Results of a prospective study. Metabol. Brain Dis. 2013, 28, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A. Clinical relevance of sarcopenia in patients with cirrhosis. World J. Gastroenterol. 2014, 20, 8061–8071. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.; Bain, V.; Sawyer, M.B. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Englesbe, M.J.; Patel, S.P.; He, K.; Lynch, R.J.; Schaubel, D.E.; Harbaugh, C.; Holcombe, S.A.; Wang, S.C.; Segev, D.L.; Sonnenday, C.J. Sarcopenia and post-liver transplant mortality. J. Am. Coll. Surg. 2010, 211, 271–278. [Google Scholar] [CrossRef]
- Davidson, H.I.; Richardson, R.; Sutherland, D.; Garden, O.J. Macronutrient preference, dietary intake, and substrate oxidation among stable cirrhotic patients. Hepatology 1999, 29, 1380–1386. [Google Scholar] [CrossRef]
- Glass, C.; Hipskind, P.; Tsien, C.; Malin, S.K.; Kasumov, T.; Shah, S.N.; Kirwan, J.P.; Dasarathy, S. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: A prospective controlled study. J. Appl. Physiol. 2013, 114, 559–565. [Google Scholar] [CrossRef]
- Tsien, C.; Davuluri, G.; Singh, D.; Allawy, A.; Ten Have, G.A.M.; Thapaliya, S.; Schulze, J.M.; Barnes, D.; McCullough, A.J.; Engelen, M.P.; et al. Branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015, 61, 2018–2029. [Google Scholar] [CrossRef]
- Jo, E.; Lee, S.-R.; Park, B.-S.; Kim, J.-S. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin. Exp. Res. 2012, 24, 412–422. [Google Scholar]
- Zaccherini, G.; Bernardi, M. The role and indications of albumin in advanced liver disease. Acta Gastroenterol. Belg. 2019, 82, 301–308. [Google Scholar] [PubMed]
- Berg, R.D.; Garlington, A.W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect. Immun. 1979, 23, 403–411. [Google Scholar] [PubMed]
- Bellot, P.; Garcia-Pagan, J.C.; Frances, R.; Abraldes, J.G.; Navasa, M.; Perez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010, 52, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Cirera, I.; Bauer, T.M.; Navasa, M.; Vila, J.; Grande, L.; Taura, P.; Fuster, J.; Garcia-Valdecasas, J.G.; Lacy, A.; Suarez, M.J.; et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatal. 2001, 34, 32–37. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, e9–e17. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Zapater, P.; Frances, R.; Gonzalez-Navajas, J.M.; de la Hoz, M.A.; Moreu, R.; Pascual, S.; Monfort, D.; Montoliu, S.; Vila, C.; Escudero, A.; et al. Serum and ascitic fluid bacterial DNA: A new independent prognostic factor in noninfected patients with cirrhosis. Hepatology 2008, 48, 1924–1931. [Google Scholar] [CrossRef]
- Salerno, F.; Guevara, M.; Bernardi, M.; Moreau, R.; Wong, F.; Angeli, P.; Garcia-Tsao, G.; Lee, S.S. Refractory ascites: Pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010, 30, 937–947. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef]
- Such, J.; Frances, R.; Munoz, C.; Zapater, P.; Casellas, J.A.; Cifuentes, A.; Rodriguez-Valera, F.; Pascual, S.; Sola-Vera, J.; Carnicer, F.; et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology 2002, 36, 135–141. [Google Scholar] [CrossRef]
- Frances, R.; Zapater, P.; Gonzalez-Navajas, J.M.; Munoz, C.; Cano, R.; Moreu, R.; Pascual, S.; Bellot, P.; Perez-Mateo, M.; Such, J. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology 2008, 47, 978–985. [Google Scholar] [CrossRef]
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS). JAMA 1995, 273, 117–123. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whitwell, J.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? JPEN J. Parenter. Enteral Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Johnson, P.E.; Bolonchuk, W.W.; Lykken, G.I. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am. J. Clin. Nutr. 1985, 41, 810–817. [Google Scholar] [CrossRef]
- Toeller, M.; Buyken, A.; Heitkamp, G.; Milne, R.; Klischan, A.; Gries, F.A. and the EURODIAB IDDM Complications Study Group. Repeatability of three-day dietary records in the EURODIAB IDDM Complications Study. Eur. J. Clin. Nutr. 1997, 51, 74–80. [Google Scholar] [CrossRef]
- Frisancho, A.R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [Google Scholar] [CrossRef]
- Carpenter, A.; Ng, V.L.; Chapman, K.; Ling, S.C.; Mouzaki, M. Predictive equations are inaccurate in the estimation of the resting energy expenditure of children with end-stage liver disease. JPEN J. Parenter. Enteral Nutr. 2017, 41, 507–511. [Google Scholar] [CrossRef]
- Tsien, C.; Rabie, R.; Wong, F. Acute kidney injury in decompensated cirrhosis. Gut 2013, 62, 131–137. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the study of liver diseases and the European Association for the Study of the liver. Hepatology 2015, 60, 715–735. [Google Scholar] [CrossRef]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V.; et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019. [Google Scholar] [CrossRef]
- Owen, O.E.; Trapp, V.E.; Reichard, G.A.; Mozzoli, M.A.; Moctezuma, J.; Paul, P.; Skutches, C.L.; Boden, G. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J. Clin. Investig. 1983, 72, 1821–1832. [Google Scholar] [CrossRef]
- Periyalwar, P.; Dasarathy, S. Malnutrition in cirrhosis: Contribution and consequences of sarcopenia on metabolic and clinical responses. Clin. Liver Dis. 2012, 16, 95–131. [Google Scholar] [CrossRef]
- Xia, Z.; Cholewa, J.; Zhao, Y.; Shang, H.-Y.; Yang, Y.-Q.; Pessoa, K.A.; Su, Q.-S.; Lima-Soares, F.; Zanchi, N.E. Targeting inflammation and downstream protein metabolism in sarcopenia: A brief up-dated description of concurrent exercise and leucine-based multimodal intervention. Front. Physiol. 2017, 8, 434. [Google Scholar] [CrossRef]
- Frost, R.A.; Lang, C.H. mTor signaling in skeletal muscle during sepsis and inflammation: Where does it all go wrong? Physiology 2011, 26, 83–96. [Google Scholar] [CrossRef]
- Balage, M.; Averous, J.; Rémond, D.; Bos, C.; Pujos-Guillot, E.; Papet, I.; Mosoni, L.; Combaret, L.; Dardevet, D. Presence of low-grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J. Nutr. Biochem. 2010, 21, 325–331. [Google Scholar] [CrossRef]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef]
| Sarcopenia Absent (n = 13) | Sarcopenia Present (n = 18) | p-Value | |
|---|---|---|---|
| Age (years) (mean, stdev) | 57.9 ± 7.4 | 55.6 ± 9.7 | 0.437 |
| Male gender (n, %) | 8, 61.5 | 13, 72.2 | 0.532 |
| BMI (kg/m2) (mean, stdev) | 30.2 ± 7.1 | 24.0 ± 3.7 | 0.059 |
| BIA—Fat mass (kg) (mean, stdev) | 30.6 ± 16.0 | 16.9 ± 8.1 | 0.059 |
| BIA-Fat-free mass (kg) (mean, stdev) | 59.6 ± 18.3 | 54.8 ± 12.4 | 0.529 |
| Triceps skinfold thickness (mm) (mean, stdev) | 15.6 ± 5.5 | 8.2 ± 2.9 | 0.005 |
| Midarm muscle area (cm2) (mean, st dev) | 45.2 ± 12.5 | 27.4 ± 5.4 | 0.007 |
| Midarm fat area (cm2) (mean, stdev) | 22.6 ± 8.5 | 9.5 ± 3.6 | 0.008 |
| Respiratory quotient (mean, stdev) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.772 |
| Measured Resting Energy Expenditure (kcal/day) (mean, stdev) | 1564.3 ± 345.6 | 1461.6 ± 258.7 | 0.584 |
| REE (% predicted) (mean, stdev) | 89.8 ± 14.1 | 93.3 ± 8.7 | 0.614 |
| Caloric intake (mean, stdev) Carbohydrates (gm) Fat (gm) Protein (gm) | 1766.0 ± 633.1 242.7 ± 91.9 53.4 ± 33.1 82.6 ± 39.4 | 1641.6 ± 458.0 210.3 ± 78.3 54.8 ± 18.2 78.5 ± 31.1 | 0.920 0.900 0.727 0.993 |
| SGA (n, %) Class A Class B Class C | 5, 38.5 6, 46.2 2, 15.4 | 2, 11.1 8, 44.4 8, 44.4 | 0.072 0.925 0.079 |
| Sarcopenia Absent (n = 13) | Sarcopenia Present (n = 18) | p-Value | |
|---|---|---|---|
| Etiology of liver disease (n, %) ETOH Viral NAFLD ETOH/viral Other | 7, 53.8 3, 23.1 1, 7.7 2, 15.4 0, 0 | 9, 50.0 4, 22.2 2, 11.1 3, 16.7 1, 5.6 | 0.860 |
| Bilirubin (umol/L) (mean, stdev) | 31.8 ± 17.9 | 30.1 ± 26.1 | 0.712 |
| INR (mean, stdev) | 1.30 ± 0.3 | 1.39 ± 0.3 | 0.349 |
| Creatinine (umol/L) (mean, stdev) | 79.4 ± 22.1 | 76.2 ± 23.3 | 0.656 |
| Albumin (mg/dL) (mean, stdev) | 35.1 ± 5.1 | 31.7 ± 5.0 | 0.083 |
| Sodium (mmol/L) (mean, stdev) | 134.9 ± 3.3 | 134.1 ± 4.2 | 0.806 |
| MELD score (mean, stdev) | 11.4 ± 3.5 | 11.7 ± 4.5 | 0.888 |
| Child-Pugh score (mean, stdev) | 8.1 ± 1.6 | 9.0 ± 1.5 | 0.767 |
| Baseline SIRS (n, %) | 1, 7.7 | 2, 11.1 | 0.677 |
| BT - Ascites (n, %) | 8, 61.5 | 10, 55.6 | 0.739 |
| BT - Serum (n, %) | 8, 61.5 | 7, 38.9 | 0.211 |
| BT -Both Serum and Ascites (n, %) | 4, 30.8 | 5, 27.8 | 0.857 |
| Sarcopenia Absent (n =13) | Sarcopenia Present (n = 18) | p-Value | Bacterial Translocation Ascites Only (n = 19) | Bacterial Translocation Serum Only (n = 15) | Bacterial Translocation Both (n = 9) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Number Events (n) | Patients with Outcome (n, %) | Total Number Events (n) | Patients with Outcome (n, %) | Total Number Events (n) | Patients with Outcome (n, %, p-Value) | Total Number Events (n) | Patients with Outcome (n, %, p-Value) | Total Number Events (n) | Patients with Outcome (n, %, p-Value) | ||
| AKI | 0 | 0, 0 | 3 | 3, 16.7 | 0.050 | 2 | 2, 10.5, 0.765 | 2 | 2, 13.3, 0.421 | 2 | 2, 22.2, 0.133 |
| Transplants | 0 | 0, 0 | 2 | 2, 11.1 | 0.132 | 1 | 1, 5.3, 0.812 | 1 | 1, 6.7, 0.962 | 1 | 1, 11.1, 0.519 |
| TIPS | 6 | 6, 46.2 | 7 | 7, 38.9 | 0.981 | 11 | 11, 57.9,0.019 | 5 | 5, 33.3, 0.551 | 4 | 4, 44.4, 0.676 |
| Infections | 5 | 2, 15.4 | 1 | 1, 5.6 | 0.364 | 3 | 1, 5.3, 0.364 | 3 | 1, 6.7, 0.579 | 3 | 1, 11.1, 0.865 |
| HE | 2 | 2, 15.4 | 2 | 1, 5.6 | 0.812 | 3 | 2, 10.5, 0.812 | 0 | 0, 0, 0.059 | 0 | 0, 0, 0.232 |
| SBP | 3 | 2, 15.4 | 1 | 1, 5.6 | 0.364 | 1 | 1, 5.3, 0.364 | 1 | 1, 6.7, 0.579 | 1 | 1, 11.1, 0.865 |
| Admission | 7 | 7, 53.8 | 6 | 4, 22.2 | 0.160 | 15 | 6, 31.6, 0.531 | 9 | 3, 20.0, 0.153 | 7 | 2, 22.2, 0.435 |
| Deaths | 2 | 2, 15.4 | 2 | 2, 11.1 | 0.429 | 1 | 1, 5.3, 0.846 | 2 | 2, 13.3, 0.076 | 1 | 1, 11.1, 0.545 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsien, C.; Antonova, L.; Such, J.; Garcia-Martinez, I.; Wong, F. Impact of Bacterial Translocation on Sarcopenia in Patients with Decompensated Cirrhosis. Nutrients 2019, 11, 2379. https://doi.org/10.3390/nu11102379
Tsien C, Antonova L, Such J, Garcia-Martinez I, Wong F. Impact of Bacterial Translocation on Sarcopenia in Patients with Decompensated Cirrhosis. Nutrients. 2019; 11(10):2379. https://doi.org/10.3390/nu11102379
Chicago/Turabian StyleTsien, Cynthia, Lilia Antonova, Jose Such, Irma Garcia-Martinez, and Florence Wong. 2019. "Impact of Bacterial Translocation on Sarcopenia in Patients with Decompensated Cirrhosis" Nutrients 11, no. 10: 2379. https://doi.org/10.3390/nu11102379
APA StyleTsien, C., Antonova, L., Such, J., Garcia-Martinez, I., & Wong, F. (2019). Impact of Bacterial Translocation on Sarcopenia in Patients with Decompensated Cirrhosis. Nutrients, 11(10), 2379. https://doi.org/10.3390/nu11102379





