A Nutraceutical Rich in Docosahexaenoic Acid Improves Portal Hypertension in a Preclinical Model of Advanced Chronic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model of Advanced Chronic Liver Disease
2.2. Docosahexaenoic Acid Administration
2.3. In Vivo Hemodynamic Analysis
2.4. Biochemical Analysis
2.5. Liver Histology
2.6. Oil Red O Staining
2.7. Immunofluorescence
2.8. Cell Culture and Docosahexaenoic Acid Treatment
2.9. RNA Isolation and Quantitative PCR
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Hepatic Fatty Acid Profile Is Deregulated in Rats with ACLD
3.2. Treatment with DHA Reestablishes a Healthy Lipid Profile
3.3. DHA Ameliorates Portal Hypertension in Rats with ACLD
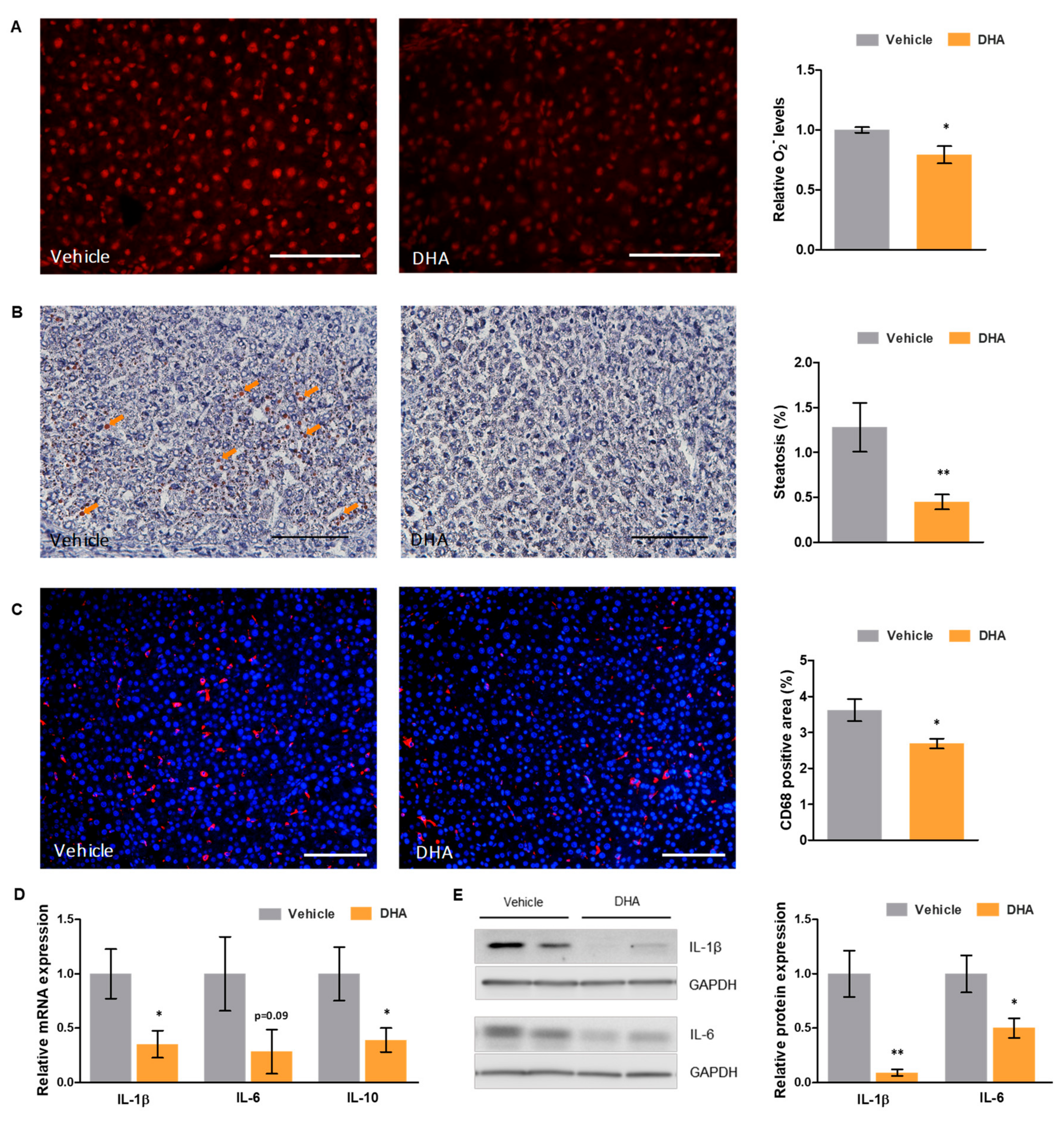
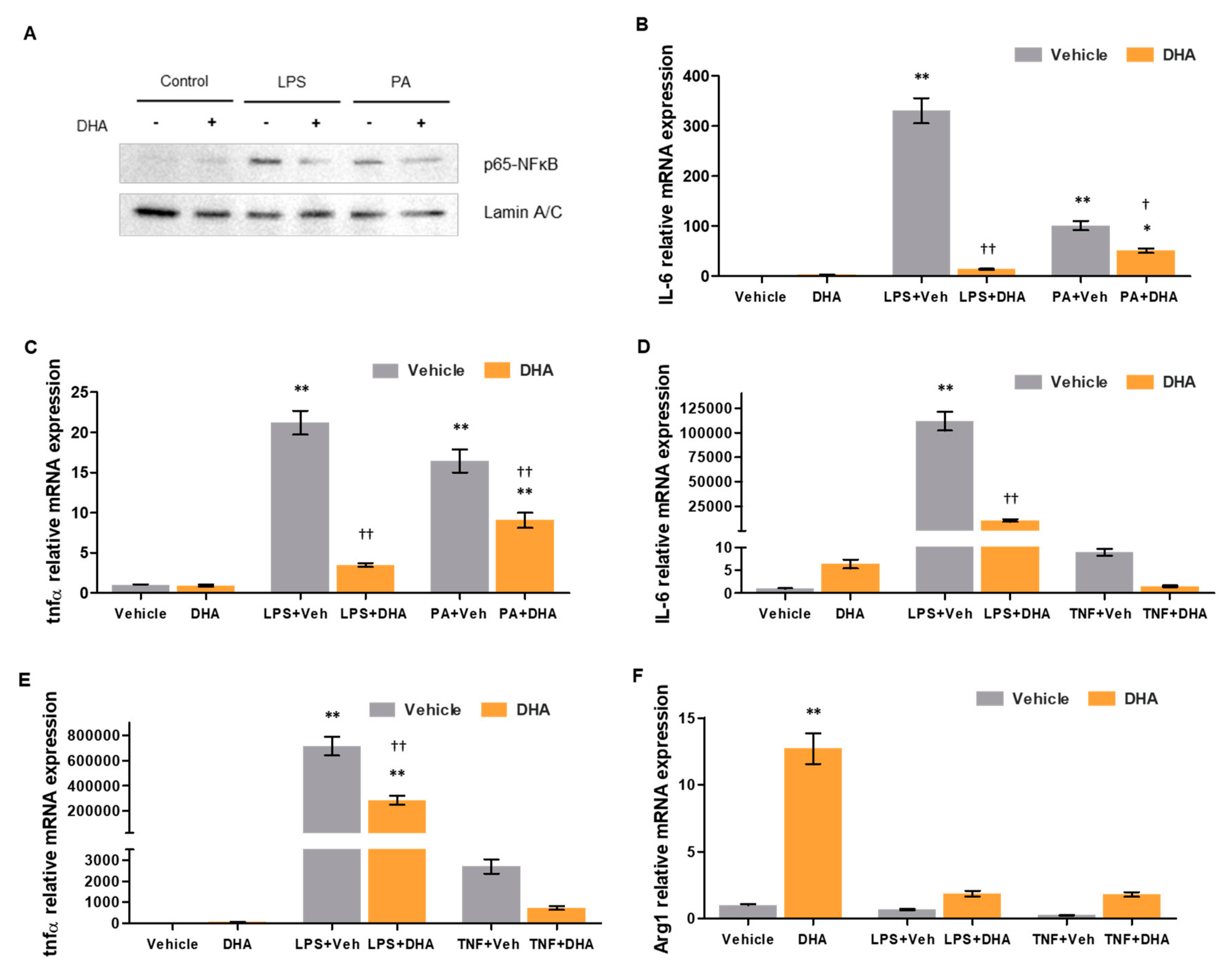
3.4. DHA Reduces Oxidative Stress and Inflammation
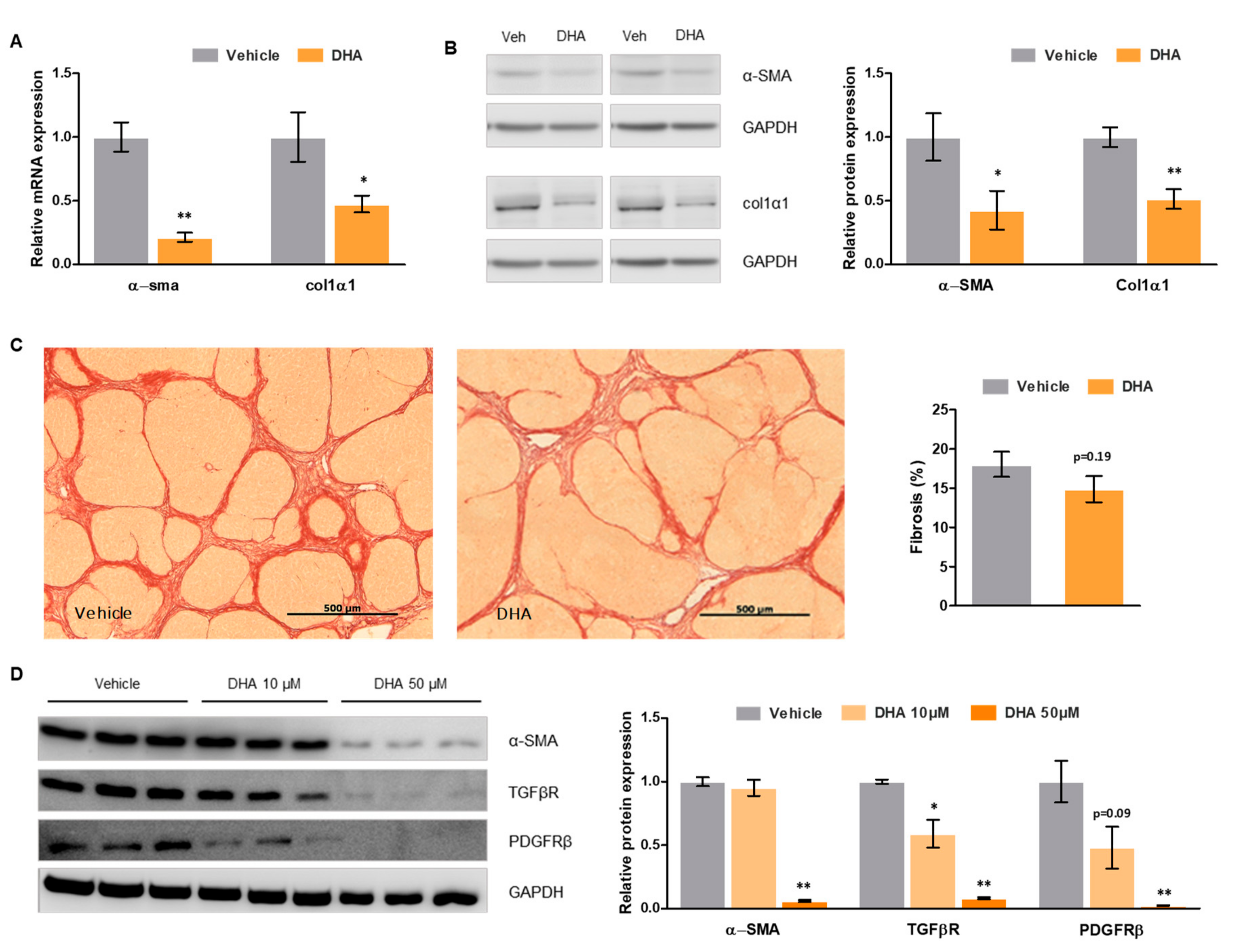
3.5. DHA Promotes HSC Deactivation in Rats with ACLD
3.6. DHA Promotes Deactivation of Human HSC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Vilaseca, M.; Guixé-Muntet, S.; Fernández-Iglesias, A.; Gracia-Sancho, J. Advances in therapeutic options for portal hypertension. Therap. Adv. Gastroenterol. 2018, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Marrone, G.; Fernández-Iglesias, A. Hepatic microcirculation and mechanisms of portal hypertension. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, H.; Thompson, M.G.; Thomas, T.; Geerts, A. Hepatic stellate cells: Role in microcirculation and pathophysiology of portal hypertension. Gut 2002, 50, 571–581. [Google Scholar] [CrossRef]
- Sarem, M.; Znaidak, R.; Macías, M.; Rey, R. Hepatic stellate cells: Its role in normal and pathological conditions. Gastroenterol. Hepatol. 2006, 29, 93–101. [Google Scholar] [CrossRef]
- Fernández-Iglesias, A.; Gracia-Sancho, J. How to Face Chronic Liver Disease: The Sinusoidal Perspective. Front. Med. 2017, 4, 7. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Bosch, J.; Blei, A.; Arroyo, V. Portal hypertension and its complications. Gastroenterology 2008, 134, 1715–1728. [Google Scholar] [CrossRef]
- El-Badry, A.M.; Graf, R.; Clavien, P.A. Omega 3–Omega 6: What is right for the liver? J. Hepatol. 2007, 47, 718–725. [Google Scholar] [CrossRef]
- Basili, S.; Raparelli, V.; Napoleone, L.; Del Ben, M.; Merli, M.; Riggio, O.; Nocella, C.; Carnevale, R.; Pignatelli, P.; Violi, F.; et al. Polyunsaturated fatty acids balance affects platelet NOX2 activity in patients with liver cirrhosis. Dig. Liver Dis. 2014, 46, 632–638. [Google Scholar] [CrossRef]
- Araya, J.; Rodrigo, R.; Videla, L.A.; Thielemann, L.; Orellana, M.; Pettinelli, P.; Poniachik, J. Increase in long-chain polyunsaturated fatty acid n−6/n−3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. 2004, 106, 635–643. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, M.; García-Calderó, H.; Lafoz, E.; García-Irigoyen, O.; Avila, M.A.; Reverter, J.C.; Bosch, J.; Hernández-Gea, V.; Gracia-Sancho, J.; García-Pagán, J.C. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology 2017, 65, 2031–2044. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.M.; Vilaseca, M.; Lafoz, E.; Garcia-Calderó, H.; Viegas Haute, G.; Fernández-Iglesias, A.; Rodrigues de Oliveira, J.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. Simvastatin Prevents Progression of Acute on Chronic Liver Failure in Rats With Cirrhosis and Portal Hypertension. Gastroenterology 2018, 155, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Manicardi, N.; Ortega-Ribera, M.; Maeso-Díaz, R.; Guixé-Muntet, S.; Fernández-Iglesias, A.; Hide, D.; García-Calderó, H.; Boyer-Díaz, Z.; Contreras, P.C.; et al. Emricasan Ameliorates Portal Hypertension and Liver Fibrosis in Cirrhotic Rats Through a Hepatocyte-Mediated Paracrine Mechanism. Hepatol. Commun. 2019, 3, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [PubMed]
- Gracia-Sancho, J.; Laviña, B.; Rodríguez-Vilarrupla, A.; García-Calderó, H.; Fernández, M.; Bosch, J.; García-Pagán, J.C. Increased oxidative stress in cirrhotic rat livers: A potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology 2008, 47, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef]
- Marrone, G.; Maeso-Díaz, R.; García-Cardena, G.; Abraldes, J.G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: Behind the molecular mechanisms of statins. Gut 2015, 64, 1434–1443. [Google Scholar] [CrossRef]
- Maeso-D, R.; Boyer-Diaz, Z.; Lozano, J.J.; Ortega-Ribera, M.; Peralta, C.; Bosch, J.; Gracia-Sancho, J. New Rat Model of Advanced NASH Mimicking Pathophysiological Features and Transcriptomic Signature of The Human Disease. Cells 2019, 8, 1062. [Google Scholar] [CrossRef]
- de Mingo Pulido, Á.; de Gregorio, E.; Chandra, S.; Colell, A.; Morales, A.; Kronenberg, M.; Marí, M. Differential role of cathepsins S and B in hepatic APC-mediated NKT cell activation and cytokine secretion. Front. Immunol. 2018, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Guixé-Muntet, S.; de Mesquita, F.C.; Vila, S.; Hernández-Gea, V.; Peralta, C.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J. Hepatol. 2017, 66, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit Rev. Eukaryot Gene Expr. 2011, 20, 87–103. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, Y.; Fang, Q.; Zhong, P.; Li, W.; Wang, L.; Fu, W.; Zhang, Y.; Xu, Z.; Li, X.; et al. Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat. Commun. 2017, 8, 13997. [Google Scholar] [CrossRef] [PubMed]
- Enguita, M.; Razquin, N.; Pamplona, R.; Quiroga, J.; Prieto, J.; Fortes, P. The cirrhotic liver is depleted of docosahexaenoic acid (DHA), a key modulator of NF-κB and TGFβ pathways in hepatic stellate cells. Cell Death Dis. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Kemuriyama, N.; Matsuzaka, T.; Kuba, M.; Ohno, H.; Han, S.; Takeuchi, Y.; Isaka, M.; Kobayashi, K.; Iwasaki, H.; Yatoh, S.; et al. Different effects of eicosapentaenoic and docosahexaenoic acids on atherogenic high- fat diet-induced non-alcoholic fatty liver disease in mice. PLoS ONE 2016, 11, e0157580. [Google Scholar] [CrossRef]
- Lytle, K.A.; Wong, C.P.; Jump, D.B. Docosahexaenoic acid blocks progression of western diet-induced nonalcoholic steatohepatitis in obese Ldlr-/- mice. PLoS ONE 2017, 12, e0173376. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Bhatia, L.; Mccormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the WELCOME* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Nobili, V.; Carpino, G.; Alisi, A.; De Vito, R.; Franchitto, A.; Alpini, G.; Onori, P.; Gaudio, E. Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. PLoS ONE 2014, 9, e88005. [Google Scholar] [CrossRef]
- González-Périz, A.; Planagumà, A.; Gronert, K.; Miquel, R.; López-Parra, M.; Titos, E.; Horrillo, R.; Ferré, N.; Deulofeu, R.; Arroyo, V.; et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: Protectin D1 and 17 S-hydroxy-DHA. FASEB J. 2006, 20, 2537–2539. [Google Scholar] [CrossRef]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic Acid Attenuates Hepatic Inflammation, Oxidative Stress, and Fibrosis without Decreasing Hepatosteatosis in a Ldlr−/− Mouse Model of Western Diet-Induced Nonalcoholic Steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Iredale, J.P.; Thompson, A.; Henderson, N.C. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yan, J.; Wang, H.; Shi, M.; Zhang, M.; Yang, W.; Peng, C.; Li, H. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS ONE 2014, 9, e83908. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Muse, A.Y.; Khan, D.M.I.O.; Ahmed, I.H.; Subhan, N.; Reza, H.M.; Alam, M.A.; Nahar, L.; Sarker, S.D. Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomed. Pharmacother. 2017, 92, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, M.; García-Calderó, H.; Lafoz, E.; Ruart, M.; López-Sanjurjo, C.I.; Murphy, M.P.; Deulofeu, R.; Bosch, J.; Hernández-Gea, V.; Gracia-Sancho, J.; et al. Mitochondria-targeted antioxidant mitoquinone deactivates human and rat hepatic stellate cells and reduces portal hypertension in cirrhotic rats. Liver Int. 2017, 37, 1002–1012. [Google Scholar] [CrossRef]
- Wang, D.; Yin, J.; Dong, R.; Zhao, J.; Wang, Q.; Wang, N.; Wang, S.; Du, X.; Lu, J. Inhibition of Janus kinase-2 signalling pathway ameliorates portal hypertensive syndrome in partial portal hypertensive and liver cirrhosis rats. Dig. Liver Dis. 2015, 47, 315–323. [Google Scholar] [CrossRef] [PubMed]
| Fatty Acid | Healthy Vehicle | Healthy DHA | Cirrhotic Vehicle | Cirrhotic DHA |
|---|---|---|---|---|
| SFA | ||||
| MA (14:0) | 0.39 ± 0.04 | 0.31 ± 0.03 | 0.43 ± 0.01 | 0.30 ± 0.02 ** |
| PA (C16:0) | 21.09 ± 0.31 | 21.48 ± 0.67 | 23.28 ± 0.41 ### | 24.58 ± 0.39 |
| SA (C18:0) | 17.82 ± 0.26 | 17.40 ± 0.72 | 14.43 ± 0.88### | 16.02 ± 0.54 |
| MUFA | ||||
| POA (C16:1 n-7) | 1.74 ± 0.17 | 1.68 ± 0.22 | 2.64 ± 0.30 | 1.74 ± 2.13 * |
| OA (C18:1 n-9) | 8.40 ± 0.54 | 7.33 ± 0.40 | 15.70 ± 1.32 ### | 8.87 ± 0.22 *** |
| VAC (C18:1 n-7) | 3.77 ± 0.29 | 3.03 ± 0.10 | 3.36 ± 0.16 | 2.85 ± 0.16 |
| EIA (C20:1 n-9) | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| NA (C24:1 n-9) | 0.27 ± 0.02 | 0.24 ± 0.01 | 0.29 ± 0.03 | 0.34 ± 0.03 |
| PUFA | ||||
| n-3 series | ||||
| ALA (C18:3 n-3) | 0.40 ± 0.04 | 0.43 ± 0.05 | 0.40 ± 0.03 | 0.25 ± 0.02 *** |
| ETE (C20:3 n-3) | 0.30 ± 0,02 | 0.22 ± 0.02 * | 0.28 ± 0.02 | 0.13 ± 0.02 ** |
| EPA (C20:5 n-3) | 0.18 ± 0.03 | 1.4 ± 0.26 ** | 0.11 ± 0.02 | 1.25 ± 0.08 *** |
| DPA (C22:5 n-3) | 0.59 ± 0.03 | 1.41 ± 0.16 ** | 0.64 ± 0.03 | 1.42 ± 0.08 *** |
| DHA (C22:6 n-3) | 5.85 ± 0.48 | 9.82 ± 0.52 ** | 1.97 ± 0.25 ### | 11.70 ± 0.45 *** |
| n-6 series | ||||
| LA (C18:2 n-6) | 17.63 ± 0.54 | 18.37 ± 0.31 | 18.13 ± 0.42 | 17.32 ± 0.29 |
| GLA (C18:3 n-6) | 0.16 ± 0.01 | 0.11 ± 0.01 * | 0.32 ± 0.02 | 0.08 ± 0.01 *** |
| HGLA (C20:3 n-6) | 0.52 ± 0.05 | 0.84 ± 0.05 *** | 0.,32 ± 0.02 | 0.82 ± 0.05 *** |
| AA (C20:4 n-6) | 18.59 ± 0.32 | 14.76 ± 0.72 *** | 14.86 ± 0.90 ### | 10.25 ± 0.34 *** |
| DTA (C22:4 n-6) | 0.49 ± 0.02 | 0.20 ± 0.02 *** | 0.73 ± 0.07 | 0.21 ± 0.01 *** |
| DPA (C22:5 n-6) | 0.26 ± 0.01 | 0.20 ± 0.02 | 1.07 ± 0.18 | 0.24 ± 0.02 *** |
| Total SFA | 40.26 ± 0.54 | 40.11 ± 0.90 | 39.01 ± 0.53 | 42.07 ± 0.35 *** |
| Total MUFA | 14.31 ± 0.93 | 12.34 ± 0.56 | 22.11 ± 1.55 ### | 13.93 ± 0.36 *** |
| Total PUFA | 45.44 ± 0.97 | 47.50 ± 0.74 | 38.88 ± 1.08 ### | 43.99 ± 0.37 *** |
| Total n-3 FA | 7.32 ± 0.54 | 12.94 ± 0.86 *** | 3.11 ± 0.24 ### | 14.74 ± 0.47 *** |
| Total n-6 FA | 38.11 ± 0.76 | 34.55 ± 0.65 *** | 35.77 ± 0.85 | 29.26 ± 0.33 *** |
| Ratios | Healthy Vehicle | Healthy DHA | Cirrhotic Vehicle | Cirrhotic DHA |
|---|---|---|---|---|
| SFA/MUFA | 2.86 ± 0.44 | 3.27 ± 0.48 | 1.81 ± 0.34 ### | 3.04 ± 0.30 *** |
| MUFA/PUFA | 0.32 ± 0.06 | 0.26 ± 0.03 | 0.58 ± 0.13 ## | 0.32 ± 0.03 *** |
| ω-6/ω-3 | 5.32 ± 0.88 | 2.72 ± 0.46 ** | 11.72 ± 1.56 ### | 2.01 ± 0.26 *** |
| ω-3 INDEX (DHA + EPA) | 6.03 ± 1.09 | 11.15 ± 1.64 *** | 2.08 ± 0.57 ### | 12.95 ± 1.49 *** |
| AA/EPA | 108.3 ± 25.2 | 12.74 ± 5.69 *** | 156.0 ± 24.2 ### | 8.65 ± 2.35 *** |
| AA/DHA | 3.26 ± 0.58 | 1.50 ± 0.33 *** | 7.87 ± 1.41 ### | 0.90 ± 0.18 *** |
| Δ5 (C20:4 n-6/C20:3 n-6) | 36.99 ± 3.95 | 17.39 ± 1.152 *** | 47.55 ± 2.12 | 12.09 ± 0.85 *** |
| Δ6 (C20:3 n-6/C18:2 n-6) | 2.99 ± 0.31 | 4.58 ± 0.27 | 1.76 ± 0.10 | 4.76 ± 0.31 *** |
| SCD1 INDEX (C16:1 n-7/C18:1 n-9) | 20.72 ± 1.81 | 22.98 ± 2.79 | 16.80 ± 1.33 | 19.74 ± 1.51 |
| SCD16 INDEX (C16:1 n-7/C16:0) | 8.24 ± 0.81 | 7.73 ± 0.78 | 11.30 ± 1.17 | 7.08 ± 0.49 |
| SCD18 INDEX (C18:1 n-9/C18:0) | 47.32 ± 3.70 | 42.72 ± 3.76 | 111.6 ± 14.1 ### | 56.02 ± 2.63 *** |
| C18:2 n-6/C20:4 n-6 | 0.95 ± 0.02 | 1.29 ± 0.08 | 1.24 ± 0.09 | 1.71 ± 0.08 |
| C18:0/C16:0 | 0.85 ± 0.01 | 0.81 ± 0.04 | 0.62 ± 0.05 # | 0.66 ± 0.03 |
| C24:0/C22:0 | 2.82 ± 0.12 | 3.39 ± 0.20 | 2.53 ± 0.22 | 3.01 ± 0.12 |
| C24:0/C20:0 | 7.77 ± 0.73 | 7.28 ± 0.58 | 3.90 ± 0.35 # | 5.73 ± 0.35 |
| C18:1 n-7/C16:1 n-7 | 2.22 ± 0,18 | 1.92 ± 0.23 | 1.34 ± 0.17 | 1.67 ± 0.11 ** |
| Parameter | Vehicle | DHA | p Value |
|---|---|---|---|
| PP (mmHg) | 13.91 ± 0.60 | 12.05 ± 0.57 | 0.03 |
| PBF (mL·min−1) | 15.66 ± 2.54 | 18.21 ± 2.31 | >0.20 |
| IHVR (mmHg·min·mL−1) | 1.05 ± 0.15 | 0.79 ± 0.11 | 0.16 |
| SMABF (mL·min−1) | 8.37 ± 0.91 | 10.03 ± 1.45 | >0.20 |
| MAP (mmHg) | 116.23 ± 5.40 | 99.92 ± 6.25 | 0.1 |
| HR (beats·min−1) | 415 ± 13 | 383 ± 17 | 0.15 |
| AST (U/L) | 155.8 ± 17.08 | 115.8 ± 9.05 | 0.04 |
| Albumin (U/L) | 31.2 ± 0.32 | 30.27 ± 0.53 | >0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyer-Diaz, Z.; Domingo, J.C.; De Gregorio, E.; Manicardi, N.; Aristu-Zabalza, P.; Cordobilla, B.; Abad-Jordà, L.; Ortega-Ribera, M.; Fernández-Iglesias, A.; Marí, M.; et al. A Nutraceutical Rich in Docosahexaenoic Acid Improves Portal Hypertension in a Preclinical Model of Advanced Chronic Liver Disease. Nutrients 2019, 11, 2358. https://doi.org/10.3390/nu11102358
Boyer-Diaz Z, Domingo JC, De Gregorio E, Manicardi N, Aristu-Zabalza P, Cordobilla B, Abad-Jordà L, Ortega-Ribera M, Fernández-Iglesias A, Marí M, et al. A Nutraceutical Rich in Docosahexaenoic Acid Improves Portal Hypertension in a Preclinical Model of Advanced Chronic Liver Disease. Nutrients. 2019; 11(10):2358. https://doi.org/10.3390/nu11102358
Chicago/Turabian StyleBoyer-Diaz, Zoe, Joan Carles Domingo, Estefanía De Gregorio, Nicolò Manicardi, Peio Aristu-Zabalza, Begoña Cordobilla, Laia Abad-Jordà, Martí Ortega-Ribera, Anabel Fernández-Iglesias, Montserrat Marí, and et al. 2019. "A Nutraceutical Rich in Docosahexaenoic Acid Improves Portal Hypertension in a Preclinical Model of Advanced Chronic Liver Disease" Nutrients 11, no. 10: 2358. https://doi.org/10.3390/nu11102358
APA StyleBoyer-Diaz, Z., Domingo, J. C., De Gregorio, E., Manicardi, N., Aristu-Zabalza, P., Cordobilla, B., Abad-Jordà, L., Ortega-Ribera, M., Fernández-Iglesias, A., Marí, M., Bosch, J., & Gracia-Sancho, J. (2019). A Nutraceutical Rich in Docosahexaenoic Acid Improves Portal Hypertension in a Preclinical Model of Advanced Chronic Liver Disease. Nutrients, 11(10), 2358. https://doi.org/10.3390/nu11102358







