Anti-Inflammatory Diets and Fatigue
Abstract
1. Introduction
2. Methods
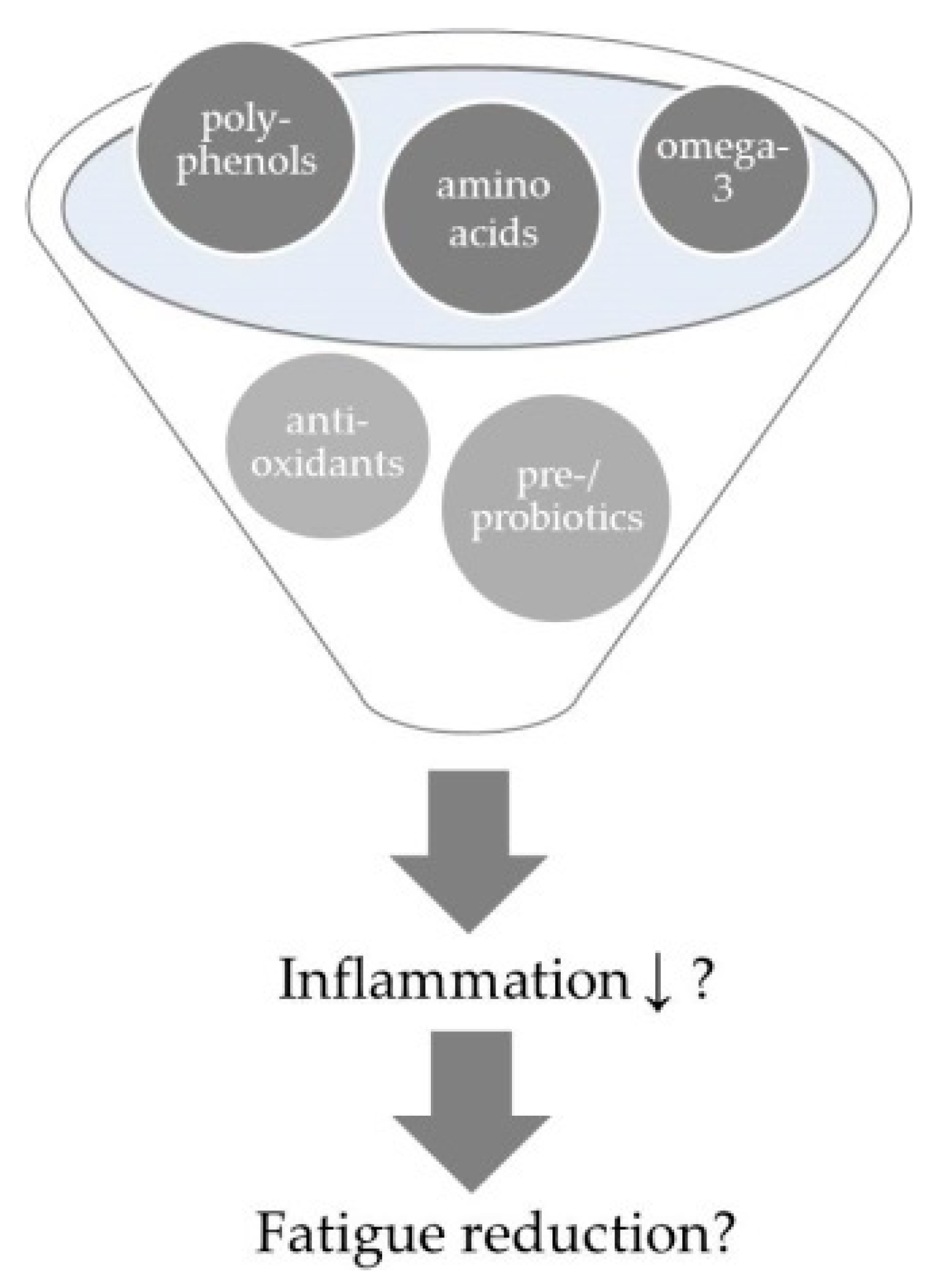
3. Anti-Inflammatory Dietary Strategies
3.1. Anti-Inflammatory Single Nutrients
3.1.1. Poly-Unsaturated Fatty Acids
3.1.2. Anti-Oxidative Vitamins
3.1.3. Vitamin D (Cholecalciferol)
3.1.4. Polyphenols
3.1.5. Protein/Amino Acids
3.2. Anti-Inflammatory Foods
3.2.1. Probiotics
3.2.2. Root Plants
3.3. Anti-Inflammatory Dietary Patterns
3.3.1. Fatigue Reduction Diet
3.3.2. Leaky Gut Diet
3.3.3. Mediterranean Diet
3.3.4. Nordic Diet
4. Discussion
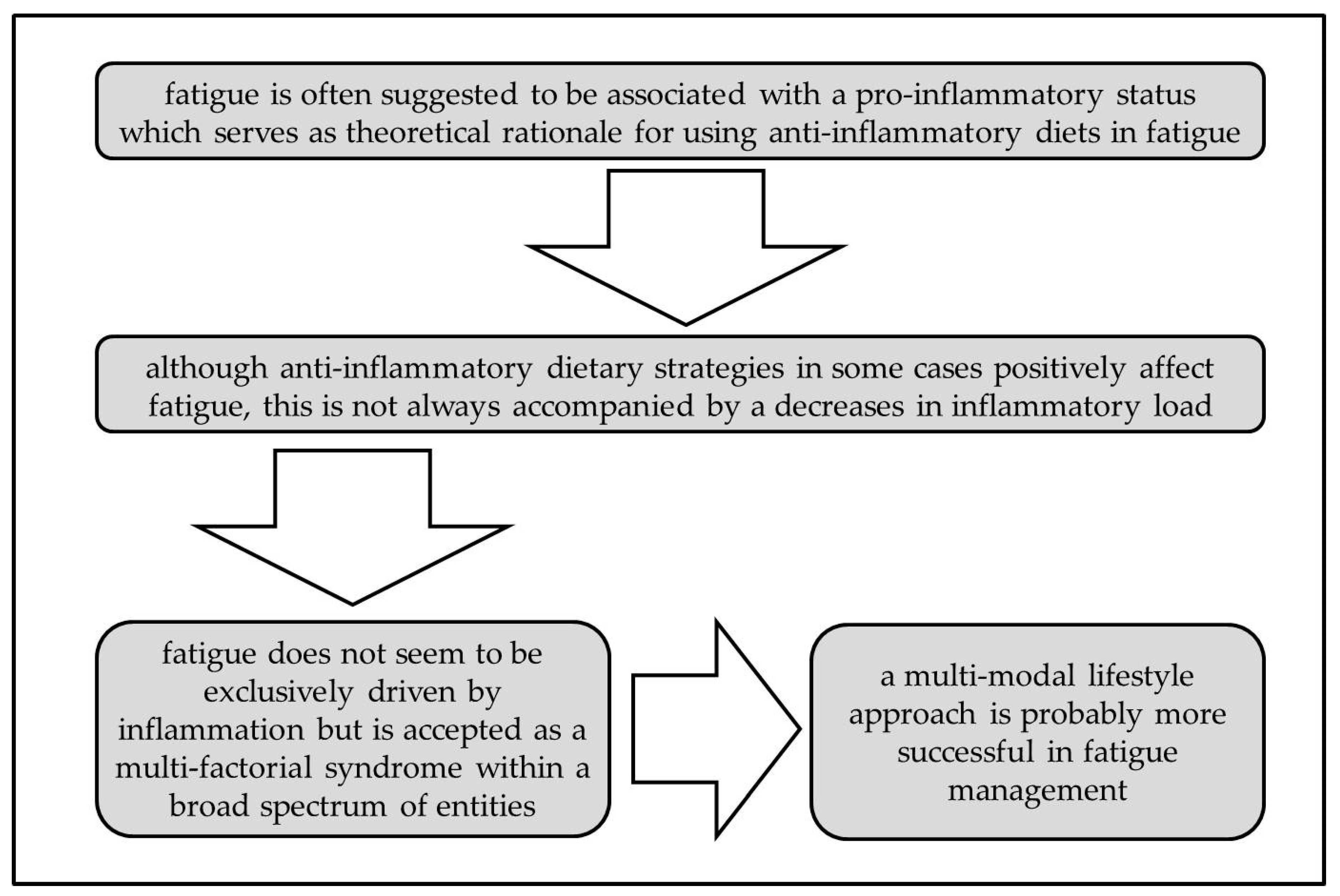
4.1. Why are Results of Anti-Inflammatory Dietary Strategies Controversial in Terms of Fatigue Management?
4.2. Future Perspectives
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| CFS | chronic fatigue syndrome |
| CNS | central nervous system |
| CRF | cancer-related fatigue |
| CRP | c-reactive protein |
| DHA | docosahexaenoic acid |
| EGCG | epigallocatechin gallate |
| EPA | eicosapentaenoic acid |
| ESR | erythrocyte sedimentation rate |
| FA | fatty acids |
| IBD | inflammatory bowel disease |
| IBS | irritable bowel syndrome |
| IgA/M | immungobulin A/M |
| IL | interleukin |
| IU | international units |
| LA | linoleic acid |
| LBP | LPS-binding protein |
| LPS | lipopolysaccharide |
| ME | myalgic encephalomyelitis |
| MS | multiple sclerosis |
| NAIOSs | natural anti-inflammatory and anti-oxidative substances |
| NF-κB | nuclear factor kappa beta |
| ONS | oral nutritional supplement |
| PUFA | poly-unsaturated fatty acid |
| QoL | quality of life |
| RA | rheumatoid arthritis |
| RCT | randomized controlled trial |
| SF-36 | a widely used QoL-measurement |
| SLE | systemic lupus erythematosus |
| TNF-α | tumor necrosis factor alpha |
Appendix A
| Study Design | Patient Sample | Treatment | Inflammation | Fatigue | Quality of Life | Commentary | References | |
|---|---|---|---|---|---|---|---|---|
| Anti-inflammatory nutrients | ||||||||
| Poly-unsaturated fatty acids (PUFA) | Randomized controlled trial (RCT) | n = 92 (♂43 ♀49) advanced non-small-cell lung cancer (NSCLC); under treatment | Intervention (n = 46): daily 2 cans oral nutritional supplement (ONS) with eicosapentaenoic acid (EPA) (2.2 g) vs. Control (n = 46): isocaloric diet duration: during first 2 cycles of chemotherapy (median 5.8 (0.3–18.4) months) | ↓interleukin (IL) -6 (p = 0.602) ✓tumor necrosis factor (TNF)-α (p = 0.541) ✓c-reactive protein (CRP) (p = 0.07) | ✓EORTC QLQ-C30 - fatigue symptoms (p = 0.04) | ⊘EORTC QLQ-C30 - global (p = 0.136) | drop-out: 8 (Intervention (I): 2/ Control (C): 6) compliance: 73% EPA-group had sign. higher energy and protein intake compared to control | [39] |
| phase III RCT 5-arm design | n = 332 (♂181 ♀151) cachectic cancer | basic treatment: daily polyphenols (300 mg), lipoic acid (300 mg), carbocysteine (2.7g), vitamin (vit) E (400 mg), vit A (30,000 IU), and vit C (500 mg) + arm 1 (n = 44): daily medroxyprogesterone (500 mg) or megestrol acetate (320 mg) vs. arm 2 (n = 25): daily 2 cans of ONS with EPA (2.2 g) vs. arm 3 (n =88): daily L-carnitine (4 g) vs. arm 4 (n = 87): daily thalidomide (200mg) vs. arm 5 (n = 88): combination of all duration: 4 months | ⊘IL-6 (p = 0.94) ⊘TNF-α (p =0.28) | ↓ Modified Fatigue Impact Scale (MFSI)-short form (SF) (p = 0.051) | ⊘EORTC - QLQ-C30 (p = 0.29) ↓EQ-5D-Index (p = 0.002) ⊘EQ-5D-VAS (p = 0.79) | drop-out: 12 (arm 1: 2/ arm 2: 2/ arm 3: 3/ arm 4: 3/ arm 5: 2) | [40] | |
| multi-center double-blind RCT | ♀97 breast cancer; survivors | omega-3 (n = 35): daily 6 capsules EPA/docosahexaenoic acid (DHA) (1.95 g/1.35 g) vs. omega-6 (n = 33): daily 6 capsules soybean oil (6 g) vs. combi (n = 29): daily EPA/DHA (1.65 g) and soybean oil (3 g) duration: 6 weeks | n/a | SI-CRF ✓omega-3: (p = 0.017) ✓omega-6: (p < 0.001) ✓combi: (p < 0.001) BFI total ✓omega-3: (p < 0.001) ✓omega-6: (p < 0.001) ✓combi: (p < 0.001) MFSI-SF ✓omega-3: (p < 0.001) ✓omega-6: (p < 0.001) ✓combi: (p < 0.001) | - | drop-out: 16 (omega-3: 5/ omega-6: 6/ combi: 5) compliance: 84.5% benefits on fatigue were more in favor of omega-6 group (p < 0.01) | [43] | |
| placebo RCT | n = 50 (♂8 ♀42) Systemic Lupus Erythematosus (SLE); median age 38.8 (19.8–57.7) years | Intervention (n = 25): daily 6 capsules EPA/DHA (2.25 g/2.25 g) vs. placebo (n = 25) duration: 6 months | ✓ erythrocyte sedimentation rate (ESR) (p = 0.008) ✓IL-13 (p = 0.033) ✓IL-12 (p = 0.058) | ⊘Fatigue Severity Scale (FSS) (p = 0.350) ⊘SF-36 - energy/ fatigue (p = 0.092) | ⊘SF-36 - emotional wellbeing (p = 0.070) | drop-out: 18 (I: 7/placebo (P): 11) compliance: I: 83%/ P: 64% | [49] | |
| double-blind, open-label RCT | n = 27 (♂4 ♀23) Multiple Sclerosis (MS); relapsing- remitting | basic treatment: daily vit E (400 international units (IU)), multi-vitamins, calcium (500 mg) + Intervention (n =14): low-fat diet (15energy%) and daily 6 capsules EPA/DHA (1.98 g/1.32 g) vs. Control (n = 13): normal-fat AHA Step I diet (≤ 30energy%) and placebo capsules (olive oil) duration: 1 year | ⊘IL-4 (n/a) ⊘IL-8 (n/a) ⊘IL-12 (n/a) ⊘IL-1ß (n/a) ⊘interferon (IFN)-γ (n/a) ⊘TNF-α (n/a) | ⊘MFIS (p = 0.059) | ⊘SF-36 (n/a) ⊘MHI (n/a) | drop-out: 8 (I: 2/ C: 6) compliance: I: 69.2%/ C: 66.7% benefits on fatigue were more in favor of placebo-group and were related to increased DHA-levels | [50] | |
| multi-center, double-blind, placebo RCT | n = 91 (♂32 ♀59) MS; relapsing- remitting | basic treatment: 3x/wk Rebif (44 µg) + Intervention (n = 46): daily 5 capsules EPA/DHA (1.35 g/0.85 g) vs. placebo (n = 45): corn oil (5 g) duration: 6 months | - | ⊘FSS (p =0.97) | ⊘SF-36 (p = 0.53) | drop-out: 11 (I: 3/ P: 8) | [51] | |
| Anti-oxidative vitamins | double-blind, placebo RCT | n = 101 (♂25 ♀76) MS; relapsing- remitting | Intervention (n = 51): daily vit A (first 6 months 25,000 IU, then 10,000 IU) vs. placebo (n = 50) duration: 12 months | - | ✓MFSI (p = 0.004) | - | drop-out: 8 (I: 4/ P: 4) | [55] |
| Vitamin D | double-blind, placebo RCT | n = 45 (♂25 ♀76) juvenile SLE | Intervention (n = 22): 1x/wk oral cholecalciferol (50,000 IU) vs. placebo (n = 23) duration: 24 weeks | ESR? CRP? | ✓kids-FSS (p = 0.012) | - | drop-out: 5 (I: 2/ P: 3) compliance: I: 85%/ P: 75% | [64] |
| Polyphenols | phase I uncontrolled study | n = 12 (♂11 ♀1) advanced/ metastatic kidney cancer; under treatment; median age 63.3 (48–83) years | Intervention: 2x/day isoquercentin (450 mg), vit C (111.6 mg), vit B3 (9 mg) vs. Control: 2x/day isoquercentin (225 mg), vit C (55.8 mg), vit B3 (4.5 mg) duration: during 2 or 4 sunitinib cycles (median 81 (75.5–86.5) days) | - | ✓ Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F) (p = 0.002) | ⊘ Functional Assessment of Cancer Therapy (FACT)-General (p = 0.069) | safety trial | [72] |
| Protein/Amino acids | phase III RCT 5-arm Design | n = 332 (♂181 ♀151) cachectic cancer | basic treatment: daily polyphenols (300 mg), lipoic acid (300 mg), carbocysteine (2.7 g), vit E (400 mg), vit A (30,000 IU), and vit C (500 mg) plus arm 1 (n = 44): daily medroxyprogesterone (500 mg) or megestrol acetate (320 mg) vs. arm 2 (n = 25): daily 2 cans of ONS with EPA (2.2 g) vs. arm 3 (n = 88): daily L-carnitine (4 g) vs. arm 4 (n = 87): daily thalidomide (200mg) vs. arm 5 (n = 88): combination of all duration: 4 months | ⊘IL-6 (p = 0.663) ⊘TNF-α (p = 0.24) | ⊘MFSI-SF (p = 0.801) | ⊘EORTC - QLQ-C30 (p = 0.832) ⊘EQ-5D-Index (p = 0.151) ⊘EQ-5D-VAS (p = 0.593) | drop-out: 12 (arm 1: 2/ arm 2: 2/ arm 3: 3/ arm 4: 3/ arm 5: 2) | [40] |
| exploratory open-label RCT | n = 90 (♂21 ♀69) chronic fatigue syndrome (CFS) | ALC (n = 30): daily acetyl-L-carnitine (2 g) vs. PLC (n = 30): daily propionyl-L-carnitine (2 g) vs. ALC+PLC (n = 30): daily acetyl-L-carnitine (2 g) + propionyl-L-carnitine (2 g) duration: 24 weeks | - | MFI-20 ⊘ALC: (p = 0.218) ✓PLC: (p = 0.004) ✓ALC+PLC: (p < 0.001) | - | drop-out: 19 (ALC: 9/ PLC: 4/ ALC+PLC: 6) | [84] | |
| Anti-inflammatory foods | ||||||||
| Probiotics | double-blind, placebo RCT | n = 28 (♂13 ♀15) irritable bowel syndrome (IBS); mean age 50 ± 8.6 years | high-dose (HD) (n = 8): daily 2 capsules synbiotics (Lactobacillus/Bifidobacterium) vs. low-dose (LD) (n = 10): daily 1 capsule synbiotics + 1 placebo vs. placebo (n = 10): daily 2 capsules duration: 8 weeks | - | ⊘FSS (p = 0.115) ✓MFI-20 (p = 0.037) ✓Fatigue-VAS (p = 0.013) | - | drop-out: 2 (HD: 2/ LD: 0/ P: 0); dose-dependent relationship | [105] |
| uncontrolled, open pilot study | n = 15 (♂5 ♀10) CFS; median age 43 (30–56) years | Intervention (n = 15): 2x/day probiotic yoghurt (L. paracasei, L. acidophilus, B. lactis) duration: 8 weeks | - | ⊘Fatigue-VAS (n/a) | ⊘SF-12 - health (n/a) | compliance has been checked via faecal samples | [106] | |
| double-blind, placebo RCT | n = 60 (♂35 ♀25) colorectal cancer; completed treatment; mean age 56.2 ± 8.9 years | Intervention (n = 32): 2x/day Lacidofil-pills (L. rhamnosus, L. acidophilus) vs. placebo (n = 28) duration: 12 weeks | - | ⊘FACT - Fatigue (p = 0.09) | ⊘FACT - General (p = 0.14) ✓FACT - cancer- related (p = 0.004) ✓FWB (p = 0.04) | drop-out: 6 (I:5/ P:1) | [107] | |
| double-blind, cross-over RCT | n = 14 (♂14 ♀1) primary sclerosing cholangitis (PCS) and IBD; median age 45 (28-70) years | Intervention: 2x/day probiotic sachet (L. acidophilus, L. casei, L. salivarius, L. lactis, B. bifidum, B. lactis) vs. placebo duration: 12 weeks | - | ⊘Fatigue-VAS (n/a) | - | drop-out: 2 cross-over after 3 months | [108] | |
| double-blind RCT | n = 63 (♂40 ♀23) spondyloarthritis, active disease; median age 43.3 (19–76) years | Intervention (n = 32): 2x/day oral probiotic powder (S. salivarius, B. lactis, L. acidophilus) vs. placebo (n = 31) duration: 12 weeks | ⊘CRP (n/a) | ⊘Multi-dimensional Assessment of Fatigue (MAF) (n/a) | ⊘ASQoL (n/a) | drop-out: 0; compliance was checked by weighing powder | [109] | |
| Root plants | single-blind RCT | n = 66 (♂56 ♀10) non-alcoholic fatty liver disease (NAFLD); mean age 49.9 ± 12.2 years | basic treatment: daily 1 capsule Silybum marianum (450 mg), 30min. aerobic exercise + Intervention (n = 35): 3x/day capsule ginsenosides (3 g) vs. placebo (n = 31) duration: 3 weeks | ✓TNF-α (p = 0.031) ⊘IL-6 (p = 0.227) | ⊘FSS (p = 0.221) | - | drop-out: 14 (I: 5/ P: 9) patients with BMI ≥ 25 showed pronounced benefit regarding TNF-α and fatigue | [114] |
| double-blind RCT | n = 90 (♂21 ♀69) CFS; median age 39.5 (20–60) years | HD (n = 30): 2x/day P. ginseng (2 g) vs. LD (n = 30): 2x/day P. ginseng (1 g) vs. placebo (n = 30) | ⊘NRS total (p = 0.068) NRS mental ✓LD: (p = 0.033) ✓HD: (p = 0.002) Fatigue-VAS ⊘LD: (p = 0.449) ✓HD: (p = 0.037) | drop-out: 2 (HD: 1/ LD: 1/ P: 0) dose-dependent amelioration | [115] | |||
| double-blind RCT | n = 51 (♂19 ♀32) cancer; under treatment; mean age 58 ± 12 years | Intervention (n = 24): 4x/day ginger extract (1.2 g; incl. gingerols 60mg) vs. placebo (n = 27) duration: during first 3 cycles of chemotherapy | ✓FACIT-Fatige (p = 0.013) | ✓FACT - General (p = 0.040) | drop-out: 17 (I: 9/ P: 8) | [117] | ||
| Anti-inflammatory dietatry patterns | ||||||||
| Fatigue reduction diet | pilot RCT | ♀30 breast cancer survivors; mean age 62.4 ± 9.7 years | Intervention (n = 15): fatigue reduction diet vs. Control (n = 15): general health curriculum duration: 3 months | - | ✓ Brief Fatigue Inventory (BFI) (p = 0.01) | - | compliance/ efficacy checked by dietary records and serum levels; control group sign. younger and leaner | [120] |
| Leaky gut diet | uncontrolled study | n = 41 (♂7 ♀34) CFS; mean age 37.9 ± 11.1 years | Intervention: leaky gut diet + NAIOs duration: 10–12 months | ✓LPS-immuno-globulin (Ig)M (p = 0.0002) ✓LPS-IgA (p = 0.0009) | ✓FF scale (p = 0.0002) | - | 63.5% responders; more benefit with shorter illness duration, younger age | [98] |
References
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Care 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Bansal, A.S.; Bradley, A.S.; Bishop, K.N.; Kiani-Alikhan, S.; Ford, B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav. Immun. 2012, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Komaroff, A.L.; Buchwald, D.S. Chronic fatigue syndrome: An update. Ann. Rev. Med. 1998, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mariman, A.; Delesie, L.; Tobback, E.; Hanoulle, I.; Sermijn, E.; Vermeir, P.; Pevernagie, D.; Vogelaers, D. Undiagnosed and comorbid disorders in patients with presumed chronic fatigue syndrome. J. Psychosom. Res. 2013, 75, 491–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bjørklund, G.; Dadar, M.; Pen, J.J.; Chirumbolo, S.; Aaseth, J. Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother. 2019, 109, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.R. Cancer-related fatigue: Causes, consequences, and management. Oncologist 2007, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.; Saez-Francas, N.; Castro-Marrero, J.; Aliste, L.; Fernandez de Sevilla, T.; Alegre, J. Gender differences in chronic fatigue syndrome. Reumatol. Clin. 2016, 12, 72–77. [Google Scholar] [CrossRef]
- Ter Wolbeek, M.; van Doornen, L.J.; Kavelaars, A.; van de Putte, E.M.; Schedlowski, M.; Heijnen, C.J. Longitudinal analysis of pro- and anti-inflammatory cytokine production in severely fatigued adolescents. Brain Behav. Immun. 2007, 21, 1063–1074. [Google Scholar] [CrossRef]
- Scheibenbogen. Chronic Fatigue Syndrome. Current concepts in pathogenesis, diagnostic approaches and treatment. Tägl. Prax. 2014, 55, 56–57. [Google Scholar]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Van Langenberg, D.R.; Della Gatta, P.; Warmington, S.A.; Kidgell, D.J.; Gibson, P.R.; Russell, A.P. Objectively measured muscle fatigue in Crohn’s disease: Correlation with self-reported fatigue and associated factors for clinical application. J. Crohn’s Colitis 2014, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef]
- Hewlett, S.; Hehir, M.; Kirwan, J.R. Measuring fatigue in rheumatoid arthritis: A systematic review of scales in use. Arth. Rheum. 2007, 57, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Heesen, C.; Nawrath, L.; Reich, C.; Bauer, N.; Schulz, K.H.; Gold, S.M. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatr. 2006, 77, 34–39. [Google Scholar] [CrossRef]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Twisk, F.N.; Kubera, M.; Ringel, K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Increased interleukin-1, tumor necrosis factor-alpha, PMN-elastase, lysozyme and neopterin. J. Affect. Disord. 2012, 136, 933–939. [Google Scholar] [CrossRef]
- Tomoda, A.; Joudoi, T.; Rabab el, M.; Matsumoto, T.; Park, T.H.; Miike, T. Cytokine production and modulation: Comparison of patients with chronic fatigue syndrome and normal controls. Psychiatr. Res. 2005, 134, 101–104. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Chen, H.J.; Lio, C.F.; Kuo, C.F.; Kao, A.C.; Wang, W.S.; Yao, W.C.; Chen, C.; Yang, T.Y. Increased risk of chronic fatigue syndrome in patients with inflammatory bowel disease: A population-based retrospective cohort study. J. Transl. Med. 2019, 17, 55. [Google Scholar] [CrossRef]
- Giloteaux, L.; Goodrich, J.K.; Walters, W.A.; Levine, S.M.; Ley, R.E.; Hanson, M.R. Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome 2016, 4, 30. [Google Scholar] [CrossRef]
- Ojo-Amaize, E.A.; Conley, E.J.; Peter, J.B. Decreased natural killer cell activity is associated with severity of chronic fatigue immune dysfunction syndrome. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1994, 18, S157–S159. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, I.; DeRuyter, M.; Rummens, J.L.; Bosmans, E.; Maes, M. Decreased expression of CD69 in chronic fatigue syndrome in relation to inflammatory markers: Evidence for a severe disorder in the early activation of T lymphocytes and natural killer cells. Neuro Endocrinol. Lett. 2007, 28, 477–483. [Google Scholar] [PubMed]
- Groven, N.; Fors, E.A.; Reitan, S.K. Patients with Fibromyalgia and Chronic Fatigue Syndrome show increased hsCRP compared to healthy controls. Brain Behav. Immun. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Bosmans, E. Not in the mind of neurasthenic lazybones but in the cell nucleus: Patients with chronic fatigue syndrome have increased production of nuclear factor kappa beta. Neuroendocrinol. Lett. 2007, 28, 456–462. [Google Scholar] [PubMed]
- Morris, G.; Maes, M. Increased nuclear factor-kappaB and loss of p53 are key mechanisms in Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med. Hypotheses 2012, 79, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Gerrity, T.R.; Papanicolaou, D.A.; Amsterdam, J.D.; Bingham, S.; Grossman, A.; Hedrick, T.; Herberman, R.B.; Krueger, G.; Levine, S.; Mohagheghpour, N.; et al. Immunologic aspects of chronic fatigue syndrome. Report on a Research Symposium convened by The CFIDS Association of America and co-sponsored by the US Centers for Disease Control and Prevention and the National Institutes of Health. Neuroimmunomodulation 2004, 11, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. Inflammatory and oxidative and nitrosative stress cascades as new drug targets in myalgic encephalomyelitis and chronic fatigue syndrome. Mod. Trends Pharmacoppsychiatr. 2013, 28, 162–174. [Google Scholar] [CrossRef]
- Grygiel-Gorniak, B.; Puszczewicz, M. Fatigue and interleukin-6—A multi-faceted relationship. Reumatologia 2015, 53, 207–212. [Google Scholar] [CrossRef]
- Tamizi far, B.; Tamizi, B. Treatment of chronic fatigue syndrome by dietary supplementation with omega-3 fatty acids—A good idea? Med. Hypotheses 2002, 58, 249–250. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K. Long-chain polyunsaturated fatty acids and the pathophysiology of myalgic encephalomyelitis (chronic fatigue syndrome). J. Clin. Pathol. 2007, 60, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Puri, B.K. The use of eicosapentaenoic acid in the treatment of chronic fatigue syndrome. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Freidin, M.B.; Wells, H.R.R.; Potter, T.; Livshits, G.; Menni, C.; Williams, F.M.K. Metabolomic markers of fatigue: Association between circulating metabolome and fatigue in women with chronic widespread pain. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marrero, J.; Zaragoza, M.C.; Domingo, J.C.; Martinez-Martinez, A.; Alegre, J.; von Schacky, C. Low omega-3 index and polyunsaturated fatty acid status in patients with chronic fatigue syndrome/myalgic encephalomyelitis. Prostaglandins Leukot. Essent. Fatty Acids 2018, 139, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Leunis, J.C. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuroendocrinol. Lett. 2005, 26, 745–751. [Google Scholar]
- Cunnane, S.C. Differential regulation of essential fatty acid metabolism to the prostaglandins: Possible basis for the interaction of zinc and copper in biological systems. Prog. Lipid Res. 1982, 21, 73–90. [Google Scholar] [CrossRef]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juarez-Hernandez, E.; Nunez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: Randomised trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- Mantovani, G.; Maccio, A.; Madeddu, C.; Serpe, R.; Massa, E.; Dessi, M.; Panzone, F.; Contu, P. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010, 15, 200–211. [Google Scholar] [CrossRef]
- Payne, C.; Wiffen, P.J.; Martin, S. Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database Syst. Rev. 2012, 1, Cd008427. [Google Scholar] [CrossRef] [PubMed]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Inglis, J.E.; Mustian, K.M.; Heckler, C.E.; Padula, G.D.A.; Mohile, S.G.; Kamen, C.S.; Culakova, E.; Lin, P.J.; Kerns, S.L.; et al. Multicenter Randomized Controlled Trial of Omega-3 Fatty Acids Versus Omega-6 Fatty Acids for the Control of Cancer-Related Fatigue Among Breast Cancer Survivors. JNCI Cancer Spectrum 2019, 3, pkz005. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Hewlett, S.; Almeida, C.; Kirwan, J.R.; Choy, E.H.; Chalder, T.; Pollock, J.; Christensen, R. Non-pharmacological interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Das Gupta, A.B.; Hossain, A.K.; Islam, M.H.; Dey, S.R.; Khan, A.L. Role of omega-3 fatty acid supplementation with indomethacin in suppression of disease activity in rheumatoid arthritis. Bangladesh Med. Res. Counc. Bull. 2009, 35, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.E.; Ramsey-Goldman, R. Fatigue in systemic lupus erythematosus. Int. J. Clin. Rheumatol. 2012, 7, 217–227. [Google Scholar] [CrossRef]
- Zonana-Nacach, A.; Roseman, J.M.; McGwin, G., Jr.; Friedman, A.W.; Baethge, B.A.; Reveille, J.D.; Alarcon, G.S. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. LUMINA Study Group. LUpus in MInority populations: NAture vs Nurture. Lupus 2000, 9, 101–109. [Google Scholar] [CrossRef]
- Wu, T.; Xie, C.; Han, J.; Ye, Y.; Weiel, J.; Li, Q.; Blanco, I.; Ahn, C.; Olsen, N.; Putterman, C.; et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS ONE 2012, 7, e37210. [Google Scholar] [CrossRef] [PubMed]
- Arriens, C.; Hynan, L.S.; Lerman, R.H.; Karp, D.R.; Mohan, C. Placebo-controlled randomized clinical trial of fish oil’s impact on fatigue, quality of life, and disease activity in Systemic Lupus Erythematosus. Nutr. J. 2015, 14, 82. [Google Scholar] [CrossRef]
- Weinstock-Guttman, B.; Baier, M.; Park, Y.; Feichter, J.; Lee-Kwen, P.; Gallagher, E.; Venkatraman, J.; Meksawan, K.; Deinehert, S.; Pendergast, D.; et al. Low fat dietary intervention with omega-3 fatty acid supplementation in multiple sclerosis patients. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 397–404. [Google Scholar] [CrossRef]
- Torkildsen, O.; Wergeland, S.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Lilleas, F.; Pedersen, T.; Bjornara, B.; et al. omega-3 fatty acid treatment in multiple sclerosis (OFAMS Study): A randomized, double-blind, placebo-controlled trial. Arch. Neurol. 2012, 69, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Manuel y Keenoy, B.; Moorkens, G.; Vertommen, J.; De Leeuw, I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001, 68, 2037–2049. [Google Scholar] [CrossRef]
- Joustra, M.L.; Minovic, I.; Janssens, K.A.M.; Bakker, S.J.L.; Rosmalen, J.G.M. Vitamin and mineral status in chronic fatigue syndrome and fibromyalgia syndrome: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0176631. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Yavari, P.; Honarvar, N.M.; Bitarafan, S.; Mahmoudi, M.; Saboor-Yaraghi, A.A. Molecular Mechanisms of the Action of Vitamin A in Th17/Treg Axis in Multiple Sclerosis. J. Mol. Neurosci. 2015, 57, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Bitarafan, S.; Saboor-Yaraghi, A.; Sahraian, M.A.; Soltani, D.; Nafissi, S.; Togha, M.; Beladi Moghadam, N.; Roostaei, T.; Mohammadzadeh Honarvar, N.; Harirchian, M.H. Effect of Vitamin A Supplementation on fatigue and depression in Multiple Sclerosis patients: A Double-Blind Placebo-Controlled Clinical Trial. Iran. J. Allergy Asthma Immunol. 2016, 15, 13–19. [Google Scholar]
- Hoeck, A.D.; Pall, M.L. Will vitamin D supplementation ameliorate diseases characterized by chronic inflammation and fatigue? Med. Hypotheses 2011, 76, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Hock, A.D. Review: Vitamin D3 deficiency results in dysfunctions of immunity with severe fatigue and depression in a variety of diseases. In vivo 2014, 28, 133–145. [Google Scholar] [PubMed]
- Berkovitz, S.; Ambler, G.; Jenkins, M.; Thurgood, S. Serum 25-hydroxy vitamin D levels in chronic fatigue syndrome: A retrospective survey. Int. J. Vitam. Nutr. Res. 2009, 79, 250–254. [Google Scholar] [CrossRef]
- Ruiz-Irastorza, G.; Gordo, S.; Olivares, N.; Egurbide, M.V.; Aguirre, C. Changes in vitamin D levels in patients with systemic lupus erythematosus: Effects on fatigue, disease activity, and damage. Arth. Care Res. 2010, 62, 1160–1165. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Hoivik, M.L.; Jahnsen, J.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, O.; Bernklev, T.; Moum, B.; et al. Fatigue is not associated with vitamin D deficiency in inflammatory bowel disease patients. World J. Gastroenterol. 2018, 24, 3293–3301. [Google Scholar] [CrossRef]
- Earl, K.E.; Sakellariou, G.K.; Sinclair, M.; Fenech, M.; Croden, F.; Owens, D.J.; Tang, J.; Miller, A.; Lawton, C.; Dye, L.; et al. Vitamin D status in chronic fatigue syndrome/myalgic encephalomyelitis: A cohort study from the North-West of England. BMJ Open 2017, 7, e015296. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Sattari, M. Vitamin D deficiency and fatigue: An unusual presentation. SpringerPlus 2015, 4, 584. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.R.; Rosa, E.P.C.; Nunes, I.; Carvalho, C. Effect of vitamin D supplementation on patients with systemic lupus erythematosus: A systematic review. Rev. Bras. Reumatol. 2017, 57, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.L.; Paupitz, J.; Aikawa, N.E.; Takayama, L.; Bonfa, E.; Pereira, R.M. Vitamin D Supplementation in Adolescents and Young Adults With Juvenile Systemic Lupus Erythematosus for Improvement in Disease Activity and Fatigue Scores: A Randomized, Double-Blind, Placebo-Controlled Trial. Arth. Care Res. 2016, 68, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Vasiadi, M.; Newman, J.; Theoharides, T.C. Isoflavones inhibit poly(I:C)-induced serum, brain, and skin inflammatory mediators—Relevance to chronic fatigue syndrome. J. Neuroinflamm. 2014, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Kuhad, A.; Tiwari, V.; Chopra, K. Epigallocatechin gallate ameliorates chronic fatigue syndrome in mice: Behavioral and biochemical evidence. Behav. Brain Res. 2009, 205, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Epigallocatechin gallate ameliorates behavioral and biochemical deficits in rat model of load-induced chronic fatigue syndrome. Brain Res. Bull. 2011, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Zheng, Z.; Zhao, Y.; Lin, W. Impact of quercetin on systemic levels of inflammation: A meta-analysis of randomised controlled human trials. Int. J. Food Sci. Nutr. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; De Placido, P.; Bruzzese, D.; Pagliuca, M.; Ungaro, P.; Bosso, D.; Ribera, D.; Iaccarino, S.; Scafuri, L.; Liotti, A.; et al. Isoquercetin as an Adjunct Therapy in Patients With Kidney Cancer Receiving First-Line Sunitinib (QUASAR): Results of a Phase I Trial. Front. Pharmacol. 2018, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, S.E.; Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Pena, M.M. Dietary quercetin reduces chemotherapy-induced fatigue in mice. Integr. Cancer Ther. 2014, 13, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, K.T.; Enos, R.T.; Narsale, A.A.; Puppa, M.J.; Davis, J.M.; Murphy, E.A.; Carson, J.A. Quercetin supplementation attenuates the progression of cancer cachexia in ApcMin/+ mice. J. Nutr. 2014, 144, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Pagliuca, M.; Perillo, T.; Zarrella, A.; Verde, A.; De Placido, S.; Buonerba, C. Complete Response and Fatigue Improvement With the Combined Use of Cyclophosphamide and Quercetin in a Patient With Metastatic Bladder Cancer: A Case Report. Medicine 2016, 95, e2598. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Thornton, J.A.; Adam, G.E.; Lieberman, H.R. Effects of 2 adenosine antagonists, quercetin and caffeine, on vigilance and mood. J. Clin. Psychopharmacol. 2010, 30, 573–578. [Google Scholar] [CrossRef]
- Stobaus, N.; Muller, M.J.; Kupferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Walrand, S.; Gryson, C.; Salles, J.; Giraudet, C.; Migne, C.; Bonhomme, C.; Le Ruyet, P.; Boirie, Y. Fast-digestive protein supplement for ten days overcomes muscle anabolic resistance in healthy elderly men. Clin. Nutr. 2016, 35, 660–668. [Google Scholar] [CrossRef]
- Moore, D.R.; Robinson, M.J.; Fry, J.L.; Tang, J.E.; Glover, E.I.; Wilkinson, S.B.; Prior, T.; Tarnopolsky, M.A.; Phillips, S.M. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009, 89, 161–168. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef] [PubMed]
- Blankfield, A. Kynurenine Pathway Pathologies: Do Nicotinamide and Other Pathway Co-Factors have a Therapeutic Role in Reduction of Symptom Severity, Including Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). Int. J. Tryptophan Res. 2013, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, R.C.; Scholte, H.R. Exploratory open label, randomized study of acetyl- and propionylcarnitine in chronic fatigue syndrome. Psychosom. Med. 2004, 66, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Teleni, L.; Opie, R.S.; Kelly, J.; Marshall, S.; Itsiopoulos, C.; Isenring, E. Efficacy and Effectiveness of Carnitine Supplementation for Cancer-Related Fatigue: A Systematic Literature Review and Meta-Analysis. Nutrients 2017, 9, 1224. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Reis, A.D.; Diniz, R.R.; Lima, F.A.; Leite, R.D.; da Silva, M.C.P.; Guerra, R.N.M.; de Moraes Vieira, E.B.; Garcia, J.B.S. Dietary supplements and fatigue in patients with breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.W.; McGregor, N.R.; Sheedy, J.R.; Buttfield, I.; Butt, H.L.; Gooley, P.R. NMR metabolic profiling of serum identifies amino acid disturbances in chronic fatigue syndrome. Clin. Chim. Acta Int. J. Clin. Chem. 2012, 413, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Leguina-Ruzzi, A. A commentary on the 2015 Canadian Clinical Practice Guidelines in glutamine supplementation to parenteral nutrition. Crit. Care 2016, 20, 7. [Google Scholar] [CrossRef]
- Coeffier, M.; Marion, R.; Leplingard, A.; Lerebours, E.; Ducrotte, P.; Dechelotte, P. Glutamine decreases interleukin-8 and interleukin-6 but not nitric oxide and prostaglandins e(2) production by human gut in-vitro. Cytokine 2002, 18, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Noe, J.E. L-glutamine use in the treatment and prevention of mucositis and cachexia: A naturopathic perspective. Integr. Cancer Ther. 2009, 8, 409–415. [Google Scholar] [CrossRef]
- Schlemmer, M.; Suchner, U.; Schapers, B.; Duerr, E.M.; Alteheld, B.; Zwingers, T.; Stehle, P.; Zimmer, H.G. Is glutamine deficiency the link between inflammation, malnutrition, and fatigue in cancer patients? Clin. Nutr. 2015, 34, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Jelsness-Jorgensen, L.P.; Bernklev, T.; Henriksen, M.; Torp, R.; Moum, B.A. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm. Bowel Dis. 2011, 17, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Leunis, J.C.; Geffard, M.; Berk, M. Evidence for the existence of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) with and without abdominal discomfort (irritable bowel) syndrome. Neuroendocrinol. Lett. 2014, 35, 445–453. [Google Scholar] [PubMed]
- Roman, P.; Carrillo-Trabalon, F.; Sanchez-Labraca, N.; Canadas, F.; Estevez, A.F.; Cardona, D. Are probiotic treatments useful on fibromyalgia syndrome or chronic fatigue syndrome patients? A systematic review. Benef. Microbes 2018, 9, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.; Marshall, T. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in the Era of the Human Microbiome: Persistent Pathogens Drive Chronic Symptoms by Interfering With Host Metabolism, Gene Expression, and Immunity. Front. Pediatr. 2018, 6, 373. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Stapleton, D.I.; Butt, H.L.; KL, D.E.M. Increased d-lactic Acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar]
- Maes, M.; Coucke, F.; Leunis, J.C. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuro Endocrinol. Lett. 2007, 28, 739–744. [Google Scholar] [PubMed]
- Maes, M.; Leunis, J.C. Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: Effects of age, duration of illness and the translocation of LPS from gram-negative bacteria. Neuroendocrinol. Lett. 2008, 29, 902–910. [Google Scholar]
- Logan, A.C.; Venket Rao, A.; Irani, D. Chronic fatigue syndrome: Lactic acid bacteria may be of therapeutic value. Med. Hypotheses 2003, 60, 915–923. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Meng, F.; Yang, W.; Hu, J.; Ye, L.; Shi, C.; Wang, C. Immunological mechanisms involved in probiotic-mediated protection against Citrobacter rodentium-induced colitis. Benef. Microbes 2016, 7, 397–407. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, K.; Kuhad, A.; Kaur, I.P. Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: Behavioral and biochemical evidences. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Moril. Soc. 2012, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J., III; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Guest, D.D.; Evans, E.M.; Rogers, L.Q. Diet components associated with perceived fatigue in breast cancer survivors. Eur. J. Cancer Care 2013, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, D.Y.; Lee, S.H.; Han, K.S.; Yang, S.W.; Kim, J.H.; Lee, S.H.; Kim, S.M.; Kim, K.N. A Randomized Clinical Trial of Synbiotics in Irritable Bowel Syndrome: Dose-Dependent Effects on Gastrointestinal Symptoms and Fatigue. Korean J. Fam. Med. 2019, 40, 2–8. [Google Scholar] [CrossRef]
- Sullivan, A.; Nord, C.E.; Evengard, B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chu, S.H.; Jeon, J.Y.; Lee, M.K.; Park, J.H.; Lee, D.C.; Lee, J.W.; Kim, N.K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Stud. Liver 2014, 46, 1126–1132. [Google Scholar] [CrossRef]
- Vleggaar, F.P.; Monkelbaan, J.F.; van Erpecum, K.J. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study. Eur. J. Gastroenterol. Hepatol. 2008, 20, 688–692. [Google Scholar] [CrossRef]
- Jenks, K.; Stebbings, S.; Burton, J.; Schultz, M.; Herbison, P.; Highton, J. Probiotic therapy for the treatment of spondyloarthritis: A randomized controlled trial. J. Rheumatol. 2010, 37, 2118–2125. [Google Scholar] [CrossRef]
- Corbitt, M.; Campagnolo, N.; Staines, D.; Marshall-Gradisnik, S. A Systematic Review of Probiotic Interventions for Gastrointestinal Symptoms and Irritable Bowel Syndrome in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). Probiotics Antimicrob. Proteins 2018, 10, 466–477. [Google Scholar] [CrossRef]
- Kim, J.H.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng Res. 2017, 41, 435–443. [Google Scholar] [CrossRef]
- Shen, H.; Gao, X.J.; Li, T.; Jing, W.H.; Han, B.L.; Jia, Y.M.; Hu, N.; Yan, Z.X.; Li, S.L.; Yan, R. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J. Ethnopharmacol. 2018, 216, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shim, H.S.; Kim, J.Y.; Kim, J.Y.; Park, S.K.; Shim, I. Ginseng Purified Dry Extract, BST204, Improved Cancer Chemotherapy-Related Fatigue and Toxicity in Mice. Evid.-Based Complement. Altern. Med. 2015, 197459. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Lee, Y.H.; Kim, S.; Suk, K.T.; Bang, C.S.; Yoon, J.H.; Baik, G.H.; Kim, D.J.; Kim, M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J. Ginseng Res. 2016, 40, 203–210. [Google Scholar] [CrossRef]
- Kim, H.G.; Cho, J.H.; Yoo, S.R.; Lee, J.S.; Han, J.M.; Lee, N.H.; Ahn, Y.C.; Son, C.G. Antifatigue effects of Panax ginseng C.A. Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Marx, W.; McCarthy, A.L.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E. The Effect of a Standardized Ginger Extract on Chemotherapy-Induced Nausea-Related Quality of Life in Patients Undergoing Moderately or Highly Emetogenic Chemotherapy: A Double Blind, Randomized, Placebo Controlled Trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef]
- George, S.M.; Alfano, C.M.; Neuhouser, M.L.; Smith, A.W.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L.; Ballard-Barbash, R. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J. Cancer Surviv. Res. Pract. 2014, 8, 680–687. [Google Scholar] [CrossRef]
- Zick, S.M.; Sen, A.; Han-Markey, T.L.; Harris, R.E. Examination of the association of diet and persistent cancer-related fatigue: A pilot study. Oncol. Nurs. Forum 2013, 40, E41–E49. [Google Scholar] [CrossRef]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue reduction diet in breast cancer survivors: A pilot randomized clinical trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef]
- Maes, M.; Leunis, J.C. Attenuation of autoimmune responses to oxidative specific epitopes, but not nitroso-adducts, is associated with a better clinical outcome in Myalgic Encephalomyelitis/chronic fatigue syndrome. Neuroendocrinol. Lett. 2014, 35, 577–585. [Google Scholar] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.I. Chronic fatigue syndrome: A personalized integrative medicine approach. Altern. Ther. Health Med. 2014, 20, 29–40. [Google Scholar] [PubMed]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Skoldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lankinen, M.; Uusitupa, M.; Schwab, U. Nordic Diet and Inflammation-A Review of Observational and Intervention Studies. Nutrients 2019, 11, 1369. [Google Scholar] [CrossRef]
- Mithril, C.; Dragsted, L.O.; Meyer, C.; Blauert, E.; Holt, M.K.; Astrup, A. Guidelines for the New Nordic Diet. Publ. Health Nutr. 2012, 15, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Ulven, S.M.; Paananen, J.; de Mello, V.; Schwab, U.; Carlberg, C.; Myhrstad, M.; Pihlajamaki, J.; Dungner, E.; Sjolin, E.; et al. Healthy Nordic diet downregulates the expression of genes involved in inflammation in subcutaneous adipose tissue in individuals with features of the metabolic syndrome. Am. J. Clin. Nutr. 2015, 101, 228–239. [Google Scholar] [CrossRef]
- Uusitupa, M.; Hermansen, K.; Savolainen, M.J.; Schwab, U.; Kolehmainen, M.; Brader, L.; Mortensen, L.S.; Cloetens, L.; Johansson-Persson, A.; Onning, G.; et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome—A randomized study (SYSDIET). J. Inter. Med. 2013, 274, 52–66. [Google Scholar] [CrossRef]
- Morelli, V. Fatigue and chronic fatigue in the elderly: Definitions, diagnoses, and treatments. Clin. Geriatr. Med. 2011, 27, 673–686. [Google Scholar] [CrossRef]
- Park, H.Y.; Jeon, H.J.; Bang, Y.R.; Yoon, I.Y. Multidimensional Comparison of Cancer-Related Fatigue and Chronic Fatigue Syndrome: The Role of Psychophysiological Markers. Psychiatr. Investig. 2019, 16, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Lin, J.M.; Reeves, W.C. Association of peripheral inflammatory markers with chronic fatigue in a population-based sample. Brain Behav. Immun. 2009, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Amel Kashipaz, M.R.; Swinden, D.; Todd, I.; Powell, R.J. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clin. Exp. Immunol. 2003, 132, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Schwander, S.K.; Donnelly, R.; Ortega, F.; Togo, F.; Broderick, G.; Yamamoto, Y.; Cherniack, N.S.; Rapoport, D.; Natelson, B.H. Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clin. Vaccine Immunol. 2010, 17, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Mullington, J.M.; Hinze-Selch, D.; Pollmacher, T. Mediators of inflammation and their interaction with sleep: Relevance for chronic fatigue syndrome and related conditions. Annal. N.Y. Acad. Sci. 2001, 933, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Milrad, S.F.; Hall, D.L.; Jutagir, D.R.; Lattie, E.G.; Ironson, G.H.; Wohlgemuth, W.; Nunez, M.V.; Garcia, L.; Czaja, S.J.; Perdomo, D.M.; et al. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J. Neuroimmunol. 2017, 303, 43–50. [Google Scholar] [CrossRef]
- Guertin, M.H.; Robitaille, K.; Pelletier, J.F.; Duchesne, T.; Julien, P.; Savard, J.; Bairati, I.; Fradet, V. Effects of concentrated long-chain omega-3 polyunsaturated fatty acid supplementation before radical prostatectomy on prostate cancer proliferation, inflammation, and quality of life: Study protocol for a phase IIb, randomized, double-blind, placebo-controlled trial. BMC Cancer 2018, 18, 64. [Google Scholar] [CrossRef]
- Aghamohammadi, D.; Ayromlou, H.; Dolatkhah, N.; Jahanjoo, F.; Shakouri, S.K. The effects of probiotic Saccharomyces boulardii on the mental health, quality of life, fatigue, pain, and indices of inflammation and oxidative stress in patients with multiple sclerosis: Study protocol for a double-blind randomized controlled clinical trial. Trials 2019, 20, 379. [Google Scholar] [CrossRef]
- Navarro-Xavier, R.A.; de Barros, K.V.; de Andrade, I.S.; Palomino, Z.; Casarini, D.E.; Flor Silveira, V.L. Protective effect of soybean oil- or fish oil-rich diets on allergic airway inflammation. J. Inflamm. Res. 2016, 9, 79–89. [Google Scholar] [CrossRef]
- Faramarzi, E.; Mahdavi, R.; Mohammad-Zadeh, M.; Nasirimotlagh, B.; Sanaie, S. Effect of conjugated linoleic acid supplementation on quality of life in rectal cancer patients undergoing preoperative Chemoradiotherapy. Pak. J. Med. Sci. 2017, 33, 383–388. [Google Scholar] [CrossRef]
- Theander, E.; Horrobin, D.F.; Jacobsson, L.T.; Manthorpe, R. Gammalinolenic acid treatment of fatigue associated with primary Sjogren’s syndrome. Scand. J. Rheumatol. 2002, 31, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell 2019, 178, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Esfandyarpour, R.; Kashi, A.; Nemat-Gorgani, M.; Wilhelmy, J.; Davis, R.W. A nanoelectronics-blood-based diagnostic biomarker for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Proc. Natl. Acad. Sci. USA 2019, 116, 10250–10257. [Google Scholar] [CrossRef] [PubMed]
- Othayoth, R.; Mathi, P.; Bheemanapally, K.; Kakarla, L.; Botlagunta, M. Characterization of vitamin-cisplatin-loaded chitosan nano-particles for chemoprevention and cancer fatigue. J. Microencapsul. 2015, 32, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.; Fuite, J.; Kreitz, A.; Vernon, S.D.; Klimas, N.; Fletcher, M.A. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav. Immun. 2010, 24, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Gottschalk, C.G.; Eddy, M.L.; Che, X.; Ukaigwe, J.E.; Peterson, D.L.; Lipkin, W.I. Immune network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome with atypical and classical presentations. Trans. Psychiatr. 2017, 7, e1080. [Google Scholar] [CrossRef] [PubMed]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: A double blind randomized clinical trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Mihaylova, I.; Kubera, M.; Uytterhoeven, M.; Vrydags, N.; Bosmans, E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009, 30, 470–476. [Google Scholar]
- Huijts, M.; Duits, A.; Staals, J.; van Oostenbrugge, R.J. Association of vitamin B12 deficiency with fatigue and depression after lacunar stroke. PLoS ONE 2012, 7, e30519. [Google Scholar] [CrossRef][Green Version]
- Nemazannikova, N.; Mikkelsen, K.; Stojanovska, L.; Blatch, G.L.; Apostolopoulos, V. Is there a Link between Vitamin B and Multiple Sclerosis? Med. Chem. 2018, 14, 170–180. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metabol. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haß, U.; Herpich, C.; Norman, K. Anti-Inflammatory Diets and Fatigue. Nutrients 2019, 11, 2315. https://doi.org/10.3390/nu11102315
Haß U, Herpich C, Norman K. Anti-Inflammatory Diets and Fatigue. Nutrients. 2019; 11(10):2315. https://doi.org/10.3390/nu11102315
Chicago/Turabian StyleHaß, Ulrike, Catrin Herpich, and Kristina Norman. 2019. "Anti-Inflammatory Diets and Fatigue" Nutrients 11, no. 10: 2315. https://doi.org/10.3390/nu11102315
APA StyleHaß, U., Herpich, C., & Norman, K. (2019). Anti-Inflammatory Diets and Fatigue. Nutrients, 11(10), 2315. https://doi.org/10.3390/nu11102315





