Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1)
Abstract
1. Introduction
2. Oleic Acid in Health and Disease
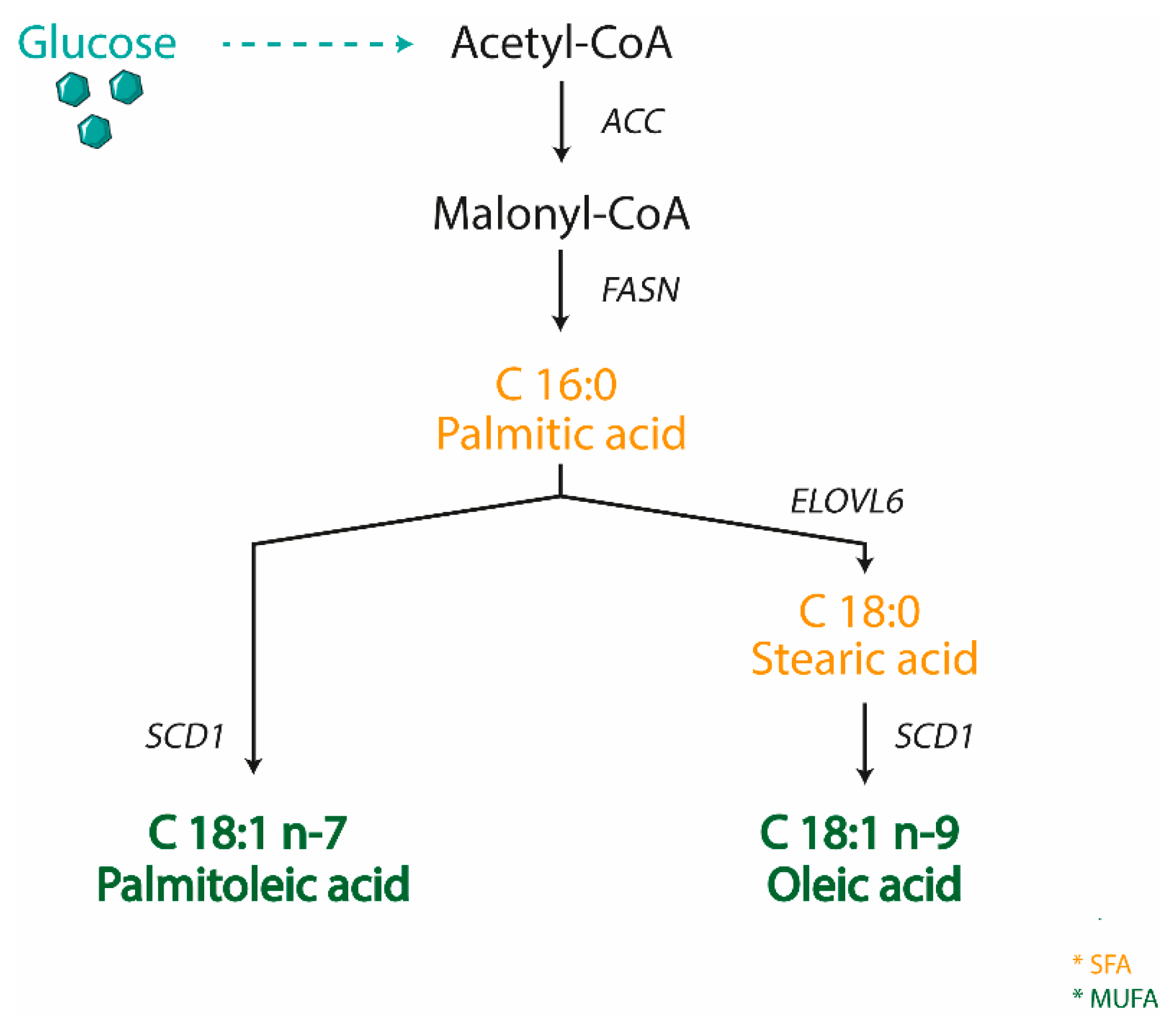
3. SCD1: The Oleic Acid Producer
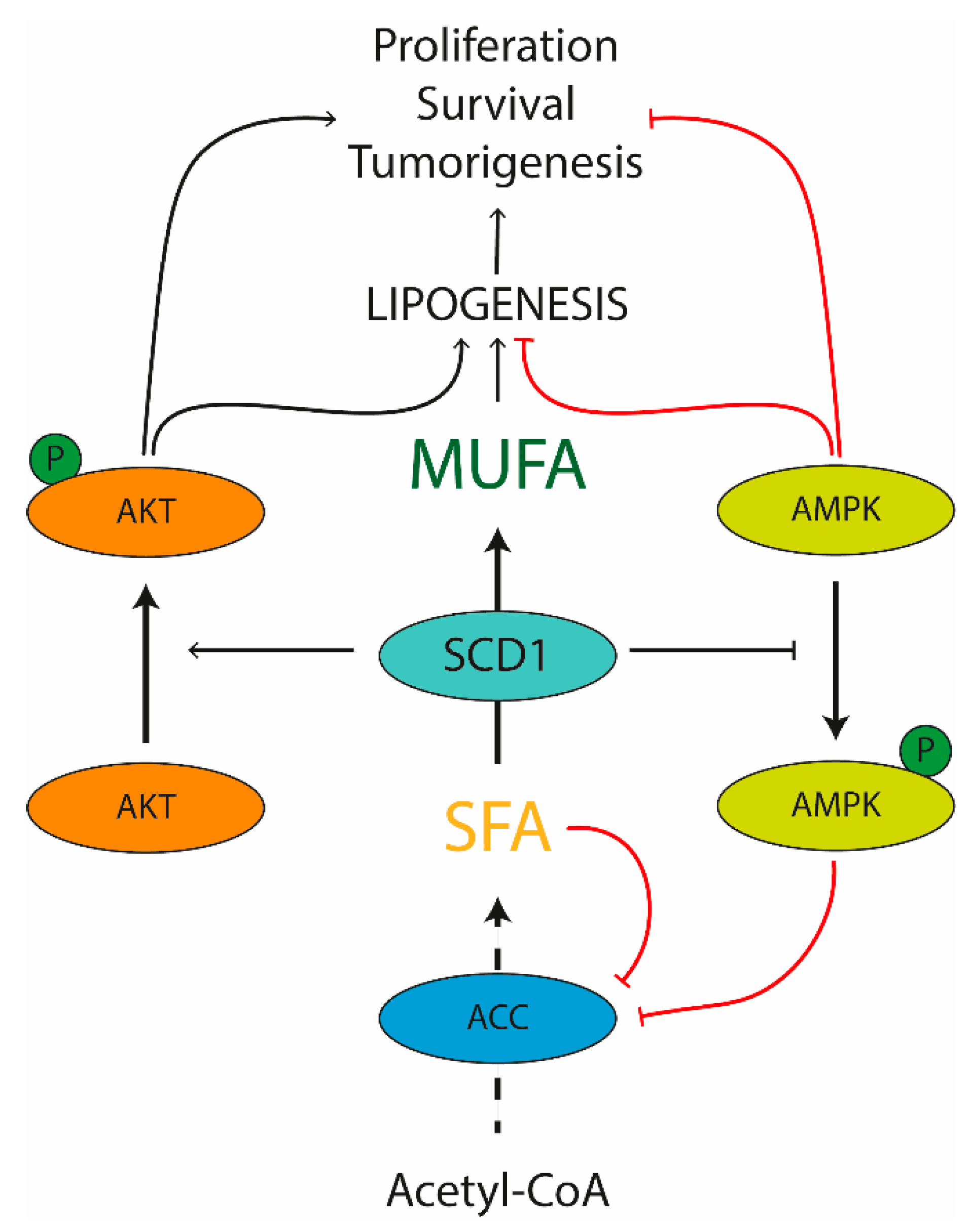
SCD1 in Pathological Conditions
4. SCD1 in the Gut-Liver Axis
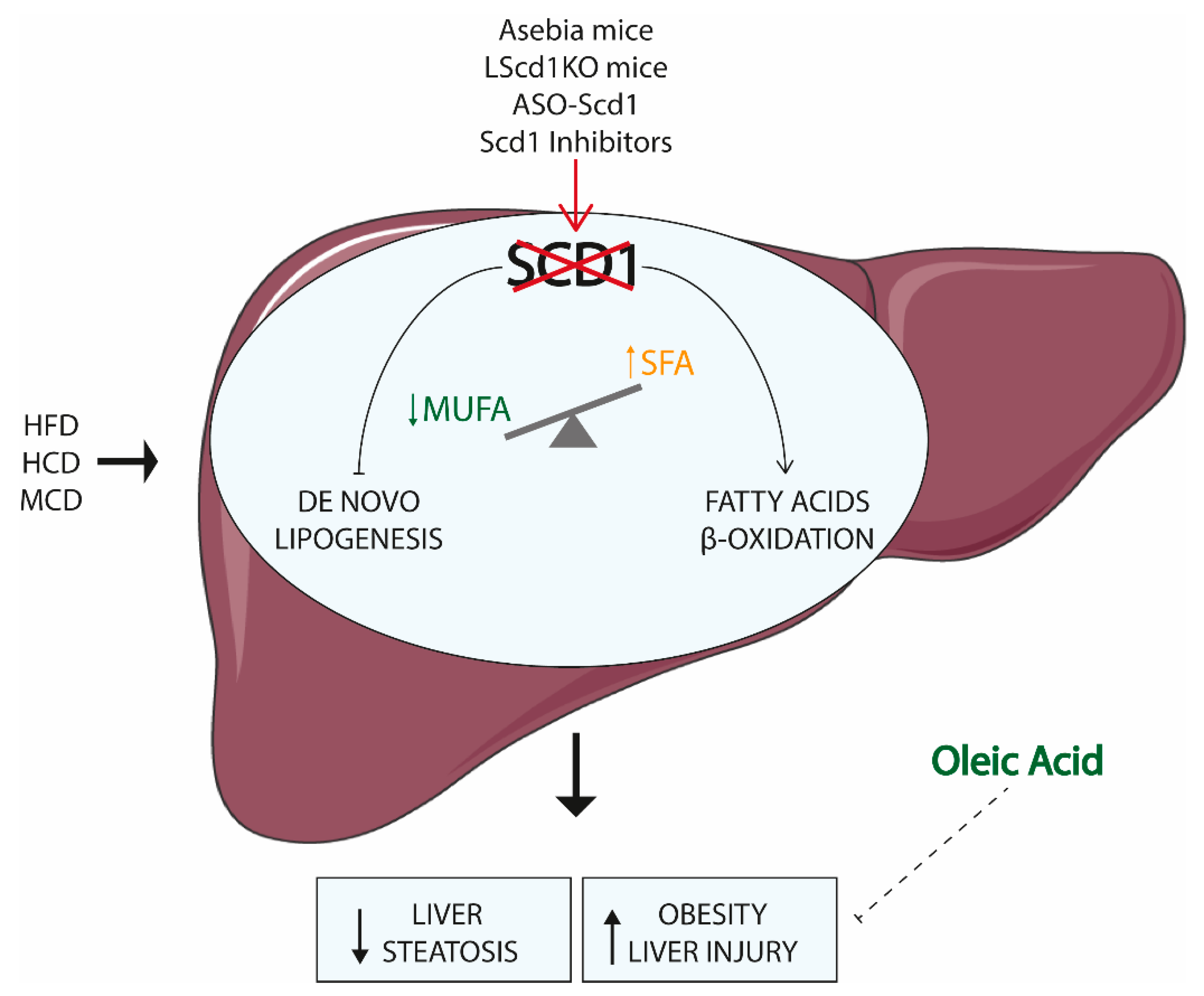
4.1. SCD1 and the Liver
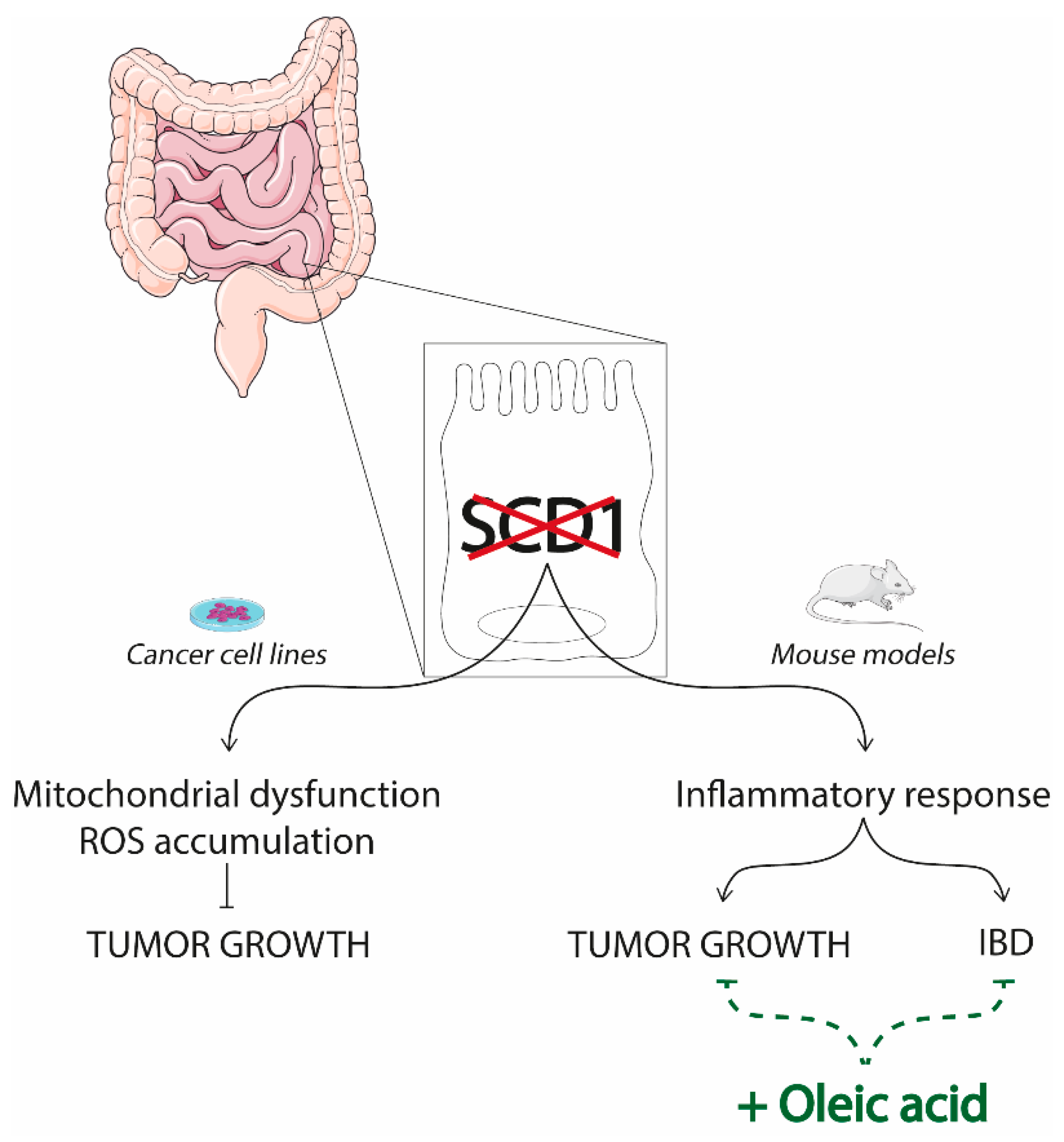
4.2. SCD1 and the Gut
4.3. SCD1 in the Gut-Liver Cross Talk
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | fatty acid |
| SFA | saturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| EVOO | extra-virgin olive oil |
| SFO | sunflower oil |
| LDL | low density lipoprotein |
| ROS | reactive oxygen species |
| SCD | stearoyl-CoA desaturase |
| SREBP | sterol regulatory element-binding protein |
| ACC | acetyl CoA carboxylase |
| FASN | fatty acid synthase |
| ELOVL6 | fatty acid elongases 6 |
| LXR | liver X receptor |
| PGC | peroxisome proliferator-activated receptors-gamma coactivator |
| AMPK | AMP-activated protein kinase |
| ARDS | acute respiratory distress syndrome |
| NAFLD | non-alcoholic liver disease |
| NASH | non-alcoholic steatohepatitis |
| HCC | hepatocellular carcinoma |
| CRC | colorectal cancer |
| IB | inflammatory bowel disease |
| HFD | high fat diet |
| HCD | high carbohydrate diet |
| MCD | methionine-choline deficient diet |
| ASO | anti-sense oligonucleotide |
| hiPSC | human induced pluripotent stem cells |
| BAT | brown adipose tissue |
References
- Vannice, G.; Rasmussen, H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. J. Acad. Nutr. Diet. 2014, 114, 136–153. [Google Scholar] [CrossRef]
- Diniz, Y.S.; Cicogna, A.C.; Padovani, C.R.; Santana, L.S.; Faine, L.A.; Novelli, E.L. Diets rich in saturated and polyunsaturated fatty acids: Metabolic shifting and cardiac health. Nutrition 2004, 20, 230–234. [Google Scholar] [CrossRef]
- Piomelli, D. A fatty gut feeling. Trends Endocrinol. Metab. 2013, 24, 332–341. [Google Scholar] [CrossRef]
- Das, U.N. Gamma-linolenic acid, arachidonic acid, and eicosapentaenoic acid as potential anticancer drugs. Nutrition 1990, 6, 429–434. [Google Scholar]
- Das, U.N. A defect in the activity of Delta6 and Delta5 desaturases may be a factor in the initiation and progression of atherosclerosis. Prostaglandins Leukot. Essent. Fatty Acids 2007, 76, 251–268. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Zare, M.; Faghih, S. Effects of Canola Oil Consumption on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J. Am. Coll. Nutr. 2019, 38, 185–196. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J.; Nutrition, C.; American Heart Association. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Trichopoulou, A. Mediterranean diet, traditional foods, and health: Evidence from the Greek EPIC cohort. Food Nutr. Bull. 2007, 28, 236–240. [Google Scholar] [CrossRef]
- Bester, D.; Esterhuyse, A.J.; Truter, E.J.; van Rooyen, J. Cardiovascular effects of edible oils: A comparison between four popular edible oils. Nutr. Res. Rev. 2010, 23, 334–348. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological Activities of Phenolic Compounds of Extra Virgin Olive Oil. Antioxidants 2013, 3, 1–23. [Google Scholar] [CrossRef]
- Bermudez, B.; Lopez, S.; Ortega, A.; Varela, L.M.; Pacheco, Y.M.; Abia, R.; Muriana, F.J. Oleic acid in olive oil: From a metabolic framework toward a clinical perspective. Curr. Pharm. Des. 2011, 17, 831–843. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Completes Review of Qualified Health Claim Petition for Oleic Acid and the Risk of Coronary Heart Disease. Available online: https://www.fda.gov/food/cfsan-constituent-updates/fda-completes-review-qualified-health-claim-petition-oleic-acid-and-risk-coronary-heart-disease (accessed on 10 August 2019).
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef]
- Navarro, M.D.; Hortelano, P.; Periago, J.L.; Pita, M.L. Effect of dietary olive and sunflower oils on the lipid composition of the aorta and platelets and on blood eicosanoids in rats. Arterioscler. Thromb. 1992, 12, 830–835. [Google Scholar] [CrossRef]
- Charnock, J.S.; Sundram, K.; Abeywardena, M.Y.; McLennan, P.L.; Tan, D.T. Dietary fats and oils in cardiac arrhythmia in rats. Am. J. Clin. Nutr. 1991, 53, 1047S–1049S. [Google Scholar] [CrossRef]
- Girardet, M.; Jacotot, B.; Mendy, F.; Piganeau, P.; Beaumont, J.L. Effects of edible oils on blood and arterial lipids in rats after one year’s balanced normolipidic diet. J. Med. 1977, 8, 261–278. [Google Scholar]
- Lambert, E.V.; Goedecke, J.H.; Bluett, K.; Heggie, K.; Claassen, A.; Rae, D.E.; West, S.; Dugas, J.; Dugas, L.; Meltzeri, S.; et al. Conjugated linoleic acid versus high-oleic acid sunflower oil: Effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br. J. Nutr. 2007, 97, 1001–1011. [Google Scholar] [CrossRef]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Prineas, R.J.; Stein, P.K.; Siscovick, D.S. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J. Am. Coll. Cardiol. 2006, 48, 478–484. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O.; Stoffersen, E.; Moncada, S.; Vane, J.R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978, 2, 117–119. [Google Scholar] [CrossRef]
- Investigators, G.P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455. [Google Scholar]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77917 Individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef]
- Casula, M.; Soranna, D.; Catapano, A.L.; Corrao, G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler. Suppl. 2013, 14, 243–251. [Google Scholar] [CrossRef]
- Djousse, L.; Akinkuolie, A.O.; Wu, J.H.; Ding, E.L.; Gaziano, J.M. Fish consumption, omega-3 fatty acids and risk of heart failure: A meta-analysis. Clin. Nutr. 2012, 31, 846–853. [Google Scholar] [CrossRef]
- Goel, A.; Pothineni, N.V.; Singhal, M.; Paydak, H.; Saldeen, T.; Mehta, J.L. Fish, Fish Oils and Cardioprotection: Promise or Fish Tale? Int. J. Mol. Sci. 2018, 19, 3703. [Google Scholar] [CrossRef]
- Kwak, S.M.; Myung, S.K.; Lee, Y.J.; Seo, H.G.; Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar] [CrossRef]
- Popoff, F.; Balaciano, G.; Bardach, A.; Comande, D.; Irazola, V.; Catalano, H.N.; Izcovich, A. Omega 3 fatty acid supplementation after myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2019, 19, 136. [Google Scholar] [CrossRef]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Weitz, D.; Weintraub, H.; Fisher, E.; Schwartzbard, A.Z. Fish oil for the treatment of cardiovascular disease. Cardiol. Rev. 2010, 18, 258–263. [Google Scholar] [CrossRef]
- Ong, A.S.; Goh, S.H. Palm oil: A healthful and cost-effective dietary component. Food Nutr. Bull. 2002, 23, 11–22. [Google Scholar] [CrossRef]
- Choo, Y.M.; Ng, M.H.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Application of supercritical fluid chromatography in the quantitative analysis of minor components (carotenes, vitamin E, sterols, and squalene) from palm oil. Lipids 2005, 40, 429–432. [Google Scholar] [CrossRef]
- Narang, D.; Sood, S.; Thomas, M.K.; Dinda, A.K.; Maulik, S.K. Effect of dietary palm olein oil on oxidative stress associated with ischemic-reperfusion injury in isolated rat heart. BMC Pharm. 2004, 4, 29. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Linseisen, J.; Welch, A.A.; Ocke, M.; Amiano, P.; Agnoli, C.; Ferrari, P.; Sonestedt, E.; Chajes, V.; Bueno-de-Mesquita, H.B.; Kaaks, R.; et al. Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: Results from the 24-h dietary recalls. Eur. J. Clin. Nutr. 2009, 63 (Suppl. 4), S61–S80. [Google Scholar] [CrossRef]
- Skeaff, C.M.; Miller, J. Dietary fat and coronary heart disease: Summary of evidence from prospective cohort and randomised controlled trials. Ann. Nutr. Metab. 2009, 55, 173–201. [Google Scholar] [CrossRef]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- Llorente-Cortes, V.; Estruch, R.; Mena, M.P.; Ros, E.; Gonzalez, M.A.; Fito, M.; Lamuela-Raventos, R.M.; Badimon, L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010, 208, 442–450. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nalsen, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001, 44, 312–319. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 August 2019).
- Lundman, P.; Boquist, S.; Samnegard, A.; Bennermo, M.; Held, C.; Ericsson, C.G.; Silveira, A.; Hamsten, A.; Tornvall, P. A high-fat meal is accompanied by increased plasma interleukin-6 concentrations. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 195–202. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 2016, 65, 193–207. [Google Scholar]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Peng, G.; Li, L.; Liu, Y.; Pu, J.; Zhang, S.; Yu, J.; Zhao, J.; Liu, P. Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinology 2011, 152, 2206–2218. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef]
- Camell, C.; Smith, C.W. Dietary oleic acid increases m2 macrophages in the mesenteric adipose tissue. PLoS ONE 2013, 8, e75147. [Google Scholar] [CrossRef]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Llor, X.; Pons, E.; Roca, A.; Alvarez, M.; Mane, J.; Fernandez-Banares, F.; Gassull, M.A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003, 22, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.I.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Motohiro, A.; Furukawa, T.; Yasumoto, K.; Inokuchi, K. Mechanisms involved in acute lung edema induced in dogs by oleic acid. Eur. Surg. Res. 1986, 18, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, D.; Li, S.; He, B.; Huang, Y.; Xu, M.; Ren, S.; Li, S.; Wang, H.; Xie, W. Activation of Liver X Receptor Attenuates Oleic Acid-Induced Acute Respiratory Distress Syndrome. Am. J. Pathol. 2016, 186, 2614–2622. [Google Scholar] [CrossRef]
- Kimura, Y. Carp oil or oleic acid, but not linoleic acid or linolenic acid, inhibits tumor growth and metastasis in Lewis lung carcinoma-bearing mice. J. Nutr. 2002, 132, 2069–2075. [Google Scholar] [CrossRef][Green Version]
- Piegari, M.; Soria, E.A.; Eynard, A.R.; Valentich, M.A. Delay of Lung Adenocarcinoma (LAC-1) Development in Mice by Dietary Oleic Acid. Nutr. Cancer 2017, 69, 1069–1074. [Google Scholar] [CrossRef]
- Romano, A.; Koczwara, J.B.; Gallelli, C.A.; Vergara, D.; Micioni Di Bonaventura, M.V.; Gaetani, S.; Giudetti, A.M. Fats for thoughts: An update on brain fatty acid metabolism. Int. J. Biochem. Cell Biol. 2017, 84, 40–45. [Google Scholar] [CrossRef]
- Bento-Abreu, A.; Velasco, A.; Polo-Hernandez, E.; Perez-Reyes, P.L.; Tabernero, A.; Medina, J.M. Megalin is a receptor for albumin in astrocytes and is required for the synthesis of the neurotrophic factor oleic acid. J. Neurochem. 2008, 106, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Friocourt, G.; Koulakoff, A.; Chafey, P.; Boucher, D.; Fauchereau, F.; Chelly, J.; Francis, F. Doublecortin functions at the extremities of growing neuronal processes. Cereb. Cortex 2003, 13, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Kim, H.J.; Man, W.C.; Ntambi, J.M. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J. Biol. Chem. 2001, 276, 39455–39461. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Velasco, A.; Granda, B.; Lavado, E.M.; Medina, J.M. Transcytosis of albumin in astrocytes activates the sterol regulatory element-binding protein-1, which promotes the synthesis of the neurotrophic factor oleic acid. J. Biol. Chem. 2002, 277, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Thibault, A.; Liu, L.; Samid, D. Lipid metabolism as a target for brain cancer therapy: Synergistic activity of lovastatin and sodium phenylacetate against human glioma cells. J. Neurochem. 1996, 66, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Gnoni, A.; Natali, F.; Testini, M.; Gnoni, G.V.; Siculella, L.; Damiano, F. Oleic Acid and Hydroxytyrosol Inhibit Cholesterol and Fatty Acid Synthesis in C6 Glioma Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9076052. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 113–121. [Google Scholar] [CrossRef]
- Miyazaki, M.; Kim, Y.C.; Ntambi, J.M. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J. Lipid Res. 2001, 42, 1018–1024. [Google Scholar]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Flowers, M.T.; Ntambi, J.M. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr. Opin. Lipidol. 2008, 19, 248–256. [Google Scholar] [CrossRef]
- Liu, X.; Strable, M.S.; Ntambi, J.M. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv. Nutr. 2011, 2, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.F.; Wilson, J.M.; Goncalves, O.; Galante-Oliveira, S.; Rocha, E.; Cunha, I. The evolutionary history of the stearoyl-CoA desaturase gene family in vertebrates. BMC Evol. Biol. 2011, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Schmidt, R.E.; Su, C.; Huang, X.; Gould, K.; Cao, G. Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Commun. 2005, 332, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Dobrzyn, A.; Elias, P.M.; Ntambi, J.M. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc. Natl. Acad. Sci. USA 2005, 102, 12501–12506. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Jacobson, M.J.; Man, W.C.; Cohen, P.; Asilmaz, E.; Friedman, J.M.; Ntambi, J.M. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J. Biol. Chem. 2003, 278, 33904–33911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Prouty, S.M.; Harmon, A.; Sundberg, J.P.; Stenn, K.S.; Parimoo, S. Scd3—A novel gene of the stearoyl-CoA desaturase family with restricted expression in skin. Genomics 2001, 71, 182–191. [Google Scholar] [CrossRef] [PubMed]
- AM, A.L.; Syed, D.N.; Ntambi, J.M. Insights into Stearoyl-CoA Desaturase-1 Regulation of Systemic Metabolism. TEM 2017, 28, 831–842. [Google Scholar] [CrossRef]
- Zheng, Y.; Eilertsen, K.J.; Ge, L.; Zhang, L.; Sundberg, J.P.; Prouty, S.M.; Stenn, K.S.; Parimoo, S. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat. Genet. 1999, 23, 268–270. [Google Scholar] [CrossRef]
- Jiang, G.; Li, Z.; Liu, F.; Ellsworth, K.; Dallas-Yang, Q.; Wu, M.; Ronan, J.; Esau, C.; Murphy, C.; Szalkowski, D.; et al. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J. Clin. Investig. 2005, 115, 1030–1038. [Google Scholar] [CrossRef]
- Dobrzyn, A.; Ntambi, J.M. The role of stearoyl-CoA desaturase in the control of metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2005, 73, 35–41. [Google Scholar] [CrossRef]
- Dobrzyn, A.; Ntambi, J.M. The role of stearoyl-CoA desaturase in body weight regulation. Trends Cardiovasc. Med. 2004, 14, 77–81. [Google Scholar] [CrossRef]
- Miyazaki, M.; Flowers, M.T.; Sampath, H.; Chu, K.; Otzelberger, C.; Liu, X.; Ntambi, J.M. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007, 6, 484–496. [Google Scholar] [CrossRef]
- Dobrzyn, A.; Dobrzyn, P.; Lee, S.H.; Miyazaki, M.; Cohen, P.; Asilmaz, E.; Hardie, D.G.; Friedman, J.M.; Ntambi, J.M. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E599–E607. [Google Scholar] [CrossRef]
- Lee, S.H.; Dobrzyn, A.; Dobrzyn, P.; Rahman, S.M.; Miyazaki, M.; Ntambi, J.M. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J. Lipid Res. 2004, 45, 1674–1682. [Google Scholar] [CrossRef]
- Sampath, H.; Flowers, M.T.; Liu, X.; Paton, C.M.; Sullivan, R.; Chu, K.; Zhao, M.; Ntambi, J.M. Skin-specific deletion of stearoyl-CoA desaturase-1 alters skin lipid composition and protects mice from high fat diet-induced obesity. J. Biol. Chem. 2009, 284, 19961–19973. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef]
- MacDonald, M.L.; van Eck, M.; Hildebrand, R.B.; Wong, B.W.; Bissada, N.; Ruddle, P.; Kontush, A.; Hussein, H.; Pouladi, M.A.; Chapman, M.J.; et al. Despite antiatherogenic metabolic characteristics, SCD1-deficient mice have increased inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 341–347. [Google Scholar] [CrossRef]
- Georgel, P.; Crozat, K.; Lauth, X.; Makrantonaki, E.; Seltmann, H.; Sovath, S.; Hoebe, K.; Du, X.; Rutschmann, S.; Jiang, Z.; et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect. Immun. 2005, 73, 4512–4521. [Google Scholar] [CrossRef]
- Chen, C.; Shah, Y.M.; Morimura, K.; Krausz, K.W.; Miyazaki, M.; Richardson, T.A.; Morgan, E.T.; Ntambi, J.M.; Idle, J.R.; Gonzalez, F.J. Metabolomics reveals that hepatic stearoyl-CoA desaturase 1 downregulation exacerbates inflammation and acute colitis. Cell Metab. 2008, 7, 135–147. [Google Scholar] [CrossRef]
- Prieur, X.; Roszer, T.; Ricote, M. Lipotoxicity in macrophages: Evidence from diseases associated with the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 327–337. [Google Scholar] [CrossRef]
- Mathieu, P.; Lemieux, I.; Despres, J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharm. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. The role of stearoyl-CoA desaturase in obesity, insulin resistance, and inflammation. Ann. N. Y. Acad. Sci. 2011, 1243, 47–53. [Google Scholar] [CrossRef]
- Cohen, P.; Miyazaki, M.; Socci, N.D.; Hagge-Greenberg, A.; Liedtke, W.; Soukas, A.A.; Sharma, R.; Hudgins, L.C.; Ntambi, J.M.; Friedman, J.M. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002, 297, 240–243. [Google Scholar] [CrossRef]
- Dobrzyn, P.; Jazurek, M.; Dobrzyn, A. Stearoyl-CoA desaturase and insulin signaling—What is the molecular switch? Biochim. Biophys. Acta 2010, 1797, 1189–1194. [Google Scholar] [CrossRef]
- McGarry, J.D. Banting lecture 2001: Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Dobrzyn, P.; Lee, S.H.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency elevates insulin-signaling components and down-regulates protein-tyrosine phosphatase 1B in muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 11110–11115. [Google Scholar] [CrossRef]
- Rahman, S.M.; Dobrzyn, A.; Lee, S.H.; Dobrzyn, P.; Miyazaki, M.; Ntambi, J.M. Stearoyl-CoA desaturase 1 deficiency increases insulin signaling and glycogen accumulation in brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E381–E387. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Igal, R.A. Stearoyl-CoA desaturase-1: A novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis 2010, 31, 1509–1515. [Google Scholar] [CrossRef]
- Mason, P.; Liang, B.; Li, L.; Fremgen, T.; Murphy, E.; Quinn, A.; Madden, S.L.; Biemann, H.P.; Wang, B.; Cohen, A.; et al. SCD1 inhibition causes cancer cell death by depleting mono-unsaturated fatty acids. PLoS ONE 2012, 7, e33823. [Google Scholar] [CrossRef]
- Goodridge, A.G. Regulation of the activity of acetyl coenzyme A carboxylase by palmitoyl coenzyme A and citrate. J. Biol. Chem. 1972, 247, 6946–6952. [Google Scholar]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Scaglia, N.; Igal, R.A. Stearoyl-CoA desaturase is involved in the control of proliferation, anchorage-independent growth, and survival in human transformed cells. J. Biol. Chem. 2005, 280, 25339–25349. [Google Scholar] [CrossRef]
- Hess, D.; Chisholm, J.W.; Igal, R.A. Inhibition of stearoylCoA desaturase activity blocks cell cycle progression and induces programmed cell death in lung cancer cells. PLoS ONE 2010, 5, e11394. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Maugeri-Sacca, M.; Fattore, L.; Bruschini, S.; De Vitis, C.; Tabbi, E.; Bellei, B.; Migliano, E.; Kovacs, D.; Camera, E.; et al. Inhibition of Stearoyl-CoA desaturase 1 reverts BRAF and MEK inhibition-induced selection of cancer stem cells in BRAF-mutated melanoma. J. Exp. Clin. Cancer Res. 2018, 37, 318. [Google Scholar] [CrossRef]
- She, K.; Fang, S.; Du, W.; Fan, X.; He, J.; Pan, H.; Huang, L.; He, P.; Huang, J. SCD1 is required for EGFR-targeting cancer therapy of lung cancer via re-activation of EGFR/PI3K/AKT signals. Cancer Cell Int. 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, Q.R.; Chan, E.; Merchant, M.; Liu, J.; French, D.; Ashkenazi, A.; Qing, J. FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res. 2012, 72, 5843–5855. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.M.; Gonzalez-Angulo, A.M.; Chen, H.; Akcakanat, A.; Do, K.A.; Fraser Symmans, W.; Pusztai, L.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F. High stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Breast Cancer Res. Treat. 2013, 137, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Knudsen, B.; True, L.D.; Hawley, S.; Etzioni, R.; Wade, C.; Gifford, D.; Coleman, I.; Nelson, P.S. Loss of stearoyl-CoA desaturase expression is a frequent event in prostate carcinoma. Int. J. Cancer 2005, 114, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Jumaa, H.; Webster, N.J. Splicing factor SRSF3 is crucial for hepatocyte differentiation and metabolic function. Nat. Commun. 2013, 4, 1336. [Google Scholar] [CrossRef]
- Ben-David, U.; Gan, Q.F.; Golan-Lev, T.; Arora, P.; Yanuka, O.; Oren, Y.S.; Leikin-Frenkel, A.; Graf, M.; Garippa, R.; Boehringer, M.; et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell 2013, 12, 167–179. [Google Scholar] [CrossRef]
- Rahimi, Y.; Mehdizadeh, A.; Nozad Charoudeh, H.; Nouri, M.; Valaei, K.; Fayezi, S.; Darabi, M. Hepatocyte differentiation of human induced pluripotent stem cells is modulated by stearoyl-CoA desaturase 1 activity. Dev. Growth Differ. 2015, 57, 667–674. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Miyazaki, M.; Stoehr, J.P.; Lan, H.; Kendziorski, C.M.; Yandell, B.S.; Song, Y.; Cohen, P.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- Feng, D.; Wang, Y.; Mei, Y.; Xu, Y.; Xu, H.; Lu, Y.; Luo, Q.; Zhou, S.; Kong, X.; Xu, L. Stearoyl-CoA desaturase 1 deficiency protects mice from immune-mediated liver injury. Lab. Investig. 2009, 89, 222–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Major, C.A.; Ryan, K.; Bennett, A.J.; Lock, A.L.; Bauman, D.E.; Salter, A.M. Inhibition of stearoyl CoA desaturase activity induces hypercholesterolemia in the cholesterol-fed hamster. J. Lipid Res. 2008, 49, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Dobrzyn, A.; Man, W.C.; Chu, K.; Sampath, H.; Kim, H.J.; Ntambi, J.M. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J. Biol. Chem. 2004, 279, 25164–25171. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Sampath, H.; Liu, X.; Flowers, M.T.; Chu, K.; Dobrzyn, A.; Ntambi, J.M. Stearoyl-CoA desaturase-1 deficiency attenuates obesity and insulin resistance in leptin-resistant obese mice. Biochem. Biophys. Res. Commun. 2009, 380, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Karahashi, M.; Ishii, F.; Yamazaki, T.; Imai, K.; Mitsumoto, A.; Kawashima, Y.; Kudo, N. Up-regulation of stearoyl-CoA desaturase 1 increases liver MUFA content in obese Zucker but not Goto-Kakizaki rats. Lipids 2013, 48, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.T.; Groen, A.K.; Oler, A.T.; Keller, M.P.; Choi, Y.; Schueler, K.L.; Richards, O.C.; Lan, H.; Miyazaki, M.; Kuipers, F.; et al. Cholestasis and hypercholesterolemia in SCD1-deficient mice fed a low-fat, high-carbohydrate diet. J. Lipid Res. 2006, 47, 2668–2680. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.T.; Keller, M.P.; Choi, Y.; Lan, H.; Kendziorski, C.; Ntambi, J.M.; Attie, A.D. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol. Genom. 2008, 33, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.T.; Ade, L.; Strable, M.S.; Ntambi, J.M. Combined deletion of SCD1 from adipose tissue and liver does not protect mice from obesity. J. Lipid Res. 2012, 53, 1646–1653. [Google Scholar] [CrossRef]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice fed a lipogenic methionine-choline-deficient diet develop hypermetabolism coincident with hepatic suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef]
- Li, Z.Z.; Berk, M.; McIntyre, T.M.; Feldstein, A.E. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: Role of stearoyl-CoA desaturase. J. Biol. Chem. 2009, 284, 5637–5644. [Google Scholar] [CrossRef]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.M.; Jiang, Q.H.; Cai, C.; Qu, M.; Shen, W. SCD1 negatively regulates autophagy-induced cell death in human hepatocellular carcinoma through inactivation of the AMPK signaling pathway. Cancer Lett. 2015, 358, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.Y.; Kweon, S.M.; Chi, F.; Hwang, E.; Kabe, Y.; Higashiyama, R.; Qin, L.; Yan, R.; Wu, R.P.; Lai, K.; et al. Stearoyl-CoA Desaturase Promotes Liver Fibrosis and Tumor Development in Mice via a Wnt Positive-Signaling Loop by Stabilization of Low-Density Lipoprotein-Receptor-Related Proteins 5 and 6. Gastroenterology 2017, 152, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.K.F.; Lau, E.Y.T.; Leung, D.H.W.; Lo, J.; Ho, N.P.Y.; Cheng, L.K.W.; Ma, S.; Lin, C.H.; Copland, J.A.; Ding, J.; et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J. Hepatol. 2017, 67, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, N.; Shimano, H.; Hasegawa, K.; Ohashi, K.; Matsuzaka, T.; Najima, Y.; Sekiya, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur. J. Cancer 2005, 41, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kuang, S.; Cao, R.; Wang, J.; Peng, Q.; Sun, C. Sorafenib kills liver cancer cells by disrupting SCD1-mediated synthesis of monounsaturated fatty acids via the ATP-AMPK-mTOR-SREBP1 signaling pathway. FASEB J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Vargas, T.; Moreno-Rubio, J.; Herranz, J.; Cejas, P.; Molina, S.; Gonzalez-Vallinas, M.; Mendiola, M.; Burgos, E.; Aguayo, C.; Custodio, A.B.; et al. ColoLipidGene: Signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget 2015, 6, 7348–7363. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, S.; Sumi, H.; Satoh, Y.; Yamamoto, Y.; Kitazawa, S.; Honda, K.; Araki, H.; Kakoi, K.; Imamura, K.; Sasaki, M.; et al. In vitro and in vivo antitumor activities of T-3764518, a novel and orally available small molecule stearoyl-CoA desaturase 1 inhibitor. Eur. J. Pharm. 2017, 807, 21–31. [Google Scholar] [CrossRef]
- Chen, L.; Ren, J.; Yang, L.; Li, Y.; Fu, J.; Li, Y.; Tian, Y.; Qiu, F.; Liu, Z.; Qiu, Y. Stearoyl-CoA desaturase-1 mediated cell apoptosis in colorectal cancer by promoting ceramide synthesis. Sci. Rep. 2016, 6, 19665. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Ye, X.; Chen, L.; Zhang, L.; Gao, Y.; Kang, J.X.; Cai, C. Characteristics of fatty acid distribution is associated with colorectal cancer prognosis. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 355–360. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Bissada, N.; Vallance, B.A.; Hayden, M.R. Absence of stearoyl-CoA desaturase-1 does not promote DSS-induced acute colitis. Biochim. Biophys. Acta 2009, 1791, 1166–1172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ducheix, S.; Peres, C.; Hardfeldt, J.; Frau, C.; Mocciaro, G.; Piccinin, E.; Lobaccaro, J.M.; De Santis, S.; Chieppa, M.; Bertrand-Michel, J.; et al. Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology 2018, 155, 1524–1538. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Hough, G.; Chattopadhyay, A.; Grijalva, V.; O’Connor, E.I.; Meriwether, D.; Wagner, A.; Ntambi, J.M.; Navab, M.; Reddy, S.T.; et al. Role of enterocyte stearoyl-Co-A desaturase-1 in LDLR-null mice. J. Lipid Res. 2018, 59, 1818–1840. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chassaing, B.; Zhang, L.; San Yeoh, B.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hormannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef] [PubMed]
| Trial Identifier | Trial Phase (Status) | Title | Intervention |
|---|---|---|---|
| NCT02647970 | Completed | Stearoyl-CoA Desaturase and Energy Metabolism in Humans | Behavioral: PUFA‒Cys/Met diet Behavioral: SFA+Cys/Met diet |
| NCT03572205 | Completed | Fatty Acid Desaturase Gene Locus Interactions with Diet (FADSDIET2) | Dietary Supplement: LA Dietary Supplement: ALA |
| NCT03282253 | Not yet recruiting | Elevated Stearoyl-CoA Desaturase-1 Expression Predicts the Disease Severity of Severe Acute Pancreatitis | |
| NCT02543216 | Completed | Gene–Diet Interactions in Fatty Acid Desaturase 1 Gene | Dietary Supplement: Sunflower oil |
| NCT03842891 | Completed | Genetic Variants Modulate Association Between Dietary n-3 LCPUFAs and DHA Proportion in Breast Milk | |
| NCT01661764 | Completed, Has Results | Fish Oil Supplementation, Nutrigenomics and Colorectal Cancer Prevention | Drug: Eicosapentanoic acid and docosahexanoic acid Drug: Oleic Acid |
| NCT02337231 | Completed | Botanical Oils Study to Determine Genetic Differences in the Way Your Body Processes Fats in Edible Oils | Dietary Supplement: soybean oil and borage oil |
| Trial Identifier | Trial Phase (Status) | Title | Intervention |
|---|---|---|---|
| NCT00715312 | Completed | Effect of Oleic Acid on Inflammation Markers and Blood Lipid Metabolites: A Randomised, Double-Blind, Crossover Study | Novel Olein |
| NCT01042340 | Completed | Energy Dense Oleic Acid Formula to Geriatric Patients | Calogen®–an energy dense oleic acid-based formula |
| NCT01124487 | Completed | The Acute Effects of Oleic Acid Enriched diets on Lipids, Insulin Sensitivity and Serum Inflammatory Markers | Dietary Supplement: The acute effects of dietary fat on lipid profile, insulin sensitivity and inflammatory markers |
| NCT02029833 | Completed | Canola Oil Multi-Centre Intervention Trial II | Other: Regular Canola Oil Other: High Oleic Canola Oil Other: Western Type Diet–Common Dietary Oils |
| NCT03054779 | Completed | Canola Oil Multi-Centre Intervention Trial II | Other: Canola Oil Other: High oleic acid canola oil Other: Western diet oil combination |
| NCT02993380 | Completed | Effect of Olive Oil on Erythrocyte Membrane Fatty Acid Contents in Hemodialysis Patients | Dietary Supplement: Stir-fried olive oil group Dietary Supplement: Natural olive oil group |
| NCT00529828 | Completed | Health Effects of CLA Versus Industrial Trans Fatty Acids | Procedure: Consumption of CLA enriched food |
| NCT01259999 | Completed | Energy Dense Formula to People Living in Old Peoples Home | Dietary Supplement: Calogen extra strawberry |
| NCT00059254 | Completed | Differential Metabolism of Dietary Fatty Acids | Dietary Supplement: Oleic acid (OA) Dietary Supplement: Palmitic Acid (PA) |
| NCT01996566 | Completed | Fatty Acid Taste thresholds: Caproic, Lauric, Oleic, Linoleic, Linolenic |
| Type of Study | SCD1 Status | Phenotype | Ref |
|---|---|---|---|
| In vivo | |||
| SCD1KO mice | Whole body SCD1 deletion | Protection from HFD and HCD-induced adiposity and hepatic steatosis. High susceptibility to DSS-induced gut inflammation | [92,122] |
| Asebia (abJ/abJ) mice | Lack of SCD1 for a naturally occurred mutation | Protection from liver steatosis and adiposity | [82,96] |
| LSCD1KO mice | Liver specific SCD1 deletion | Protection from HCD-induced adiposity and hepatic steatosis. Susceptibility to HFD-induced obesity and hepatic steatosis. | [85] |
| LASCD1KO mice | Adipose and liver combined SCD1 deletion | Susceptibility from diet induced obesity | [130] |
| iSCD1KO mice | Intestinal specific SCD1 deletion | Susceptibility to CRC when crossed with ApcMin mice and fed with oleic acid deficient diet | [144] |
| Hamster treated with SCD1 inhibitor | Inhibition of SCD1 activity | Liver protection from cholesterol enriched diet and susceptibility to atherogenic risk | [124] |
| Ex Vivo | |||
| HCC specimens | High SCD1 expression | Shorter disease-free survival and sorafenib resistance in HCC | [136] |
| CRC specimens | Low SCD1 activity | [142] | |
| CRC specimens | High SCD1 expression | Worse clinical CRC outcome | [139,141] |
| In vitro | |||
| Cell culture treated with SCD1 inhibitor | Inhibition of SCD1 activity | Suppression of tumor cell proliferation and apoptosis induction | [106,141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. https://doi.org/10.3390/nu11102283
Piccinin E, Cariello M, De Santis S, Ducheix S, Sabbà C, Ntambi JM, Moschetta A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients. 2019; 11(10):2283. https://doi.org/10.3390/nu11102283
Chicago/Turabian StylePiccinin, Elena, Marica Cariello, Stefania De Santis, Simon Ducheix, Carlo Sabbà, James M. Ntambi, and Antonio Moschetta. 2019. "Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1)" Nutrients 11, no. 10: 2283. https://doi.org/10.3390/nu11102283
APA StylePiccinin, E., Cariello, M., De Santis, S., Ducheix, S., Sabbà, C., Ntambi, J. M., & Moschetta, A. (2019). Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients, 11(10), 2283. https://doi.org/10.3390/nu11102283






