Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Population Study
- Age < 18 years and > 40 years;
- Menopause (defined as amenorrhea for ≥3 years or amenorrhea for ≥1 but <3 years and plasma follicle-stimulating hormone concentrations elevated to the postmenopausal range); pregnancy or lactation in the past 6 months;
- Hyperandrogenism and/or biochemical hyperandrogenemia, oligomenorrhea due to secondary etiologies as per the Endocrine Society Clinical Practice Guidelines and previous publications including endocrine disorders (congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome, hyperprolactinaemia, thyroid dysfunction and adrenal disorders) [24];
- Pre-existing systemic or psychiatric disease and use of medications that impact carbohydrate or lipid metabolism (oral contraceptive pills, metformin, anti-epileptics, anti-psychotics, statins and fish oil);
- Specific nutritional regimens or hypocaloric diet in the last three months, including vegan or vegetarian diets; supplementation with antioxidants, vitamins or minerals;
- Occasional or current of use of drugs that could influence fluid balance, including non-steroidal anti-inflammatory drugs, diuretics, laxative use; and,
- Women patients with implanted pacemakers or defibrillators because of the theoretical possibility of interference with the device activity due to the field of current induced by the impedance measurements.
2.3. Sample Size Justification and Power
2.4. Lifestyle Habits and Anthropometric Measurements
2.5. Nutritional Assessments
2.6. Body Composition
2.7. Assay Methods
2.8. Clinical Hyperandrogenism
2.9. Statistical Analysis
3. Results
Correlation Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Laven, J.S.; Imani, B.; Eijkemans, M.J.; Fauser, B.C. New approach to polycystic ovary syndrome and other forms of anovulatory infertility. Obstet. Gynecol. Surv. 2002, 57, 755–767. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam, E.A.; ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar]
- Amiri, M.; Ramezani Tehrani, F.; Nahidi, F.; Bidhendi Yarandi, R.; Behboudi-Gandevani, S.; Azizi, F. Association between biochemical hyperandrogenism parameters and Ferriman-Gallwey score in patients with polycystic ovary syndrome: A systematic review and meta-regression analysis. Clin. Endocrinol. (Oxf.) 2017, 87, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Economou, F.; Palimeri, S.; Christakou, C. Metformin in polycystic ovary syndrome. Ann. N. Y. Acad. Sci. 2010, 1205, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Yadav, A.; Dang, A.S. Sex hormone binding globulin—An important biomarker for predicting PCOS risk: A systematic review and meta-analysis. Syst. Biol. Reprod. Med. 2018, 64, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Tosi, F.; Castello, R.; Magnani, C.M.; Negri, C.; Brun, E.; Furlani, L.; Caputo, M.; Muggeo, M. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: Evidence that androgens impair insulin action in women. J. Clin. Endocrinol. Metab. 1996, 81, 952–960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lord, J.; Thomas, R.; Fox, B.; Acharya, U.; Wilkin, T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Geronikolou, S.A.; Bacopoulou, F.; Cokkinos, D. Bioimpedance Measurements in Adolescents with Polycystic Ovary Syndrome: A Pilot Study. Adv. Exp. Med. Biol. 2017, 987, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch. Biochem. Biophys. 2009, 486, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Breslin, P.A.; Beauchamp, G.K.; Keast, R.S. Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra virgin olive oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Singh, N.P.; Singh, B.; Hofseth, L.J.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S. Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(-/-) mice. Brain Behav. Immun. 2012, 26, 72–82. [Google Scholar] [CrossRef]
- Rajaram, S.; Connell, K.M.; Sabate, J. Effect of almond-enriched high-monounsaturated fat diet on selected markers of inflammation: A randomised, controlled, crossover study. Br. J. Nutr. 2010, 103, 907–912. [Google Scholar] [CrossRef]
- Morken, T.; Bohov, P.; Skorve, J.; Ulvik, R.; Aukrust, P.; Berge, R.K.; Livden, J.K. Anti-inflammatory and hypolipidemic effects of the modified fatty acid tetradecylthioacetic acid in psoriasis—A pilot study. Scand. J. Clin. Lab. Investig. 2011, 71, 269–273. [Google Scholar] [CrossRef]
- Douglas, C.C.; Norris, L.E.; Oster, R.A.; Darnell, B.E.; Azziz, R.; Gower, B.A. Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil. Steril. 2006, 86, 411–417. [Google Scholar] [CrossRef]
- Savastano, S.; Belfiore, A.; Di Somma, C.; Mauriello, C.; Rossi, A.; Pizza, G.; De Rosa, A.; Prestieri, G.; Angrisani, L.; Colao, A. Validity of bioelectrical impedance analysis to estimate body composition changes after bariatric surgery in premenopausal morbidly women. Obes. Surg. 2010, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, U.; Pall, M.; Mathur, R.; Azziz, R. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Hestiantoro, A.; Kapnosa Hasani, R.D.; Shadrina, A.; Situmorang, H.; Ilma, N.; Muharam, R.; Sumapraja, K.; Wiweko, B. Body fat percentage is a better marker than body mass index for determining inflammation status in polycystic ovary syndrome. Int. J. Reprod. Biomed. (Yazd) 2018, 16, 623–628. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine, S. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, D.; Versace, V.; Tagliaferri, V.; De Cicco, S.; Immediata, V.; Apa, R.; Guido, M.; Lanzone, A. The resting metabolic rate in women with polycystic ovary syndrome and its relation to the hormonal milieu, insulin metabolism, and body fat distribution: A cohort study. J. Endocrinol. Investig. 2019, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Di Somma, C.; Altieri, B.; Vecchiarini, M.; Orio, F.; Spinosa, T.; Colao, A.; Savastano, S. Patient empowerment and the Mediterranean diet as a possible tool to tackle prediabetes associated with overweight or obesity: A pilot study. Hormones (Athens) 2019, 18, 75–84. [Google Scholar] [CrossRef]
- Savastano, S.; Di Somma, C.; Colao, A.; Barrea, L.; Orio, F.; Finelli, C.; Pasanisi, F.; Contaldo, F.; Tarantino, G. Preliminary data on the relationship between circulating levels of Sirtuin 4, anthropometric and metabolic parameters in obese subjects according to growth hormone/insulin-like growth factor-1 status. Growth Horm. IGF Res. Off. J. Growth Horm. Res. Soc. Int. IGF Res. Soc. 2015, 25, 28–33. [Google Scholar] [CrossRef]
- Savanelli, M.C.; Scarano, E.; Muscogiuri, G.; Barrea, L.; Vuolo, L.; Rubino, M.; Savastano, S.; Colao, A.; Di Somma, C. Cardiovascular risk in adult hypopituitaric patients with growth hormone deficiency: Is there a role for vitamin D? Endocrine 2016, 52, 111–119. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. A New Light on Vitamin D in Obesity: A Novel Association with Trimethylamine-N-Oxide (TMAO). Nutrients 2019, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Body Mass Index. Available online: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 1 August 2019).
- Anthropometry Procedures Manual. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/Anthropometry_Procedures_Manual.pdf (accessed on 1 August 2019).
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Tarantino, G.; Somma, C.D.; Muscogiuri, G.; Macchia, P.E.; Falco, A.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxid. Med. Cell. Longev. 2017, 2017, 6101254. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Altieri, B.; Muscogiuri, G.; Laudisio, D.; Annunziata, G.; Colao, A.; Faggiano, A.; Savastano, S. Impact of Nutritional Status on Gastroenteropancreatic Neuroendocrine Tumors (GEP-NET) Aggressiveness. Nutrients 2018, 10, 1854. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Macchia, P.E.; Tarantino, G.; Di Somma, C.; Pane, E.; Balato, N.; Napolitano, M.; Colao, A.; Savastano, S. Nutrition: A key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J. Transl. Med. 2015, 13, 303. [Google Scholar] [CrossRef]
- Barrea, L.; Di Somma, C.; Macchia, P.E.; Falco, A.; Savanelli, M.C.; Orio, F.; Colao, A.; Savastano, S. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin. Nutr. 2017, 36, 293–301. [Google Scholar] [CrossRef]
- Savanelli, M.C.; Barrea, L.; Macchia, P.E.; Savastano, S.; Falco, A.; Renzullo, A.; Scarano, E.; Nettore, I.C.; Colao, A.; Di Somma, C. Preliminary results demonstrating the impact of Mediterranean diet on bone health. J. Transl. Med. 2017, 15, 81. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Annunziata, G.; Megna, M.; Falco, A.; Balato, A.; Colao, A.; Savastano, S. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018, 92, 1831–1845. [Google Scholar] [CrossRef]
- Turconi, G.; Guarcello, M.; Berzolari, F.G.; Carolei, A.; Bazzano, R.; Roggi, C. An evaluation of a colour food photography atlas as a tool for quantifying food portion size in epidemiological dietary surveys. Eur. J. Clin. Nutr. 2005, 59, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [CrossRef] [PubMed]
- Savastano, S.; Barbato, A.; Di Somma, C.; Guida, B.; Pizza, G.; Barrea, L.; Avallone, S.; Schiano di Cola, M.; Strazzullo, P.; Colao, A. Beyond waist circumference in an adult male population of Southern Italy: Is there any role for subscapular skinfold thickness in the relationship between insulin-like growth factor-I system and metabolic parameters? J. Endocrinol. Investig. 2012, 35, 925–929. [Google Scholar] [CrossRef]
- Barrea, L.; Macchia, P.E.; Di Somma, C.; Napolitano, M.; Balato, A.; Falco, A.; Savanelli, M.C.; Balato, N.; Colao, A.; Savastano, S. Bioelectrical phase angle and psoriasis: A novel association with psoriasis severity, quality of life and metabolic syndrome. J. Transl. Med. 2016, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Macchia, P.E.; Di Somma, C.; Falco, A.; Savanelli, M.C.; Colao, A.; Savastano, S. Mediterranean Diet and Phase Angle in a Sample of Adult Population: Results of a Pilot Study. Nutrients 2017, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Fabbrocini, G.; Annunziata, G.; Muscogiuri, G.; Donnarumma, M.; Marasca, C.; Colao, A.; Savastano, S. Role of Nutrition and Adherence to the Mediterranean Diet in the Multidisciplinary Approach of Hidradenitis Suppurativa: Evaluation of Nutritional Status and Its Association with Severity of Disease. Nutrients 2018, 11, 57. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gomez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Kushner, R.F. Bioelectrical impedance analysis: A review of principles and applications. J. Am. Coll. Nutr. 1992, 11, 199–209. [Google Scholar]
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Danielzik, S.; Dorhofer, R.P.; Later, W.; Wiese, S.; Muller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. J. Parenter. Enter. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Somma, C.D.; Salzano, C.; Pugliese, G.; Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A Possible Biomarker to Quantify Inflammation in Subjects with Obesity and 25(OH)D Deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ferriman, D.; Gallwey, J.D. Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 1961, 21, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Brodell, L.A.; Mercurio, M.G. Hirsutism: Diagnosis and management. Gend. Med. 2010, 7, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Yeung, E.H.; Mumford, S.L.; Zhang, C.; Gaskins, A.J.; Wactawski-Wende, J.; Schisterman, E.F.; BioCycle Study, G. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur. J. Clin. Nutr. 2013, 67, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Abiemo, E.E.; Alonso, A.; Nettleton, J.A.; Steffen, L.M.; Bertoni, A.G.; Jain, A.; Lutsey, P.L. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA). Br. J. Nutr. 2013, 109, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 136,846 participants. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Rimm, E.B.; Medina-Remon, A.; Martinez-Gonzalez, M.A.; Lopez-Sabater, M.C.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; et al. Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Sia, C.L.; Shepard, M.K.; Rote, N.S.; Minium, J. The altered mononuclear cell-derived cytokine response to glucose ingestion is not regulated by excess adiposity in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, E2244–E2251. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Cook, N.R.; Stampfer, M.J.; Ridker, P.M.; Rexrode, K.M.; Buring, J.E.; Manson, J.E.; Liu, S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008, 57, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Marzullo, P.; Muscogiuri, G.; Di Somma, C.; Scacchi, M.; Orio, F.; Aimaretti, G.; Colao, A.; Savastano, S. Source and amount of carbohydrate in the diet and inflammation in women with polycystic ovary syndrome. Nutr. Res. Rev. 2018, 31, 291–301. [Google Scholar] [CrossRef]
- Gonzalez, F. Nutrient-Induced Inflammation in Polycystic Ovary Syndrome: Role in the Development of Metabolic Aberration and Ovarian Dysfunction. Semin. Reprod. Med. 2015, 33, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Kalgaonkar, S.; Almario, R.U.; Gurusinghe, D.; Garamendi, E.M.; Buchan, W.; Kim, K.; Karakas, S.E. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur. J. Clin. Nutr. 2011, 65, 386–393. [Google Scholar] [CrossRef]
- Berbert, A.A.; Kondo, C.R.; Almendra, C.L.; Matsuo, T.; Dichi, I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition 2005, 21, 131–136. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Bao, T.; Ge, J. Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2018, 16, 27. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Ebrahimi, F.A.; Hashemi, T.; Taghizadeh, M.; Razavi, M.; Sanami, M.; Asemi, Z. The effects of vitamin D and omega-3 fatty acid co-supplementation on glycemic control and lipid concentrations in patients with gestational diabetes. J. Clin. Lipidol. 2017, 11, 459–468. [Google Scholar] [CrossRef]
- Johansen, K.L.; Kaysen, G.A.; Young, B.S.; Hung, A.M.; da Silva, M.; Chertow, G.M. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am. J. Clin. Nutr. 2003, 77, 842–846. [Google Scholar] [CrossRef]
- Norman, K.; Stobaus, N.; Zocher, D.; Bosy-Westphal, A.; Szramek, A.; Scheufele, R.; Smoliner, C.; Pirlich, M. Cutoff percentiles of bioelectrical phase angle predict functionality, quality of life, and mortality in patients with cancer. Am. J. Clin. Nutr. 2010, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Stobaus, N.; Pirlich, M.; Valentini, L.; Schulzke, J.D.; Norman, K. Determinants of bioelectrical phase angle in disease. Br. J. Nutr. 2012, 107, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobaus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, A.; Beisenherz, A.; Romer, K.; Kremer, G.; Salzberger, B.; Elia, M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am. J. Clin. Nutr. 2000, 72, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Lowrie, E.G.; Wilmore, D.W.; Gonzalez, J.; Lew, N.L.; Ling, J.; Leboff, M.S.; Gottlieb, M.N.; Huang, W.; Zebrowski, B.; et al. Nutritional assessment with bioelectrical impedance analysis in maintenance hemodialysis patients. J. Am. Soc. Nephrol. 1995, 6, 75–81. [Google Scholar] [PubMed]
- Hoidrup, S.; Andreasen, A.H.; Osler, M.; Pedersen, A.N.; Jorgensen, L.M.; Jorgensen, T.; Schroll, M.; Heitmann, B.L. Assessment of habitual energy and macronutrient intake in adults: Comparison of a seven day food record with a dietary history interview. Eur. J. Clin. Nutr. 2002, 56, 105–113. [Google Scholar] [CrossRef]
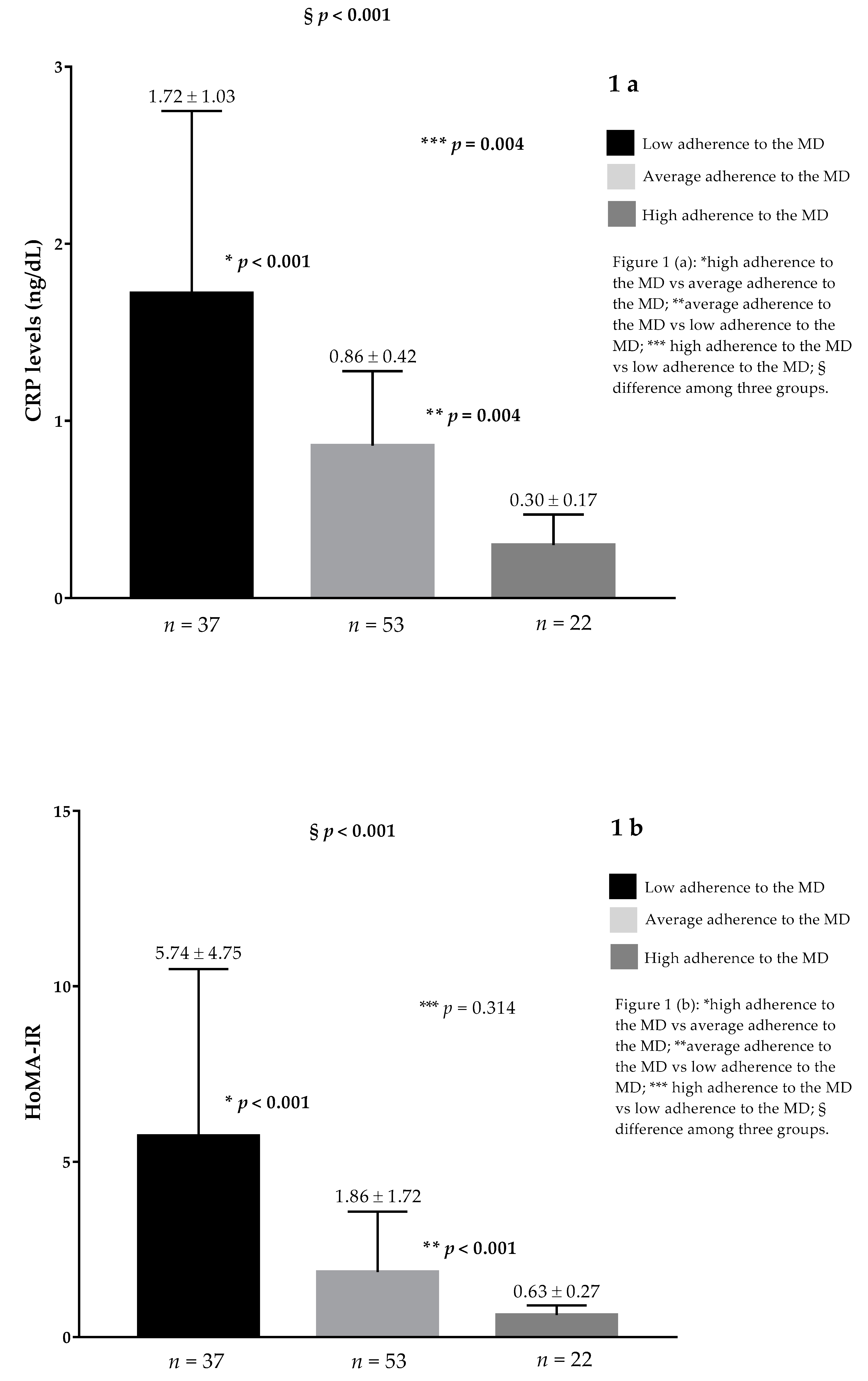
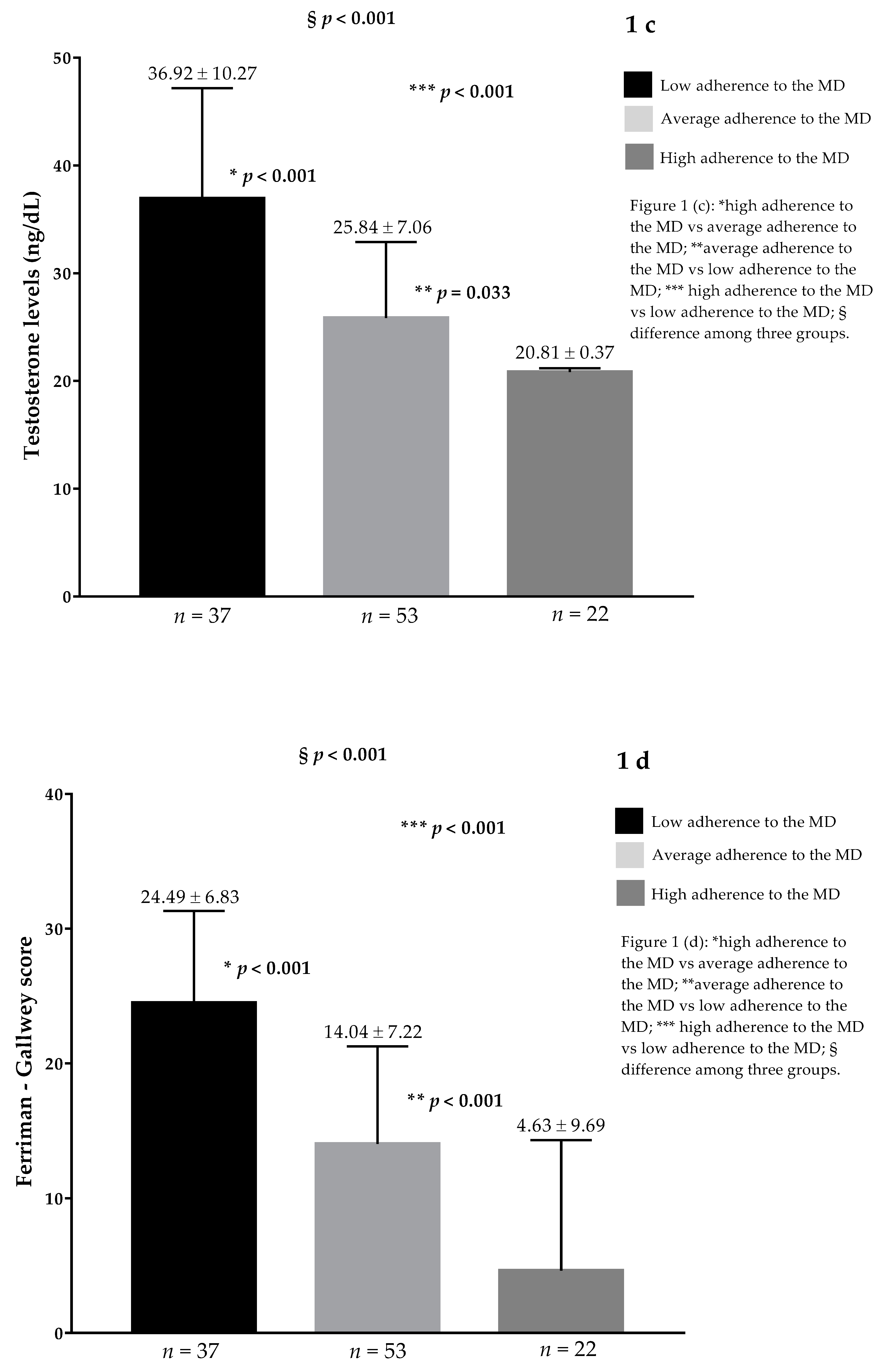
| Parameters | PCOS Patients n = 112 | Control Group n = 112 | p Values |
|---|---|---|---|
| Lifestyle Habits | |||
| Age (years) | 24.21 ± 5.47 | 24.07 ± 5.05 | 0.721 |
| Smoking | χ2 = 0.10, p = 0.756 | ||
| Yes (n, %) | 29, 25.9% | 26, 23.2% | |
| No (n, %) | 83, 74.1% | 86, 76.8% | |
| Physical activity | χ2 = 0.08, p = 0.777 | ||
| Sedentary (n, %) | 76, 67.9% | 73, 65.2% | |
| Moderate (n, %) | 36, 32.1% | 39, 34.8% | |
| Anthropometric measurements | |||
| BMI (kg/m2) | 30.95 ± 5.66 | 30.76 ± 5.60 | 0.273 |
| Normal-weight (n, %) | 24, 21.4% | 24, 21.4% | χ2 = 0.00, p = 1.000 |
| Over-weight (n, %) | 29, 25.9% | 29, 25.9% | |
| Obesity I (n, %) | 24, 21.4% | 24, 21.4% | |
| Obesity II (n, %) | 35, 31.3% | 35, 31.3% | |
| WC (cm) | 101.09 ± 16.29 | 92.54 ± 14.17 | <0.001 |
| WC< cut-off | 20, 17.9% | 43, 38.4% | χ2 = 10.69, p = 0.001 |
| WC > cut-off | 92, 82.1% | 69, 61.6% | |
| Adherence to the MD | |||
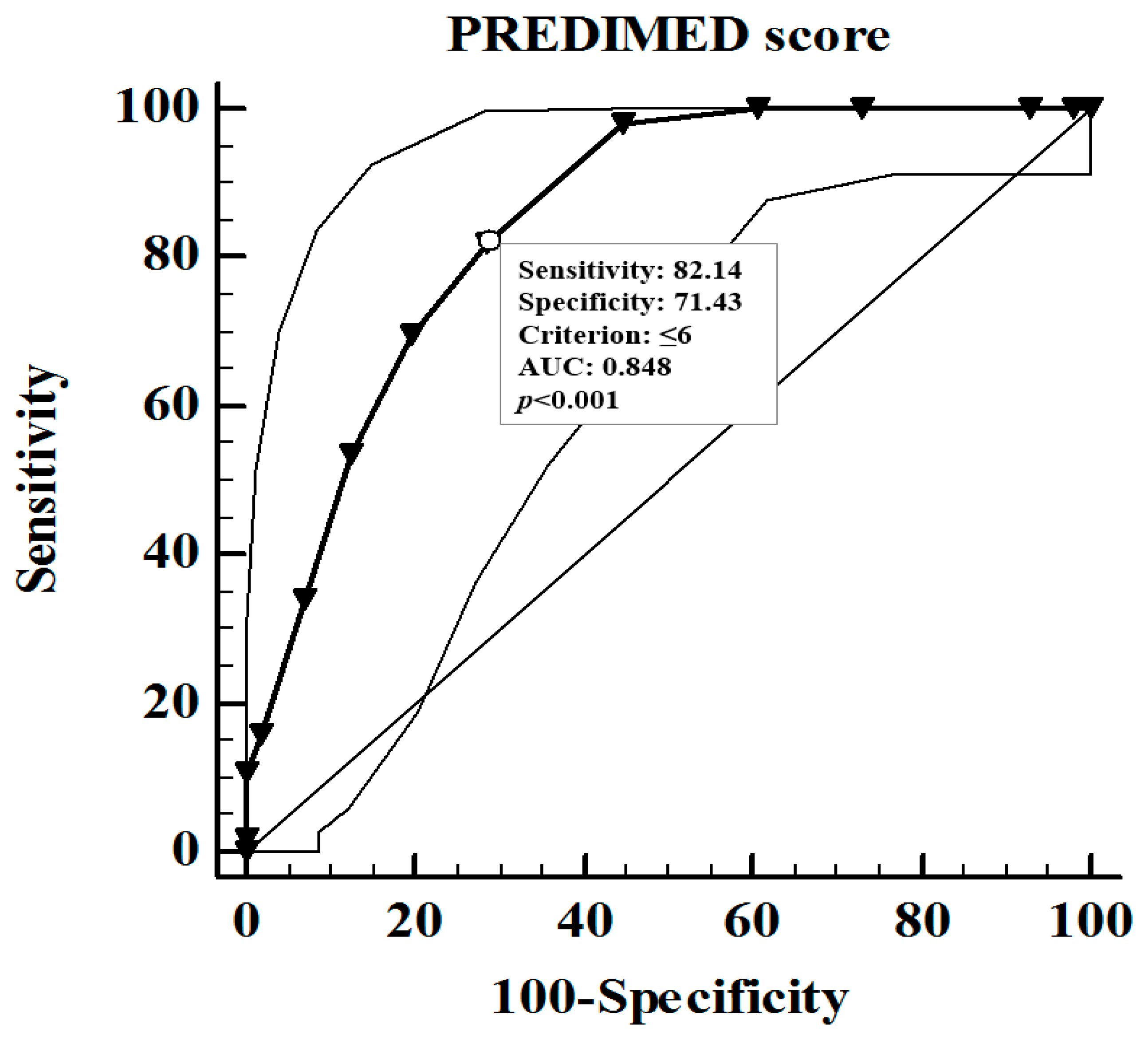
| PREDIMED score | 6.97 ± 2.72 | 8.12 ± 2.80 | <0.001 |
| Low adherence to the MD | 37, 33.0% | 21, 18.8% | χ2 = 5.24, p = 0.022 |
| Average adherence to the MD | 53, 47.3% | 58, 51.8% | χ2 = 0.29, p = 0.593 |
| High adherence to the MD | 22, 19.6% | 33, 29.5% | χ2 = 2.41, p = 0.121 |
| Inflammatory parameter | |||
| CRP levels (ng/mL) | 1.03 ± 0.84 | 0.58 ± 0.44 | <0.001 |
| Hormonal and biochemical parameters | |||
| Testosterone (ng/dL) | 28.51 ± 9.82 | 10.21 ± 4.39 | <0.001 |
| Insulin (μU/mL) | 11.61 ± 12.97 | 6.19 ± 7.79 | <0.001 |
| Fasting glucose (mg/dL) | 94.92 ± 11.70 | 89.03 ± 10.89 | <0.001 |
| HoMA-IR | 2.90 ± 3.59 | 1.45 ± 1.95 | <0.001 |
| Clinical Hyperandrogenism | |||
| Ferriman-Gallwey score | 15.64 ± 9.69 | 2.47 ± 1.68 | <0.001 |
| Questions PREDIMED Questionnaire | PCOS Patients n = 112 | Control Group n = 112 | χ2 | p Values | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Use of extra-virgin olive oil as main culinary lipid | 88 | 78.6 | 108 | 96.4 | 14.74 | <0.001 |
| Extra virgin olive oil >4 tablespoons | 64 | 57.1 | 55 | 49.1 | 1.15 | 0.284 |
| Vegetables ≥2 servings/day | 54 | 48.2 | 62 | 55.4 | 0.87 | 0.349 |
| Fruits ≥3 servings/day | 67 | 59.8 | 66 | 58.9 | 0.01 | 1.000 |
| Red/processed meats <1/day | 62 | 55.4 | 57 | 50.9 | 0.28 | 0.592 |
| Butter, cream, margarine <1/day | 47 | 42.0 | 55 | 49.1 | 0.88 | 0.348 |
| Soda drinks <1/day | 53 | 47.3 | 58 | 51.8 | 0.28 | 0.593 |
| Wine glasses ≥ 7/week | 30 | 26.8 | 35 | 31.3 | 0.35 | 0.555 |
| Legumes ≥ 3/week | 60 | 53.6 | 81 | 72.3 | 7.66 | 0.006 |
| Fish/seafood ≥ 3/week | 35 | 31.3 | 76 | 67.9 | 28.57 | <0.001 |
| Commercial sweets and confectionery ≤ 2/week | 59 | 52.7 | 56 | 50.0 | 0.07 | 0.789 |
| Tree nuts ≥ 3/week | 31 | 27.7 | 76 | 67.6 | 34.64 | <0.001 |
| Poultry more than red meats | 63 | 56.3 | 51 | 45.5 | 2.16 | 0.142 |
| Use of sofrito sauce ≥ 2/week | 68 | 60.7 | 73 | 65.2 | 0.31 | 0.580 |
| Parameters | PCOS Patients n = 112 | Control Group n = 112 | p-Value |
|---|---|---|---|
| Dietary Intake | |||
| Total energy (kcal) | 2245.31 ± 290.75 | 2254.84 ± 272.37 | 0.711 |
| Protein (g of total kcal) | 86.98 ± 10.15 | 88.43 ± 9.96 | 0.261 |
| Carbohydrate (g of total kcal) | 307.98 ± 42.03 | 310.47 ± 37.42 | 0.518 |
| Complex (g of total kcal) | 174.21 ± 25.43 | 191.48 ± 23.60 | <0.001 |
| Simple (g of total kcal) | 133.77 ± 34.01 | 118.99 ± 33.62 | <0.001 |
| Fiber (g/day) | 15.43 ± 3.66 | 17.22 ± 4.19 | <0.001 |
| Fat (g of total kcal) | 73.94 ± 13.59 | 70.07 ± 10.73 | <0.001 |
| SFA (g of total kcal) | 24.55 ± 7.51 | 17.39 ± 10.71 | <0.001 |
| Unsaturated fat (g of total kcal) | 49.38 ± 8.63 | 52.67 ± 8.12 | 0.002 |
| MUFA (g of total kcal) | 38.21 ± 4.56 | 43.68 ± 5.86 | <0.001 |
| PUFA (g of total kcal) | 11.16 ± 6.88 | 8.99 ± 4.69 | 0.005 |
| n-6 PUFA (g/day) | 7.82 ± 6.83 | 4.67 ± 3.87 | <0.001 |
| n-3 PUFA (g/day) | 3.34 ± 2.24 | 4.32 ± 3.29 | <0.001 |
| Body Composition | |||
| R (Ω) | 488.71 ± 82.59 | 477.73 ± 71.79 | 0.289 |
| Xc (Ω) | 49.05 ± 10.09 | 51.58 ± 9.58 | 0.028 |
| PhA (°) | 5.76 ± 0.71 | 6.20 ± 0.79 | <0.001 |
| FM (%) | 34.47 ± 9.63 | 29.75 ± 9.88 | <0.001 |
| FFM (%) | 65.44 ± 9.67 | 69.89 ± 10.07 | <0.001 |
| BCM (%) | 49.92 ± 8.67 | 52.03 ± 9.92 | 0.082 |
| TBW (%) | 47.97 ± 7.06 | 49.99 ± 7.44 | 0.001 |
| ECW (%) | 47.20 ± 3.50 | 45.85 ± 3.92 | 0.004 |
| ICW (%) | 52.81 ± 3.51 | 54.15 ± 3.92 | 0.004 |
| Parameters | Testosterone (ng/dL) | ||
|---|---|---|---|
| < 22.27 ng/dL | > 22.27 ng/dL | p-Value | |
| n = 56 | n = 56 | ||
| Age (years) | 24.02 ± 5.74 | 24.41 ± 5.21 | 0.706 |
| BMI (kg/m2) | 27.39 ± 4.88 | 34.53 ± 3.85 | <0.001 |
| Normal-weight (n, %) | 24, 42.9% | 0, 0% | <0.001 |
| Over-weight (n, %) | 20, 35.7% | 9, 16.1% | 0.031 |
| Obesity I (n, %) | 3, 5.4% | 21, 37.5% | <0.001 |
| Obesity II (n, %) | 9, 16.1% | 26, 46.4% | 0.001 |
| WC (cm) | 92.76 ± 12.69 | 109.42 ± 15.29 | <0.001 |
| WC > cut-off | 37, 66.1% | 55, 98.2% | <0.001 |
| Adherence to the MD | |||
| PREDIMED score | 8.61 ± 2.37 | 5.34 ± 1.96 | <0.001 |
| Low adherence to the MD | 7, 12.5% | 0, 0% | <0.001 |
| Average adherence to the MD | 27, 48.2% | 30, 53.6% | 0.999 |
| High adherence to the MD | 22, 39.3% | 26, 46.4% | <0.001 |
| Inflammatory parameter | |||
| CRP levels (ng/dL) | 0.51 ± 0.31 | 1.56 ± 0.88 | <0.001 |
| Hormonal and biochemical parameters | |||
| Insulin (μU/mL) | 6.06 ± 7.85 | 17.16 ± 14.67 | <0.001 |
| Fasting glucose (mg/dL) | 90.36 ± 10.97 | 99.48 ± 10.64 | <0.001 |
| HoMA-IR | 1.39 ± 2.11 | 4.41 ± 4.13 | <0.001 |
| Dietary Intake | |||
| Total energy (kcal) | 2141.83 ± 230.24 | 2348.78 ± 309.52 | <0.001 |
| Protein (g of total kcal) | 88.01 ± 10.69 | 85.96 ± 9.55 | 0.287 |
| Carbohydrate (g of total kcal) | 292.29 ± 31.87 | 323.67 ± 45.26 | <0.001 |
| Complex (g of total kcal) | 173.01 ± 20.24 | 175.41 ± 29.88 | 0.619 |
| Simple (g of total kcal) | 119.28 ± 26.68 | 148.25 ± 34.58 | <0.001 |
| Fiber (g/day) | 17.08 ± 3.48 | 13.79 ± 3.04 | <0.001 |
| Fat (g of total kcal) | 68.96 ± 10.70 | 78.92 ± 14.42 | <0.001 |
| SFA (g of total kcal) | 20.51 ± 6.14 | 28.60 ± 6.54 | <0.001 |
| Unsaturated fat (g of total kcal) | 48.45 ± 6.73 | 50.32 ± 10.16 | 0.254 |
| MUFA (g of total kcal) | 39.49 ± 3.45 | 36.94 ± 5.18 | 0.003 |
| PUFA (g of total kcal) | 8.96 ± 5.10 | 13.38 ± 7.71 | 0.001 |
| n-6 PUFA (g/day) | 5.51 ± 4.92 | 10.14 ± 7.69 | <0.001 |
| n-3 PUFA (g/day) | 3.44 ± 2.28 | 3.23 ± 2.21 | 0.631 |
| Body Composition | |||
| R (Ω) | 504.93 ± 73.69 | 472.50 ± 88.31 | 0.037 |
| Xc (Ω) | 52.27 ± 8.96 | 45.83 ± 10.20 | 0.001 |
| PhA (°) | 5.94 ± 0.65 | 5.57 ± 0.72 | 0.006 |
| FM (%) | 30.07 ± 9.19 | 38.86 ± 7.99 | <0.001 |
| FFM (%) | 69.74 ± 9.33 | 61.14 ± 7.99 | <0.001 |
| BCM (%) | 51.53 ± 7.95 | 48.31 ± 9.12 | 0.049 |
| TBW (%) | 51.19 ± 6.72 | 44.76 ± 5.85 | <0.001 |
| ECW (%) | 46.29 ± 2.99 | 48.13 ± 3.76 | 0.005 |
| ICW (%) | 53.74 ± 3.02 | 51.88 ± 3.74 | <0.001 |
| Testosterone Levels (ng/dL) | ||||
|---|---|---|---|---|
| Parameters | Simple Correlation | After Adjusted for BMI and Total Energy | ||
| r | p-Value | r | p-Value | |
| Age (years) | −0.034 | 0.725 | −0.057 | 0.555 |
| BMI (kg/m2) | 0.720 | <0.001 | - | - |
| WC (cm) | 0.722 | <0.001 | 0.399 | <0.001 |
| PREDIMED score | −0.716 | <0.001 | −0.357 | <0.001 |
| CRP levels (ng/dL) | 0.774 | <0.001 | 0.677 | <0.001 |
| Insulin (μU/mL) | 0.721 | <0.001 | 0.490 | <0.001 |
| Fasting glucose (mg/dL) | 0.517 | <0.001 | 0.176 | 0.066 |
| HoMA-IR | 0.792 | <0.001 | 0.485 | <0.001 |
| Total energy (kcal) | 0.492 | <0.001 | ||
| Protein (g of total kcal) | −0.167 | 0.078 | −0.264 | 0.005 |
| Carbohydrate (g of total kcal) | 0.490 | <0.001 | −0.003 | 0.979 |
| Complex (g of total kcal) | −0.0.18 | 0.849 | −0.365 | <0.001 |
| Simple (g of total kcal) | 0.620 | <0.001 | 0.419 | <0.001 |
| Fiber (g/day) | −0.575 | <0.001 | −0.381 | <0.001 |
| Fat (g of total kcal) | 0.551 | <0.001 | 0.158 | 0.100 |
| SFA (g of total kcal) | 0.691 | <0.001 | 0.321 | 0.001 |
| Unsaturated fat (g of total kcal) | 0.267 | 0.004 | −0.018 | 0.850 |
| MUFA (g of total kcal) | −0.244 | 0.010 | −0.444 | <0.001 |
| PUFA (g of total kcal) | 0.497 | <0.001 | 0.288 | 0.002 |
| n-6 PUFA (g/day) | 0.522 | <0.001 | 0.412 | <0.001 |
| n-3 PUFA (g/day) | −0.068 | 0.476 | −0.314 | 0.001 |
| R (Ω) | −0.253 | 0.007 | −0.071 | 0.460 |
| Xc (Ω) | −0.482 | <0.001 | −0.152 | 0.114 |
| PhA (°) | −0.452 | <0.001 | −0.192 | 0.095 |
| FM (%) | 0.483 | <0.001 | −0.121 | 0.207 |
| FFM (%) | −0.474 | <0.001 | 0.117 | 0.223 |
| BCM (%) | −0.322 | 0.001 | 0.005 | 0.957 |
| TBW (%) | −0.483 | <0.001 | 0.121 | 0.209 |
| ECW (%) | 0.474 | 0.001 | 0.179 | 0.061 |
| ICW (%) | −0.475 | 0.001 | −0.181 | 0.058 |
| Parameters | Multiple Regression Analysis | |||
|---|---|---|---|---|
| Model 1 | R2 | β | t | p Value |
| CRP levels | 0.704 | −0.841 | 16.28 | <0.001 |
| PREDIMED SCORE | 0.724 | −0.212 | −2.96 | <0.001 |
| MUFA (g of total kcal) | 0.738 | −0.133 | −2.61 | 0.001 |
| Variable excluded: WC, HoMA-IR, protein, complex carbohydrate, simple carbohydrate, SFA, PUFA, n-6 PUFA, n-3 PUFA, fiber. | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrea, L.; Arnone, A.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Salzano, C.; Pugliese, G.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients 2019, 11, 2278. https://doi.org/10.3390/nu11102278
Barrea L, Arnone A, Annunziata G, Muscogiuri G, Laudisio D, Salzano C, Pugliese G, Colao A, Savastano S. Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients. 2019; 11(10):2278. https://doi.org/10.3390/nu11102278
Chicago/Turabian StyleBarrea, Luigi, Angela Arnone, Giuseppe Annunziata, Giovanna Muscogiuri, Daniela Laudisio, Ciro Salzano, Gabriella Pugliese, Annamaria Colao, and Silvia Savastano. 2019. "Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS)" Nutrients 11, no. 10: 2278. https://doi.org/10.3390/nu11102278
APA StyleBarrea, L., Arnone, A., Annunziata, G., Muscogiuri, G., Laudisio, D., Salzano, C., Pugliese, G., Colao, A., & Savastano, S. (2019). Adherence to the Mediterranean Diet, Dietary Patterns and Body Composition in Women with Polycystic Ovary Syndrome (PCOS). Nutrients, 11(10), 2278. https://doi.org/10.3390/nu11102278









