Microbial Metabolites of Flavan-3-Ols and Their Biological Activity
Abstract
1. Introduction
2. Origin
3. Biological Properties of Flavan-3-Ol Microbial Metabolites
3.1. Phenyl-γ-valerolactones and Phenylvaleric Acids
3.1.1. Anti-adhesive Activity
3.1.2. Anti-inflammatory Activity
3.1.3. Cardiovascular Protective Effect
3.1.4. Chemopreventive Effect
3.1.5. Immunostimulatory Activity
3.1.6. Neuroprotective Effect
3.2. Phenolic Acids
3.2.1. Anti-adhesive Activity
3.2.2. Antidiabetic Effect
3.2.3. Antiglycative Activity
3.2.4. Anti-inflammatory Activity
3.2.5. Antioxidant Activity
3.2.6. Anti-proliferative Activity and Cytotoxicity
3.2.7. Cardiovascular Protective Effect
3.2.8. Chemopreventive Effect
3.2.9. Modulation of Drug Metabolizing Enzymes
3.2.10. Modulation of Intestinal Microbiota
3.2.11. Modulation of Lipid Metabolism
3.2.12. Neuroprotective Effect
3.2.13. Osteoprotective Effect
3.2.14. Renoprotective Effects
4. Predictive Potential
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.H.; Luben, R.N.; Spencer, J.P.E.; Schroeter, H.; Khaw, K.-T.; Kuhnle, G.G.C. Flavonoid Intake in European Adults (18 to 64 Years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.; Bibiloni, M.D.M.; Tur, J.A. Polyphenol estimated intake and dietary sources among older adults from Mallorca Island. PLoS ONE 2018, 13, e0191573. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: Synthesis, analysis, bioavailability, and bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodríguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C. Nutrition epidemiology of flavan-3-ols: The known unknowns. Mol. Asp. Med. 2018, 61, 2–11. [Google Scholar] [CrossRef]
- Raman, G.; Shams-White, M.; Avendano, E.E.; Chen, F.; Novotny, J.A.; Cassidy, A. Dietary intakes of flavan-3-ols and cardiovascular health: A field synopsis using evidence mapping of randomized trials and prospective cohort studies. Syst. Rev. 2018, 7, 100. [Google Scholar] [CrossRef]
- Lei, L.; Yang, Y.; He, H.; Chen, E.; Du, L.; Dong, J.; Yang, J. Flavan-3-ols consumption and cancer risk: A meta-analysis of epidemiologic studies. Oncotarget 2016, 7, 73573–73592. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Cordero, C.; Salvatore, S.; Pellegrini, N.; Brighenti, F. Bioavailability and catabolism of green tea flavan-3-ols in humans. Nutrition 2010, 26, 1110–1116. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic Acid, Quercetin-3-Rutinoside and Black Tea Phenols Are Extensively Metabolized in Humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lee, M.J.; Sheng, S.; Meng, X.; Prabhu, S.; Winnik, B.; Huang, B.; Chung, J.Y.; Yan, S.; Ho, C.T.; et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000, 13, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Stalmach, A.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. Green tea flavan-3-ols: Colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010, 58, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-(14) C] (-)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.-P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef]
- Castello, F.; Costabile, G.; Bresciani, L.; Tassotti, M.; Naviglio, D.; Luongo, D.; Ciciola, P.; Vitale, M.; Vetrani, C.; Galaverna, G.; et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch. Biochem. Biophys. 2018, 646, 1–9. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Boeres, A.; Massacessi, L.; Istas, G.; Ventura, M.R.; Nunes dos Santos, C.; Heiss, C.; Rodriguez-Mateos, A. Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols. Arch. Biochem. Biophys. 2016, 599, 31–41. [Google Scholar] [CrossRef]
- Mena, P.; González de Llano, D.; Brindani, N.; Esteban-Fernández, A.; Curti, C.; Moreno-Arribas, M.V.; Del Rio, D.; Bartolomé, B. 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone and its sulphate conjugates, representative circulating metabolites of flavan-3-ols, exhibit anti-adhesive activity against uropathogenic Escherichia coli in bladder epithelial cells. J. Funct. Foods 2017, 29, 275–280. [Google Scholar] [CrossRef]
- Uhlenhut, K.; Högger, P. Facilitated cellular uptake and suppression of inducible nitric oxide synthase by a metabolite of maritime pine bark extract (Pycnogenol). Free Radic. Biol. Med. 2012, 53, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Nanjo, F. Effects of Metabolites Produced from (−)-Epigallocatechin Gallate by Rat Intestinal Bacteria on Angiotensin I-Converting Enzyme Activity and Blood Pressure in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2015, 63, 8262–8266. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Kim, J.H.; Kim, J.S.; Oh, Y.S.; Han, S.M.; Park, J.H.Y.; Lee, K.W.; Lee, C.Y. 5-(3′,4′-Dihydroxyphenyl-γ-valerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int. J. Mol. Sci. 2017, 18, 1363. [Google Scholar] [CrossRef] [PubMed]
- Hara-Terawaki, A.; Takagaki, A.; Kobayashi, H.; Nanjo, F. Inhibitory Activity of Catechin Metabolites Produced by Intestinal Microbiota on Proliferation of HeLa Cells. Biol. Pharm. Bull. 2017, 40, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, I.J.; Granica, S.; Piwowarski, J.P.; Szawkało, J.; Wiązecki, K.; Czarnocki, Z.; Kiss, A.K. The Activity of Urolithin A and M4 Valerolactone, Colonic Microbiota Metabolites of Polyphenols, in a Prostate Cancer in Vitro Model. Planta Med. 2019, 85, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4(+) T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Pervin, M.; Nakagawa, A.; Iguchi, K.; Hara, A.; Takagaki, A.; Nanjo, F.; Minami, A.; Nakamura, Y. Blood-Brain Barrier Permeability of Green Tea Catechin Metabolites and their Neuritogenic Activity in Human Neuroblastoma SH-SY5Y Cells. Mol. Nutr. Food Res. 2017, 61, 1700294. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary Tract Infection Syndromes: Occurrence, Recurrence, Bacteriology, Risk Factors, and Disease Burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- De Llano, D.G.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martínlvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Varì, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPARγ activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Millán, E.; Ramos, S.; Alvarez, C.; Bravo, L.; Goya, L.; Martín, M.Á. Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways. Food Chem. Toxicol. 2014, 66, 245–253. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Gotteland, M.; Castillo, R.L.; Chen, C. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp. Cell Res. 2015, 334, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Martín, M.Á.; Ramos, S. (-)-Epicatechin and the Colonic 2,3-Dihydroxybenzoic Acid Metabolite Regulate Glucose Uptake, Glucose Production, and Improve Insulin Signaling in Renal NRK-52E Cells. Mol. Nutr. Food Res. 2018, 62, 1700470. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Martín, M.Á.; Ramos, S. Protective effects of (-)-epicatechin and the colonic metabolite 3,4-dihydroxyphenylacetic acid against glucotoxicity-induced insulin signalling blockade and altered glucose uptake and production in renal tubular NRK-52E cells. Food Chem. Toxicol. 2018, 120, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bitner, B.F.; Ray, J.D.; Kener, K.B.; Herring, J.A.; Tueller, J.A.; Johnson, D.K.; Tellez Freitas, C.M.; Fausnacht, D.W.; Allen, M.E.; Thomson, A.H.; et al. Common gut microbial metabolites of dietary flavonoids exert potent protective activities in β-cells and skeletal muscle cells. J. Nutr. Biochem. 2018, 62, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55 (Suppl 1), S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61, 1600475. [Google Scholar] [CrossRef]
- Yang, X.-W.; Wang, N.; Li, W.; Xu, W.; Wu, S. Biotransformation of 4,5-O-dicaffeoylquinic acid methyl ester by human intestinal flora and evaluation on their inhibition of NO production and antioxidant activity of the products. Food Chem. Toxicol. 2013, 55, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Di Gesso, J.L.; Kerr, J.S.; Zhang, Q.; Raheem, S.; Yalamanchili, S.K.; O’Hagan, D.; Kay, C.D.; O’Connell, M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015, 59, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wang, H.; Wang, J.-H.; Wang, Q.; Ma, Q.-F.; Chen, Y.-Y. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-κB and MAPKs Signaling Pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Fu, S.; Wang, C.; Zhou, B. Preventive Effects of Protocatechuic Acid on LPS-Induced Inflammatory Response in Human Gingival Fibroblasts via Activating PPAR-γ. Inflammation 2015, 38, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Crespo, I.; San-Miguel, B.; Mauriz, J.L.; Ortiz de Urbina, J.J.; Almar, M.; Tuñón, M.J.; González-Gallego, J. Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway. Nutrients 2017, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Wangensteen, H.; Barsett, H. Elderberry and Elderflower Extracts, Phenolic Compounds, and Metabolites and Their Effect on Complement, RAW 264.7 Macrophages and Dendritic Cells. Int. J. Mol. Sci. 2017, 18, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-κB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef]
- Biskup, I.; Golonka, I.; Gamian, A.; Sroka, Z. Antioxidant activity of selected phenols estimated by ABTS and FRAP methods. Postep. Hig Med. Dosw (Online) 2013, 67, 958–963. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.D.S.; Jordão, N.A.; da Costa Pereira Soares, N.; deMesquita, J.F.; Monteiro, M.; Teodoro, A.J. Pharmacokinetic, Anti-proliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using in Vitro and in Silico Approaches. Molecules 2018, 23, 2569. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Santangelo, C.; Filesi, C.; Galvano, F.; D’Archivio, M.; Masella, R.; Giovannini, C. Protocatechuic Acid Prevents oxLDL-Induced Apoptosis by Activating JNK/Nrf2 Survival Signals in Macrophages. Oxid Med. Cell. Longev. 2015, 2015, 351827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-D.; Sun, X.; Zhang, Y.; Wu, H.-J.; Wang, H.; Yang, R. Protocatechuic acid inhibits TGF-β1-induced proliferation and migration of human airway smooth muscle cells. J. Pharmacol. Sci. 2019, 139, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bae, O.N.; Lim, K.M.; Noh, J.Y.; Kang, S.; Chung, K.Y.; Chung, J.H. Novel anti-platelet activity of protocatechuic acid through the inhibition of high shear stress-induced platelet aggregation. J. Pharmacol. Exp. Ther. 2012, 343, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Kumfu, S.; Pannangpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J. Endocrinol. 2014, 223, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl) propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; De Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Babu, P.V.A.; Symons, J.D.; Jalili, T. Metabolites of flavonoid compounds preserve indices of endothelial cell nitric oxide bioavailability under glucotoxic conditions. Nutr. Diabetes 2017, 7, e286. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Ramos, S.; Goya, L.; Martín, M.Á. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem. Toxicol. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Pourová, J.; Najmanová, I.; Vopršalová, M.; Migkos, T.; Pilařová, V.; Applová, L.; Nováková, L.; Mladěnka, P. Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vasc. Pharmacol. 2018, 111, 36–43. [Google Scholar] [CrossRef]
- Applová, L.; Karlíčková, J.; Warncke, P.; Macáková, K.; Hrubša, M.; Macháček, M.; Tvrdý, V.; Fischer, D.; Mladěnka, P. 4-Methylcatechol, a Flavonoid Metabolite with Potent Anti-platelet Effects. Mol. Nutr. Food Res. 2019, e1900261. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.-P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef]
- López de Las Hazas, M.-C.; Mosele, J.I.; Macià, A.; Ludwig, I.A.; Motilva, M.-J. Exploring the Colonic Metabolism of Grape and Strawberry Anthocyanins and Their in Vitro Apoptotic Effects in HT-29 Colon Cancer Cells. J. Agric. Food Chem. 2017, 65, 6477–6487. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Huang, J.J.; Cheung, P.C.K. Mechanistic Study of the in Vitro and in Vivo Inhibitory Effects of Protocatechuic Acid and Syringic Acid on VEGF-Induced Angiogenesis. J. Agric. Food Chem. 2018, 66, 6742–6751. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Li, X.-Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res 2018, 32, 2256–2263. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97). Nutr. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kurita, A.; Nakashima, S.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotechnol. Biochem. 2017, 81, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Protocatechuic acid protects against menadione-induced liver damage by up-regulating nuclear erythroid-related factor 2. Drug Chem. Toxicol. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, K.; Lv, L.; Wu, S.; Guo, Z. Ferulic acid ameliorates nonalcoholic fatty liver disease and modulates the gut microbiota composition in high-fat diet fed ApoE-/-mice. Biomed. Pharmacother. 2019, 113, 108753. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Yousef, A.I.; Abd El-Twab, S.M.; Abdel Reheim, E.S.; Ashour, M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017, 32, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández, A.; Rendeiro, C.; Spencer, J.P.E.; Del Coso, D.G.; de Llano, M.D.G.; Bartolomé, B.; Moreno-Arribas, M.V. Neuroprotective Effects of Selected Microbial-Derived Phenolic Metabolites and Aroma Compounds from Wine in Human SH-SY5Y Neuroblastoma Cells and Their Putative Mechanisms of Action. Front. Nutr. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Brenner, M.C.; Punessen, N.; Snodgrass, M.; Byars, C.; Arora, Y.; Linseman, D.A. Comparison of the Neuroprotective and Anti-Inflammatory Effects of the Anthocyanin Metabolites, Protocatechuic Acid and 4-Hydroxybenzoic Acid. Oxid. Med. Cell. Longev. 2017, 2017, 6297080. [Google Scholar] [CrossRef] [PubMed]
- Kho, A.R.; Choi, B.Y.; Lee, S.H.; Hong, D.K.; Lee, S.H.; Jeong, J.H.; Park, K.-H.; Song, H.K.; Choi, H.C.; Suh, S.W. Effects of Protocatechuic Acid (PCA) on Global Cerebral Ischemia-Induced Hippocampal Neuronal Death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef]
- Siddiqui, S.; Kamal, A.; Khan, F.; Jamali, K.S.; Saify, Z.S. Gallic and vanillic acid suppress inflammation and promote myelination in an in vitro mouse model of neurodegeneration. Mol. Biol. Rep. 2018, 46, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, J.-Y.; Cheon, Y.-H.; Baek, J.M.; Ahn, S.-J.; Yoon, K.-H.; Lee, M.S.; Oh, J. Protocatechuic Acid Attenuates Osteoclastogenesis by Downregulating JNK/c-Fos/NFATc1 Signaling and Prevents Inflammatory Bone Loss in Mice. Phytother. Res. 2016, 30, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-X.; Wu, T.-Y.; Xu, B.-B.; Xu, X.-Y.; Chen, H.-G.; Li, X.-Y.; Wang, G. Protocatechuic acid inhibits osteoclast differentiation and stimulates apoptosis in mature osteoclasts. Biomed. Pharmacother. 2016, 82, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Martín, M.Á.; Goya, L.; Ramos, S. (−)-Epicatechin and the colonic metabolite 3,4-dihydroxyphenylacetic acid protect renal proximal tubular cell against high glucose-induced oxidative stress by modulating NOX-4/SIRT-1 signalling. J. Funct. Foods 2018, 46, 19–28. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F.; Yang, S.; Chen, B.; Shi, J. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomed. Pharmacother. 2018, 98, 18–22. [Google Scholar] [PubMed]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [PubMed]
- Nakamura, Y.; Miyoshi, N. Electrophiles in Foods: The Current Status of Isothiocyanates and Their Chemical Biology. Biosci. Biotechnol. Biochem. 2010, 74, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Hazim, S.; Curtis, P.J.; Schär, M.Y.; Ostertag, L.M.; Kay, C.D.; Minihane, A.-M.; Cassidy, A. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 694–702. [Google Scholar] [PubMed]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61, 1500900. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.R.; Wiseman, H.; Sanders, T.A.B.; Adlercreutz, H.; Bowey, E.A. Interindividual Variation in Metabolism of Soy Isoflavones and Lignans: Influence of Habitual Diet on Equol Production by the Gut Microflora. Nutr. Cancer 2000, 36, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ludwig, I.A.; Tomatis, V.B.; Acharjee, A.; Calani, L.; Rosi, A.; Brighenti, F.; Ray, S.; Griffin, J.L.; Bluck, L.J.; et al. Inter-individual variability in the production of flavan-3-ol colonic metabolites: Preliminary elucidation of urinary metabotypes. Eur. J. Nutr. 2019, 58, 1529–1543. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Espín, J.C.; García-Villalba, R. The human metabolism of nuts proanthocyanidins does not reveal urinary metabolites consistent with distinctive gut microbiota metabotypes. Mol. Nutr. Food Res. 2019, 63, e1800819. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Effect of food structure and processing on (poly)phenol–gut microbiota interactions and the effects on human health. Annu. Rev. Food Sci. Technol. 2019, 10, 221–238. [Google Scholar] [CrossRef] [PubMed]
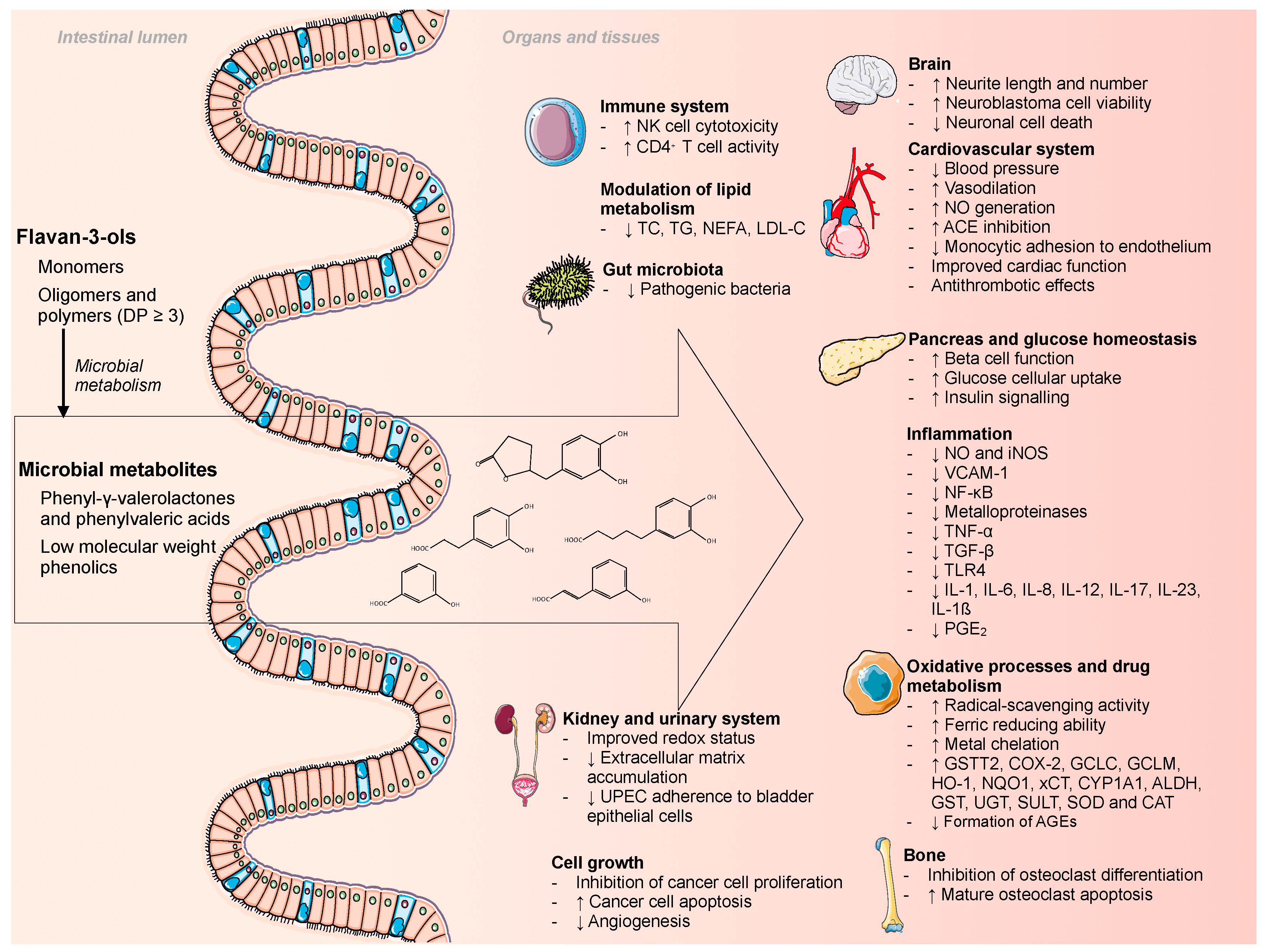
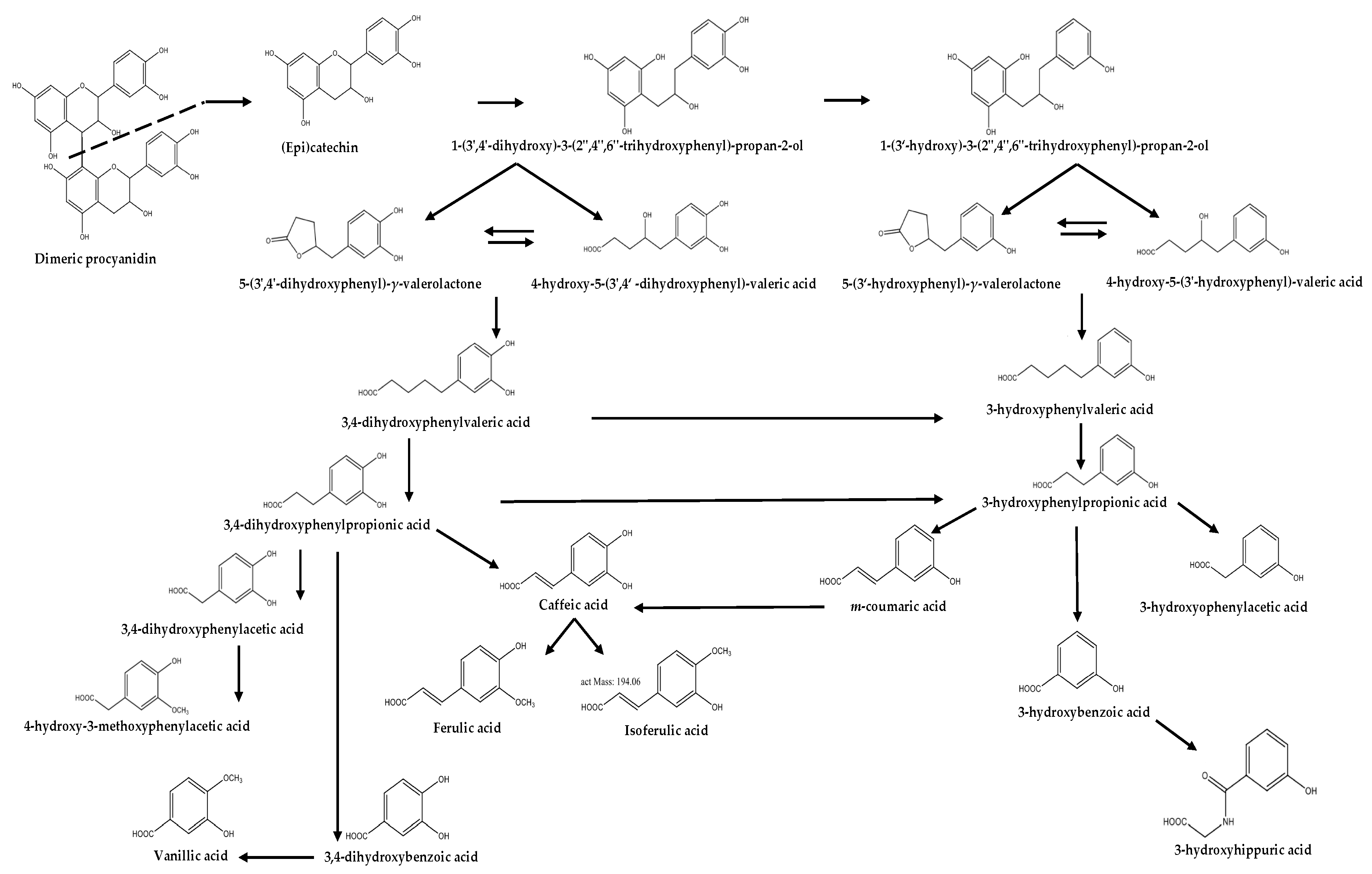
| Test/Model | Microbial Metabolite | Concentration/Dose | Results | Ref. |
|---|---|---|---|---|
| Anti-adhesive activity | ||||
| Adherence of uropathogenic Escherichia coli (UPEC) to T24 bladder epithelial cells | (R)-5-(3’,4’-dihydroxyphenyl)-γ-VL; (R)-5-phenyl-γ-VL-3’,4’-di-O-sulphate; (R)-5-(4’-hydroxyphenyl)-γ-VL-3’-O-sulphate; (R)-5-(3’-Hydroxyphenyl)-γ-VL-4’-O-sulphate | 50–100 µM | All metabolites inhibited adherence of UPEC at 100 µM. (R)-5-(3’-hydroxyphenyl)-γ-VL-4’-O-sulphate also inhibited the adhesion at 50 µM. | [21] |
| Anti-inflammatory activity | ||||
| NO formation and iNOS expression in LPS-exposed RAW 264.7 macrophage; binding to RAW 264.7, EA.hy 926 endothelial cell and human monocyte | 5-(3’,4’-dihydroxyphenyl)-γ-VL | IC50 = 1.3–3.8 µg/mL | NO production and iNOS expression were inhibited in a concentration-dependent manner. High binding capacity to RAW 264.7, EA.hy 926 and human monocytes, which was reduced in the presence of phloretin. | [22] |
| Cardiovascular protective effect | ||||
| Systolic blood pressure in spontaneously hypertensive rats and ACE activity | 5-(3,4,5-trihydroxyphenyl)-γ-VL; 5-(3,5-dihydroxyphenyl)-γ-VL; 4-hydroxy-5-(3,4,5-trihydroxyphenyl)ValA; 4-hydroxy-5-(3,5-dihydroxyphenyl)-ValA; 5-(3,4,5-trihydroxyphenyl)ValA; 5-(3,5-dihydroxyphenyl)ValA; 5-(3-hydroxyphenyl)ValA | 150–200 mg/kg; IC50 = 1.51–19.59 µM | Systolic blood pressure decreased 2 h after 150 mg/kg 5-(3,4,5-trihydroxyphenyl)-γ-VL intake, and 4 h after 200 mg/kg 5-(3,5-dihydroxyphenyl)-γ-VL. The order of ACE inhibitory activity was: EGCG > 5-(3,4,5-trihydroxyphenyl)ValA > 5-(3,5-dihydroxyphenyl)ValA > 5-(3,4,5-trihydroxyphenyl)-γ-VL ≅ 5-(3-hydroxyphenyl)ValA > EC > 4-hydroxy-5-(3,4,5-trihydroxyphenyl)ValA >> 4-hydroxy-5-(3,5-dihydroxyphenyl)-ValA >> 5-(3,5-dihydroxyphenyl)-γ-VL. | [23] |
| THP-1 monocyte adhesion to TNF-α-stimulated human umbilical vein endothelial cells | 5-(3’,4’-dihydroxyphenyl)-γ-VL | 7.5–30 µM | The endothelial adhesion was prevented. Downregulation of VCAM-1 and MCP-1 expression, as well as of NF-κB promoter activity and IKK and IκBα phosphorylation. | [24] |
| Chemopreventive effect | ||||
| Proliferation of human cervical cancer cell (HeLa) | 4-hydroxy-5-(3,5-dihydroxyphenyl)VA; 5-(3,5-dihydroxyphenyl)-γ-VL; 4-hydroxy-5-(3,4,5- trihydroxyphenyl)ValA; 5-(3,4,5-tri-hydroxyphenyl)-γ-VL; 5-(3,4,5-trihydroxy-phenyl)ValA; 5-(3,5-dihydroxyphenyl)ValA; 5-(3-hydroxyphenyl)ValA; 4-hydroxy-5-(3,4-dihydroxyphenyl)ValA; 5-(3,4-dihydroxyphenyl)ValA | 50 µg/mL | 4-hydroxy-5-(3,4,5-trihydroxyphenyl)ValA, 5-(3,4,5-trihydroxyphenyl)ValA and 5-(3,4-dihydroxyphenyl)ValA inhibited the proliferation of HeLa cells by 71.9%, 13.5% and 53.9%, respectively (relative to negative control set at 100, DMSO). 5-(3,4,5-trihydroxyphenyl)ValA had the strongest inhibitory activity among the metabolites (IC50 = 5.58 µM). | [25] |
| Proliferation of androgen-dependent human prostate cancer cells (LNCaP) | 5-(3’,4’,5’-trihydroxyphenyl)-γ-VL | IC50 = 117 μM | Inhibition of LNCaP proliferation. DHT-induced nuclear translocation of AR was inhibited in 54.5 ± 4.7% of cells. | [26] |
| Immunomodulatory activity | ||||
| NK cell cytotoxicity against murine lymphoma YAC-1 target cells in mouse splenocytes treated in vivo; activation of mice splenic CD4⁺ T cells | 5-(3’,5’-dihydroxyphenyl)-γ-VL; 4-hydroxy-5-(3’,5’-dihydroxyphenyl)ValA; 4-hydroxy-5-(3’,4’,5’-trihydroxy-phenyl)ValA; 5-(3’,4’,5’-trihydroxyphenyl)-γ-VL; 5-(3’,5’-dihydroxyphenyl)ValA; 5-(3’,4’,5’-trihydroxyphenyl)ValA and 5-(3’-hydroxyphenyl)ValA | 10 mg/kg; 10 µM | NK cell cytotoxic activity increased in the 5-(3’,5’-dihydroxyphenyl)-γ-VL intake group. IFN-γ production was also dose-dependently increased. The order of CD4⁺ T cell activity (ATP) was: 5-(3’-hydroxyphenyl)ValA > 4-hydroxy-5-(3’,5’-dihydroxyphenyl)ValA = 5-(3’,5’-dihydroxyphenyl)-γ-VL > 5-(3’,5’-dihydroxyphenyl)ValA > 4’-dehydroxylated EGC. | [27] |
| Neuroprotective effect | ||||
| Human SH-SY5Y neuroblastoma cells growth and neurite outgrowth | 5-(3’,5’-dihydroxyphenyl)-γ-VL and its conjugated forms (glucuronide and sulfate forms) | 0.05 µM | 5-(3’,5’-dihydroxyphenyl)-γ-VL enhanced SH-SY5Y cell number. Neurite length and number was significantly increased by 5-(3’,5’-dihydroxyphenyl)-γ-VL and its sulfated form. Glucuronide only increased neurite number. | [28] |
| Test/Model | Microbial Metabolite | Concentration/Dose | Results | Ref. |
|---|---|---|---|---|
| Anti-adhesive activity | ||||
| Adherence of uropathogenic E. coli to T24 epithelial bladder cells | Catechol; BA; 3-HB; PCA; VA; GA; PA; 3-HPA; 3,4-DHPA; 3-PP; 3-HPP and 3,4-DHPP | 100–500 µM | Catechol, BA, VA, PA and 3,4-DHPA inhibited E. coli adherence in a concentration-dependent manner. GA and PA had the strongest effect, followed by 3,4-DHPA. | [32] |
| Antidiabetic effects | ||||
| Glucose transport in human and murine 3T3-L1 adipocytes stimulated or not with insulin | PCA | 100 µmol/L | PCA reversed the oxLDL-induced drop in glucose uptake and GLUT4 translocation. PCA also prevented the oxLDL-induced reduction of adiponectin mRNA expression and secretion, as well of PPARγ mRNA expression and activity. | [33] |
| Beta cell function of rat INS-1E pancreatic beta cells and isolated rat pancreatic islets | 3,4-DHPA; 2,3-DHB and 3-HPP | 1–5 µM | 3,4-DHPA and 3-HPP significantly increased glucose-induced insulin secretion (5 and 1 µM, respectively). In presence of oxidative stress, 3,4-DHPA and 3-HPP reduced ROS and carbonyl group production, and glucose-stimulated insulin secretion was restored to control levels. The phosphorylation of PKC and ERKs was enhanced. | [34] |
| Beta cell function of Min6 pancreatic beta cells incubated with cholesterol | 3,4-DHPA | 10–250 µM | 3,4-DHPA prevented impaired insulin secretion induced by cholesterol by protecting pancreatic beta cells against oxidative stress, apoptosis and mitochondrial dysfunction. | [35] |
| Insulin signalling and glucose uptake and production in rat renal NRK-52E cells | 2,3-DHB; 3,4-DHPA; 3-HPP and VA | 20 µM | Glucose uptake and production decreased after treatment with 2,3-DHB, and PEPCK levels as well. IR and IRS-1 phosphorylated and total protein levels were increased. The inhibition of the PI3K/Akt pathway was restrained. | [36] |
| Insulin signalling and glucose uptake and production in rat renal NRK-52E cells treated with high glucose | 3,4-DHPA; 2,3-DHB and 3-HPP | 10 µM | 3,4-DHPA restored the altered glucose uptake and production caused by high glucose, and tyrosine phosphorylated and total levels of IR increased. The PI3K/Akt pathway and AMPK were activated, while the PEPCK expression was decreased. | [37] |
| Beta cell function and glucose utilization in human skeletal muscle and rat INS-1 beta cells | HA; HVA and 5-PVA | 5–100 µM | HA and 5-PVA stimulated glucose oxidation in skeletal muscle and preserved skeletal mitochondrial function after oxidative insult. In beta cells, all metabolites induced glucose-stimulated insulin secretion without affecting beta cell mitochondrial respiration or electron transport chain components’ expression. | [38] |
| Antiglycative activity | ||||
| Formation of AGEs in BSA/glucose system and glyoxal trapping ability | PG; 3,4-DHPP; DHFA; 3-HPA; 3,4-DHPA and HVA | 2.0–50 µmol/L | Only DHFA at 10 µmol/L had a significant impact inhibiting albumin glycation, and a combination of 3-HPA, 3,4-DHPA and HVA inhibited glycation at 2.0 µmol/L. PG, 3,4-DHPP and 3,4-DHPA showed a glyoxal trapping ability of approximately 60%, 90% and 65%, respectively. | [39] |
| Formation of AGEs in BSA/glucose and BSA/MGO systems | 3,4-DHPA; 3-HPA and HVA | 1 mM | The order of inhibitory activity against AGEs was: rutin > quercetin > 3,4-DHPA > aminoquanidine > 3-HPA > HVA | [40] |
| Anti-inflammatory activity | ||||
| NO production in LPS-activated RAW264.7 cells | 3-HPP; CA and 3,4-DHPP | IC50 = 224.85–689.91 µM | CA and 3,4-DHPP inhibited the NO production significantly stronger than 3-HPP. | [41] |
| Inflammatory response in LPS-stimulated human THP-1 monocytic cells | 4-HBA; BA-glucuronide; BA-sulfate; PCA; PCA-3-glucuronide; PCA-4-glucuronide; PCA-3-sulfate; PCA-4-sulfate; VA; VA-glucuronide and VA-sulfate | 0.1–10 µM | LPS-induced TNF-α secretion was inhibited by BA-sulfate, VA-glucuronide and PCA-3-sulfate, as well as by four combinations of metabolites that included 4-HBA and/or PCA with a stronger effect than the individual metabolites. 4-HBA significantly reduced IL-1ß secretion. | [42] |
| Inflammatory response in LPS-stimulated BV2 microglia | PCA | 5–20 µM | PCA dose-dependently inhibited LPS-induced TNF-α, IL-6, IL-1ß and PGE2 production, and suppressed LPS-induced TLR4 expression, NF-κB and MAPKs activation. | [43] |
| Inflammatory response in LPS-stimulated human gingival fibroblasts | PCA | 5–20 µM | PCA inhibited LPS-induced IL-6 and IL-8 production and NF-κB activation. PPAR-γ antagonist GW9662 reversed the prevention of IL-6 and IL-8 production by PCA. | [44] |
| Colitis mice model induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS) | PCA | 30 and 60 mg/kg | PCA improved TNBS-induced colitis in mice, reduced the GSSG/GSH ratio and expression of proinflammatory cytokines, and increased the expression of antioxidant enzymes and Nrf2. The SphK/S1P axis and the related NF-κB and STAT3 signaling pathway were abrogated. | [45] |
| NO production in LPS-Stimulated RAW 264.7 macrophages and dendritic D2SC/I cells | p-CoA; HVA; 4-HB; HA; FA; PCA; CA; VA; 3-HPA; 3,4-DHPA | 0.1–100 µM | CA, 3,4-DHPA and PCA were the most active metabolites inhibiting NO production in RAW 264.7 cells. In D2SC/I cells, 3,4-DHPA, CA, and p-CoA were the most potent metabolites. | [46] |
| Inflammatory response of HIEC-6 human intestinal epithelial cells after IL-1β-induced ulcerative colitis, and of mice after TNBS-induced ulcerative colitis | GA | 20–60 mg/kg | Anti-inflammatory cytokines (IL-4 and IL-10) increased and the proinflammatory ones (IL-1, IL-6, IL-12, IL-17, IL-23, TGF-β and TNF-α) decreased in HIEC-6 cells and in mice. Apoptosis was reduced in GA treated groups and the colonic inflammation in mice was attenuated. GA inhibited NF-κB activation. | [47] |
| Antioxidant activity | ||||
| DPPH radical scavenging activity | 3-HPP; CA and 3,4-DHPP | IC50 = 5.02–5.91 µM | CA and 3,4-DHPP had the stronger scavenging radical activity, while 3-HPP had no antioxidant activity. | [41] |
| ABTS assay | 4-HPA; 3,4-DHPA; PCA; 2,3-DHB; PG and GA | IC50 = 4.332–852.713 µM | The ability to scavenge 50% of free radical ABTS• + was stronger for GA, PG and 3,4-DHPA but weaker for 4-HPA. | [48] |
| Ferric-reducing antioxidant potential (FRAP) | 4-HPA; 3,4-DHPA; PCA; 2,3-DHB; PG and GA | 1.00 x 10-3 mg/mL | The strongest antioxidant activity was shown by 3,4-DHPA, PG, GA and PCA. | [48] |
| DPPH radical scavenging activity | 3,4-DHPA; 3-HPA and HVA | 1 mM | The order of antioxidant activity was: quercetin > rutin = 3,4-DHPA > HVA >> 3-HPA. | [40] |
| Ferric-reducing antioxidant potential (FRAP) | 3,4-DHPA; 3-HPA and HVA | 1 mM | The order of reducing activity was: quercetin > HVA > 3,4-DHPA > rutin >> 3-HPA. | [40] |
| Cyclic voltammetry (CV) | 3,4-DHPA; 3-HPA and HVA | 1 mM | The order of reducing activity was: quercetin > rutin > 3,4-DHPA > HVA > 3-HPA. | [40] |
| Ferrozine assay | 3,4-DHPA; 3-HPA and HVA | 1 mM | The order of chelating activity was: rutin > quercetin > HVA >> 3-HPA >> 3,4-DHPA. | [40] |
| DPPH radical scavenging assay | 3,4-DHPA; 3-HPA; PCA and HA | 2–10 µM | The order of antioxidant activity was: 3,4-DHPA = quercetin > PCA > 3-HPA ≅ HA. | [49] |
| Superoxide scavenging assay | 3,4-DHPA; 3-HPA; PCA and HA | 50 µM | The order of superoxide scavenging activity was: quercetin > 3,4-DHPA > PCA >> 3-HPA ≅ HA. | [49] |
| DPPH radical scavenging assay | 3,4-DHPA; p-CoA; VA and FA | 25 µM | The order of antioxidant activity was: 3,4-DHPA > VA > FA > p-CoA. | [50] |
| Ferric-reducing antioxidant potential (FRAP) | 3,4-DHPA; p-CoA; VA and FA | 5 µM | The order of reducing activity was: 3,4-DHPA > VA > FA > p-CoA. | [50] |
| ABTS assay | 3,4-DHPA; p-CoA; VA and FA | 5 µM | The order of antioxidant activity was: 3,4-DHPA > FA > p-CoA > VA. | [50] |
| ORAC assay | 3,4-DHPA; p-CoA; VA and FA | 3 µM | The order of antioxidant activity was: p-CoA > 3,4-DHPA > VA > FA. | [50] |
| Anti-proliferative activity and cytotoxicity | ||||
| Apoptosis and cellular oxidative stress of oxLDL-exposed J774A.1 cells | PCA | 25 µM | OxLDL-induced cell death was prevented, as well as ROS production and GSH depletion. The activation of p53 was prevented, and therefore the overexpression of p53-target genes decreased. p38MAPK and PKC∂ activation was reversed. PCA induced JNK activation and increased nuclear Nrf2 content. | [51] |
| TGF-ß1-induced proliferation and migration of human airway smooth muscle cells (ASMCc) | PCA | 1–50 nM | PCA inhibited the proliferation and migration of ASMCs and the expression of type I collagen and fibronectin. The Smad2/3 activation in ASMCs exposed to TGF-ß1 was downregulated. | [52] |
| Cardiovascular protective effect | ||||
| Antithrombotic efficacy under high shear stress in vitro in human platelets as well as in an in vivo arterial thrombosis model | PCA | 5–25 µM | PCA significantly decreased stress-induced platelet aggregation by blocking the interaction between von Willebrand factor (vWF) and glycoprotein Ib. Intracellular calcium increase was attenuated, shear-induced granular secretion from dense and α-granules was inhibited and glycoprotein IIb/IIIa activation was attenuated. The antithrombotic effects of PCA were confirmed in vivo. | [53] |
| Cardiac function and cardiac autonomic balance in STZ-induced diabetic rats | PCA | 50 and 100 mg/kg | %FS and %LVEF increased and LF:HF decreased compared with untreated diabetic rats. Plasma HbA1c decreased as well as cardiac MDA and cardiac mitochondrial ROS. Mitochon-drial membrane depolarization and swelling was prevented and cardiac anti-apoptotic BCL2 protein levels increased | [54] |
| Vasodilation of pre-contracted isolated aortic rings; blood pressure in normotensive and spontaneously hypertensive rats | 3-PP; 4-HPP; 3,4-DHPP; 4-HPA; 3,4-DHPA; HVA; 3-HB; PhG; 4-MC; m-CoA; 3-HPP and 3-HPA | 100 nM; 2.5–25 mg/kg and 5 mg/kg/50 µL/min | 3-HPP had the highest vasodilatory activity, which was NO and endothelium-dependent. In vivo, 3-HPP lowered arterial blood pressure in normotensive and spontaneously hypertensive rats. | [55] |
| Diabetic cardiomyopathy in type 2 diabetic rats | PCA | 50 and 100 mg/kg | PCA was protective against diabetic cardiomyopathy through hypoglycemic, insulin-sensitizing, anti-inflammatory and antioxidant effects. | [56] |
| Insulin-stimulated NO production by human aortic endothelial cells under high glucose conditions | 3-HPP | 1 µM | Under glucotoxic conditions, 3-HPP preserved insulin-stimulated increases in NO production, and phosphorylation of Akt and eNOS. The rise in ROS and RNS was prevented. | [57] |
| Endothelial function and oxidative stress in human Ea.hy926 endothelial cells | 3,4-DHPA; 2,3-DHB and 3-HPP | 10–12 µM | 3,4-DHPA and a mix of the metabolites increased the NO generation and phosphorylation of eNOS, Akt and AMPK. Under oxidative stress, metabolites enhanced cell viability and prevented reduced eNOS phosphorylation. ROS generation and phosphorylation of ERK and JNK were prevented. | [58] |
| Relaxation of pre-contracted rat artery rings and blood pressure in spontaneously hypertensive rats | 3,4-DHPA; 4-MC and 3-HPP | EC50 = 22.4–49.1 µM; 0.5–25 mg/kg and 5 mg/kg/min | The vasorelaxant activity of 3,4-DHPA and 4-MC was similar in aorta and mesenteric artery. The effect of 3,4-DHPA depended on endothelium, NO, prostaglandin and Ca2+-activa-ted K+ channels. Both metabolites dose-dependently decreased blood pressure after bolus and infusion administration. | [59] |
| Whole blood platelet aggregation induced by arachidonic acid and ex ovo hen’s egg model of thrombosis | 4-MC and PG | IC50 = 3–25 µM; 5 mM | PG showed a comparable anti-platelet effect to that of acetylsalicylic acid, while that of 4-MC was significantly lower. 4-MC interfered with calcium intracellular signalling, being this the possible mechanism of action. In the ex vivo experiment, the anti-platelet effect of 4-MC was confirmed by significantly increasing the survival of the eggs. | [60] |
| Chemopreventive effect | ||||
| ATP production by HCT-116 colon cancer cells | 3,4-DHPA; 3-HPA; 4-HPA; HVA and 3-OMGA | IC50 = 260 µmol/L | 3-OMGA inhibited cell proliferation. Combining 3,4-DHPA (100, 200 and 300 µmol/L) and EGCG (40 µmol/L) increased the anti-proliferative effect compared to individual treatments. | [61] |
| Apoptosis of HT-29 colon cancer cells | 4-HPA and VA | 100 µM | 4-HPA enhanced the late-stage apoptosis and the percentage of dead cells compared to control cells. | [62] |
| Angiogenesis in HUVEC cells treated with VEGF and in zebrafish model | PCA | 6.25–100 μM; 25 µM | PCA inhibited the proliferation, migration, invasion and capillary structure formation of HUVECs, and blocked the VEGFR2-dependent Akt/MMP2 and ERK pathways. In vivo, the anti-angiogenic effect of PCA was possibly due to downregulation of VEGFα-VEGFR2 and Ang2-Tie2 pathways. | [63] |
| Proliferation and apoptosis of HT-29 colon cancer cells | 3,4-DHPA; p-CoA; VA and FA | 0.1–100 µM | 3,4-DHPA had the strongest effect reducing cell viability. All metabolites reduced cell number in S phase, and p-CoA and FA increased apoptosis. | [50] |
| Proliferation, cell-cycle arrest and apoptosis of Caco-2 cell | CA; 3-PP and BA | 100–1000 µM and EC50 = 460–500 µM | Only CA reduced cell proliferation by 50%, while 3-PPA and BA decreased it at 1000 μM. CA and 3-PP induced cell-cycle arrest at the S-phase. CA activated caspase-3 and 3-PPA decreased mitochondrial DNA content. | [64] |
| Apoptosis and autophagy in OVCAR-3 ovarian cancer cells | PCA | 5–30 µM | PCA inhibited cell proliferation by inducing apoptosis and autophagy. PCA modulated proapoptotic and anti-apoptotic proteins (Bax, Bcl-2, PARP and caspase-3) and upregulated autophagy-related protein LC3-II. | [65] |
| Modulation of drug metabolizing enzymes | ||||
| GSTT2 and COX-2 expression in LT97 human colorectal adenoma cells | 3,4-DHPA and 3,4-DHPP | 2.5–25 µM | GSTT2 mRNA expression was enhanced up to 1.8-fold. COX-2 mRNA and protein expression were reduced, as well as CumOOH-induced DNA damage. | [66] |
| Gene expression of drug-metabolizing enzymes in Hepa1c1c7 mouse hepatoma cells | 3,4-DHPA; 3-HPA; PCA and HA | 5–250 µM | 3,4-DHPA increased GCLC, HO-1, NQO1, xCT and CYP1A1 gene expression. PCA at 50 µM increased the gene expression of NQO1. Peroxide-induced cytotoxicity was inhibited. | [49] |
| Acetaminophen-induced liver injury in mice | 3,4-DHPA | 10–50 mg/kg | Acetaminophen-induced hepatotoxicity was attenuated by 3,4-DHPA. Nrf-2 translocation to the nucleus was increased, as well as the expression of phase-II (UGT, SULT, GCLC and GCLM) and antioxidant enzymes. | [67] |
| ALDH activity and gene expression in Hepa1c1c7 mouse hepatoma cells | 3,4-DHPA | 5–50 µM | Concentration-dependent enhancement of the ALDH activity, as well as expression of ALDH1A1, ALDH2 and ALDH3A. Nuclear levels of Nrf2 and AhR increased significantly at 20 µM, while those of NF-κB decreased. | [68] |
| Menadione-induced liver damage in rats | PCA | 10 and 20 mg/kg | PCA prevented menadione mediated-alterations in hepatocellular markers and it also increased the activities of antioxidant enzymes (SOD and CAT) and phase II detoxifying enzymes (GST and NQO-1), and Nrf-2. | [69] |
| Modulation of intestinal microbiota | ||||
| Alteration of the composition in fecal microbiota of ApoE-/- mice fed on a high-fat diet | FA | 30 mg/kg | FA significantly lowered the ratio of Firmicutes to Bacteroidetes when compared to obese control group. | [70] |
| Growth inhibition of pathogenic and probiotic bacteria | GA; VA; FA and PCA | MIC = 20–35 mmol/L MBC = 20–30 mmol/L | MIC against E.coli and Staphylococcus Typhimurium was similar among metabolites (15-20 mmol/L). VA and PCA had the lowest MBC (20 mmol/L). Lactobacillus acidophilus and L. rhamnosus were also inhibited but at higher concentrations (MIC > 35 mmol/L) and their MBC was also > 35 mmol/L. | [71] |
| Modulation of lipid metabolism | ||||
| Lipid metabolism in HFD-induced obesity in mice | FA | 25 and 50 mg/kg | FA reduced serum TC, TG and NEFA levels as compared to the obese control mice. Liver TC and TG significantly decreased as well. SREBP1c, FAS and ACC were reduced, while CPT1a and PPARα were up-regulated. | [72] |
| Lipid metabolism in HFD-induced obesity in mice | FA | 30 mg/kg | Serum TC, TG and LDL-C decreased when compared to obese control group, and liver TC and TG levels as well. FA significantly increased the mRNA expression of AHR and decreased that of FAS and SREBP-1c. | [70] |
| Neuroprotective effect | ||||
| Human neuroblastoma SK-N-MC cell viability after DMNQ-induced oxidative stress | PG; 3,4-DHPP; DHFA; 3-HPA; 3,4-DHPA and HVA | 0.1–20 µmol/L | The greatest increase in cell survival was induced by DHFA, followed by PG and 3,4-DHPA. Combination of metabolites also increased cell survival after oxidative stress. | [39] |
| Type 2 diabetes-induced neurodegeneration in rats | GA and p-CoA | 20–40 mg/kg | Treatment of diabetic rats with GA and p-CoA enhanced the histology of the hippocampus and glucose tolerance, prevented brain oxidative stress, improved antioxidant status, reduced inflammation and inhibited apoptosis. | [73] |
| Neuroinflammation model based on SIN-1 stress-induced injury in human SH-SY5Y neuroblastoma cells | 3,4-DHPA; 3-HPP and 3-HPA | 0.1–10 µM | Metabolites increased cell viability, probably through inhibition of ERK1/2, modulation of p38 MAPK kinases (3-HPA), and reduction of caspase-3 activation (3-HPP). | [74] |
| Apoptosis of human SH-SY5Y neuroblastoma cells previous or during H2O2 exposition. | 3,4-DHPP; 3,4-DHPA and GA | 5–10 µM | All metabolites decreased late apoptosis, but 3,4-DHPP had the strongest effect. ROS levels decreased and REDOX activity increased. All metabolites attenuated H2O2-induced activation of caspases-3 and -9. | [75] |
| Apoptosis of rat cerebellar granule neurons under H2O2-induced oxidative stress, nitrosative stress and excitotoxicity | 4-HBA and PCA | 10–300 µM | Both 4-HBA and PCA mitigated oxidative stress induced by H2O2. Under conditions of nitrosative stress only PCA was neuroprotective, but under conditions of excitotoxicity only 4-HBA reduced cell death. | [76] |
| Ischemia-induced hippocampal neuronal death in rats | PCA | 30 mg/kg | PCA decreased neuronal cell death, oxidative stress, microglial activation, astrocyte activation and BBB disruption compared with the control group after ischemia. GSH glutathione reduced concentration was recovered. | [77] |
| Lysolecithin (LPC)-induced model of inflammation in mouse hippocampal neurons co-cultured with glial cells | GA and VA | 0.2–1 µM | GA and VA increased neurite outgrowth and upregulated myelin protein in neurites and oligodendrocyte cell bodies. COX-2, NFκB, TN-C, CSPGs and GFAP expression in astrocytes decreased. GA and VA reversed the reduction in sustained repetitive firing induced by LPC. | [78] |
| Osteoprotective effects | ||||
| Osteoclast differentiation and function in mouse bone marrow macrophages treated with RANKL; inflammatory bone destruction in LPS-treated mice | PCA | 25 µM; 25 mg/kg | PCA inhibited osteoclastogenesis and the bone-resorbing activity of mature osteoclasts. LPS-mediated bone loss in vivo was also restored by PCA. | [79] |
| Osteoclast differentiation and apoptosis in RAW264.7 murine macrophage cells treated with RANKL | PCA | 8 µM | PCA inhibited osteoclast differentiation by regulating oxidative stress and inflammation, and induced apoptosis in mature osteoclasts by inducing mitochondrial membrane potential, Cyt c release and caspase activation. | [80] |
| Renoprotective effects | ||||
| Redox status in high-glucose-exposed rat renal proximal tubular NRK-52E cell | 3,4-DHPA | 10 µM | 3,4-DHPA reversed the increase in ROS levels and the decreased antioxidant defense. SIRT-1 increased, and the high glucose-induced increase of phosphorylated MAPKs and NOX-4 were restored. | [81] |
| Extracellular matrix accumulation in high glucose-induced human mesangial cells | PCA | 5 and 10 µM | PCA inhibited high glucose-induced proliferation of mesangial cells and protected them against high glucose damage inhibiting the p38 MAPK signaling pathway. | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez Campos, E.; Stehle, P.; Simon, M.-C. Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients 2019, 11, 2260. https://doi.org/10.3390/nu11102260
Márquez Campos E, Stehle P, Simon M-C. Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients. 2019; 11(10):2260. https://doi.org/10.3390/nu11102260
Chicago/Turabian StyleMárquez Campos, Estefanía, Peter Stehle, and Marie-Christine Simon. 2019. "Microbial Metabolites of Flavan-3-Ols and Their Biological Activity" Nutrients 11, no. 10: 2260. https://doi.org/10.3390/nu11102260
APA StyleMárquez Campos, E., Stehle, P., & Simon, M.-C. (2019). Microbial Metabolites of Flavan-3-Ols and Their Biological Activity. Nutrients, 11(10), 2260. https://doi.org/10.3390/nu11102260






