Olive Oil Effects on Colorectal Cancer
Abstract
:1. Introduction
2. Effects of Olive Oil Phenols on CRC
3. Anti-Inflammatory, Immunomodulatory and Other Anticancer Properties of Olive Oil
4. Effects of Olive Oil Fatty Acids on CRC
5. Effects of Olive Oil on Gut Microbiota
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fact Sheets by Cancer. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 8 September 2018).
- American Cancer Society. Cancer Facts & Figures 2007; American Cancer Society: Atlanta, GA, USA, 2007. [Google Scholar]
- Shike, M. Diet and lifestyle in the prevention of colorectal cancer: An overview. Am. J. Med. 1999, 106, 11S–15S, discussion 50S–51S. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Godos, J.; Platania, A.; Galvano, F.; Mistretta, A.; Grosso, G. Mediterranean diet adherence in the Mediterranean healthy eating, aging and lifestyle (MEAL) study cohort. Int. J. Food Sci. Nutr. 2018, 69, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13 (Suppl. 2), S14. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Bes-Rastrollo, M.; Toledo, E.; Gea, A.; Fresán, U.; Barbagallo, M.; Martínez-González, M.A. Dietary fiber intake and mortality in a Mediterranean population: The “Seguimiento Universidad de Navarra” (SUN) project. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Biondi, A.; Galvano, F.; Mistretta, A.; Marventano, S.; Buscemi, S.; Drago, F.; Basile, F. Factors associated with colorectal cancer in the context of the Mediterranean diet: A case-control study. Nutr. Cancer 2014, 66, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2016, 5, 9–17. [Google Scholar] [CrossRef]
- Fernandez, A.G.; Adams, M.R.; Fernandez-Diez, M.J. Table Olives: Production and Processing; Springer: New York, USA, 1997; ISBN 978-0-412-71810-6. [Google Scholar]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Tsimidou, M.Z. Antioxidants in Greek Virgin Olive Oils. Antioxidants 2014, 3, 387–413. [Google Scholar] [CrossRef] [Green Version]
- Bassani, B.; Rossi, T.; Stefano, D.D.; Pizzichini, D.; Corradino, P.; Macrì, N.; Noonan, D.M.; Albini, A.; Bruno, A. Potential chemopreventive activities of a polyphenol rich purified extract from olive mill wastewater on colon cancer cells. J. Funct. Foods 2016, 27, 236–248. [Google Scholar] [CrossRef]
- Gimeno, E.; de la Torre-Carbot, K.; Lamuela-Raventós, R.M.; Castellote, A.I.; Fitó, M.; de la Torre, R.; Covas, M.-I.; López-Sabater, M.C. Changes in the phenolic content of low density lipoprotein after olive oil consumption in men. A randomized crossover controlled trial. Br. J. Nutr. 2007, 98, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Spencer, J.P.E.; Dessì, M.A. Extra virgin olive oil phenolics: Absorption, metabolism, and biological activities in the GI tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Sotiroudis, T.G.; Kyrtopoulos, S.A. Anticarcinogenic compounds of olive oil and related biomarkers. Eur. J. Nutr. 2008, 47 (Suppl. 2), 69–72. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; García-Villalba, R.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Metabolism and Bioavailability of Olive Oil Polyphenols. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; InTech: London, UK, 2012. [Google Scholar] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How heating affects extra virgin olive oil quality indexes and chemical composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef]
- Purcaro, G.; Navas, J.A.; Guardiola, F.; Conte, L.S.; Moret, S. Polycyclic aromatic hydrocarbons in frying oils and snacks. J. Food Prot. 2006, 69, 199–204. [Google Scholar] [CrossRef]
- Perumalla Venkata, R.; Subramanyam, R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicol. Rep. 2016, 3, 636–643. [Google Scholar] [CrossRef]
- Galeone, C.; Talamini, R.; Levi, F.; Pelucchi, C.; Negri, E.; Giacosa, A.; Montella, M.; Franceschi, S.; La Vecchia, C. Fried foods, olive oil and colorectal cancer. Ann. Oncol. 2007, 18, 36–39. [Google Scholar] [CrossRef]
- Diggs, D.L.; Huderson, A.C.; Harris, K.L.; Myers, J.N.; Banks, L.D.; Rekhadevi, P.V.; Niaz, M.S.; Ramesh, A. Polycyclic Aromatic Hydrocarbons and digestive tract cancers—A perspective. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2011, 29, 324–357. [Google Scholar] [CrossRef]
- Turesky, R.J.; Vouros, P. Formation and analysis of heterocyclic aromatic amine-DNA adducts in vitro and in vivo. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 802, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Casal, S.; Malheiro, R.; Sendas, A.; Oliveira, B.P.P.; Pereira, J.A. Olive oil stability under deep-frying conditions. Food Chem. Toxicol. 2010, 48, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Rossi, T.; Bassani, B.; Gallo, C.; Maramotti, S.; Noonan, D.M.; Bruno, A. Effect of a Purified Extract of Olive Mill Waste water on Endothelial Cell Proliferation, Apoptosis, Migration and Capillary-Like Structure in vitro and in vivo. J. Bioanal. Biomed. 2015, S12, 006. [Google Scholar]
- Mateos, R.; Pereira-Caro, G.; Bacon, J.R.; Bongaerts, R.; Sarriá, B.; Bravo, L.; Kroon, P.A. Anticancer activity of olive oil hydroxytyrosyl acetate in human adenocarcinoma Caco-2 cells. J. Agric. Food Chem. 2013, 61, 3264–3269. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Nannelli, G.; Frosini, M.; Giachetti, A.; Ziche, M.; Donnini, S. Inhibition of cell cycle progression by the hydroxytyrosol–cetuximab combination yields enhanced chemotherapeutic efficacy in colon cancer cells. Oncotarget 2017, 8, 83207–83224. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, H.K.; Castellon, R. Oleuropein, a non-toxic olive iridoid, is an anti-tumor agent and cytoskeleton disruptor. Biochem. Biophys. Res. Commun. 2005, 334, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón de la Lastra, C. Oleuropein, a secoiridoid derived from olive tree, inhibits the proliferation of human colorectal cancer cell through downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef]
- Bartolí, R.; Fernández-Bañares, F.; Navarro, E.; Castellà, E.; Mañé, J.; Alvarez, M.; Pastor, C.; Cabré, E.; Gassull, M.A. Effect of olive oil on early and late events of colon carcinogenesis in rats: Modulation of arachidonic acid metabolism and local prostaglandin E(2) synthesis. Gut 2000, 46, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, S.; Song, Y.; Yao, J.; Huang, K.; Zhu, X. Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncol. Lett. 2016, 11, 3075–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin sensitizes colon cancer cells to anti-tumor activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, B.; Gao, F.; Shi, R. Modulation of G2/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncol. Lett. 2018, 15, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Wu, R.; Xiao, X.; Yang, C.; Yang, Y.; Wang, C.; Lin, L.; Kong, A.-N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell. Biochem. 2018, 119, 9573–9582. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; García-Salguero, L.; Peragón, J.; Medina, P.P.; Parra, A.; Cascante, M.; Lupiáñez, J.A. Maslinic Acid, a Natural Triterpene, Induces a Death Receptor-Mediated Apoptotic Mechanism in Caco-2 p53-Deficient Colon Adenocarcinoma Cells. PLoS ONE 2016, 11, e0146178. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Zurita, F.J.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Cascante, M. Maslinic acid, a natural triterpene from Olea europaea L., induces apoptosis in HT29 human colon-cancer cells via the mitochondrial apoptotic pathway. Cancer Lett. 2009, 273, 44–54. [Google Scholar] [CrossRef]
- Baskar, A.A.; Ignacimuthu, S.; Paulraj, G.M.; Al Numair, K.S. Chemopreventive potential of beta-Sitosterol in experimental colon cancer model--an in vitro and In vivo study. BMC Complement. Altern. Med. 2010, 10, 24. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97). Nutr. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef]
- Losso, J.N.; Bansode, R.R.; Trappey, A.; Bawadi, H.A.; Truax, R. In vitro anti-proliferative activities of ellagic acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef]
- Grosso, G. Effects of Polyphenol-Rich Foods on Human Health. Nutrients 2018, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J.G. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1638–1656. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and Cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in Olive and its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 114, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Tunca, B.; Tezcan, G.; Cecener, G.; Egeli, U.; Ak, S.; Malyer, H.; Tumen, G.; Bilir, A. Olea europaea leaf extract alters microRNA expression in human glioblastoma cells. J. Cancer Res. Clin. Oncol. 2012, 138, 1831–1844. [Google Scholar] [CrossRef]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Farr, S.A.; Price, T.O.; Dominguez, L.J.; Motisi, A.; Saiano, F.; Niehoff, M.L.; Morley, J.E.; Banks, W.A.; Ercal, N.; Barbagallo, M. Extra virgin olive oil improves learning and memory in SAMP8 mice. J. Alzheimers Dis. 2012, 28, 81–92. [Google Scholar] [CrossRef]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef]
- Catalán, Ú.; López de Las Hazas, M.-C.; Rubió, L.; Fernández-Castillejo, S.; Pedret, A.; de la Torre, R.; Motilva, M.-J.; Solà, R. Protective effect of hydroxytyrosol and its predominant plasmatic human metabolites against endothelial dysfunction in human aortic endothelial cells. Mol. Nutr. Food Res. 2015, 59, 2523–2536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Echeverría, F.; Valenzuela, R.; Bustamante, A.; Álvarez, D.; Ortiz, M.; Soto-Alarcon, S.A.; Muñoz, P.; Corbari, A.; Videla, L.A. Attenuation of High-Fat Diet-Induced Rat Liver Oxidative Stress and Steatosis by Combined Hydroxytyrosol-(HT-)Eicosapentaenoic Acid Supplementation Mainly Relies on HT. Oxidative Med. Cell. Longev. 2018, 2018, 5109503. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef]
- Polyphenols in Olive Related Health Claims. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/2033 (accessed on 13 November 2018).
- Li, S.; Han, Z.; Ma, Y.; Song, R.; Pei, T.; Zheng, T.; Wang, J.; Xu, D.; Fang, X.; Jiang, H.; et al. Hydroxytyrosol inhibits cholangiocarcinoma tumor growth: An in vivo and in vitro study. Oncol. Rep. 2014, 31, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Zhu, D.-J.; Chen, X.-W.; Chen, Q.-K.; Luo, Z.-T.; Liu, C.-C.; Wang, G.-X.; Zhang, W.-J.; Liao, N.-Z. Curcumin enhances the effects of irinotecan on colorectal cancer cells through the generation of reactive oxygen species and activation of the endoplasmic reticulum stress pathway. Oncotarget 2017, 8, 40264–40275. [Google Scholar] [CrossRef]
- Yang, S.; Li, W.; Sun, H.; Wu, B.; Ji, F.; Sun, T.; Chang, H.; Shen, P.; Wang, Y.; Zhou, D. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer 2015, 15, 969. [Google Scholar] [CrossRef]
- Amiot, M.J.; Fleuriet, A.; Macheix, J.J. Importance and evolution of phenolic compounds in olive during growth and maturation. J. Agric. Food Chem. 1986, 34, 823–826. [Google Scholar] [CrossRef]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.G.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef]
- Valli, A.; Rodriguez, M.; Moutsianas, L.; Fischer, R.; Fedele, V.; Huang, H.-L.; Van Stiphout, R.; Jones, D.; Mccarthy, M.; Vinaxia, M.; et al. Hypoxia induces a lipogenic cancer cell phenotype via HIF1α-dependent and -independent pathways. Oncotarget 2015, 6, 1920–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giner, E.; Andújar, I.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Oleuropein Ameliorates Acute Colitis in Mice. J. Agric. Food Chem. 2011, 59, 12882–12892. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Albutti, A.S.; Aly, S.M. Therapeutics role of olive fruits/oil in the prevention of diseases via modulation of anti-oxidant, anti-tumour and genetic activity. Int. J. Clin. Exp. Med. 2014, 7, 799–808. [Google Scholar] [PubMed]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.D.; Quesada-Gómez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Chimento, A.; De Luca, A.; Casaburi, I.; Rizza, P.; Onofrio, A.; Iacopetta, D.; Puoci, F.; Andò, S.; Maggiolini, M.; et al. Oleuropein and hydroxytyrosol inhibit MCF-7 breast cancer cell proliferation interfering with ERK1/2 activation. Mol. Nutr. Food Res. 2010, 54, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Margarucci, L.; Monti, M.C.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Tosco, A.; Casapullo, A. Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor. Chem. Commun. 2013, 49, 5844–5846. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Annali dell’Istituto Superiore di Sanità 2007, 43, 394–405. [Google Scholar]
- Godos, J.; Biondi, A.; Galvano, F.; Basile, F.; Sciacca, S.; Giovannucci, E.L.; Grosso, G. Markers of systemic inflammation and colorectal adenoma risk: Meta-analysis of observational studies. World J. Gastroenterol. 2017, 23, 1909–1919. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Nolfo, F.; Rametta, S.; Marventano, S.; Grosso, G.; Mistretta, A.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Pharmacological and dietary prevention for colorectal cancer. BMC Surg. 2013, 13 (Suppl. 2), S16. [Google Scholar] [CrossRef]
- García-Rodríguez, L.A.; Huerta-Alvarez, C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology 2001, 12, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Andrewes, P.; Busch, J.L.H.C.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A.; Bränning, C.; Molin, G.; Adawi, D.; Hagslätt, M.-L.; Nyman, M.; Jeppsson, B.; Ahrné, S. Colorectal Oncogenesis and Inflammation in a Rat Model Based on Chronic Inflammation due to Cycling DSS Treatments. Gastroenterol. Res. Pract. 2011, 2011, 924045. [Google Scholar] [CrossRef]
- Basseri, R.J.; Basseri, B.; Papadakis, K.A. Dysplasia and cancer in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 59–66. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Guo, B. IL-10 Modulates Th17 Pathogenicity during Autoimmune Diseases. J. Clin. Cell Immunol. 2016, 7, 400. [Google Scholar] [CrossRef]
- Abraham, C.; Dulai, P.S.; Vermeire, S.; Sandborn, W.J. Lessons Learned from Trials Targeting Cytokine Pathways in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 374–388. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Shi, J.; Yang, P.; Jia, B.; Wu, H.; Zhang, X.; Li, Z. Apigenin Restrains Colon Cancer Cell Proliferation via Targeted Blocking of Pyruvate Kinase M2-Dependent Glycolysis. J. Agric. Food Chem. 2017, 65, 8136–8144. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 2016, 25, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Quesada, C.; López-Biedma, A.; Gaforio, J.J. Maslinic Acid Enhances Signals for the Recruitment of Macrophages and Their Differentiation to M1 State. Evid.-Based Complement. Altern. Med. 2015, 2015, 654721. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, M.; Fang, Y.-J.; Lu, M.-S.; Pan, Z.-Z.; Huang, W.-Q.; Chen, Y.-M.; Zhang, C.-X. Association between phytosterol intake and colorectal cancer risk: A case-control study. Br. J. Nutr. 2017, 117, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Hirose, Y.; Indranie, C.; Reddy, B.S. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Res. 2001, 61, 1927–1933. [Google Scholar] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.L. Squalene, olive oil, and cancer risk: A review and hypothesis. Cancer Epidemiol. Biomark. Prev. 1997, 6, 1101–1103. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Urbano, G.; López-Jurado, M.; Nestares, T.; Gomez, M.C.; Mir, A.; Ros, E.; Mataix, J.; Gil, A. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J. Nutr. 1999, 129, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Llor, X.; Pons, E.; Roca, A.; Alvarez, M.; Mañé, J.; Fernández-Bañares, F.; Gassull, M.A. The effects of fish oil, olive oil, oleic acid and linoleic acid on colorectal neoplastic processes. Clin. Nutr. 2003, 22, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Yuan, J.-M.; Huang, J.Y.; Su, J.; Wang, R.; Koh, W.-P.; Ong, C.-N. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis. Oncol. 2017, 1, 38. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the Human Microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampa, A.; Silvi, S.; Servili, M.; Montedoro, G.; Orpianesi, C.; Cresci, A. In vitro modulatory effects of colonic microflora by olive oil iridoids. Microb. Ecol. Health Dis. 2006, 18, 147–153. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res. Int. 2014, 64, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Diet, Gut Microbiota, and Colorectal Cancer Prevention: A Review of Potential Mechanisms and Promising Targets for Future Research. Curr. Colorectal Cancer Rep. 2017, 13, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.S.; Wagner, K. Influence of dietary phytochemicals and microbiota on colon cancer risk. J. Agric. Food Chem. 2012, 60, 6728–6735. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and Cancer Chemoprevention. Evid-Based Complement. Altern. Med. 2011, 2011, 591356. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Corollaro, M.L.; Vitaglione, P.; Napolitano, A.; Ferracane, R.; Travaglia, F.; Arlorio, M.; Costabile, A.; Klinder, A.; Gibson, G. In vitro bioaccessibility and gut biotransformation of polyphenols present in the water-insoluble cocoa fraction. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S44–S55. [Google Scholar] [CrossRef]
- Stoneham, M.; Goldacre, M.; Seagroatt, V.; Gill, L. Olive oil, diet and colorectal cancer: An ecological study and a hypothesis. J. Epidemiol. Community Health 2000, 54, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; O’Keefe, S.J. Influence of Bile Acids on Colorectal Cancer Risk: Potential Mechanisms Mediated by Diet—Gut Microbiota Interactions. Curr. Nutr. Rep. 2017, 6, 315–322. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Design (Cancer Type) | Intervention and Substances Supplementation | Dosage | Effects |
|---|---|---|---|---|
| Bassani et al. (2016) [13] | in vitro (CT-26 CRC cell line) in vivo (syngenic BalbC mice with CT-26 CRC cell line) | Purified extracts from OMWW rich in HT | HT: 2.7–5.72 g/L | ↓VEGF, ↓IL-8 ↓cell migration and invasion ↓tumor cell growth ↓cell adhesion |
| Rossi et al. (2015) [27] | in vitro (human umbilical vein endothelial cells) in vivo (matrigel sponge assay) | A009 (phenol rich purified extract from OMWW) | 1/1000 to 1/250 dilution HT: 2.7–5.72 g/L | Anti-angiogenetic and pro-apoptotic effects ↓endothelial cell proliferation, migration and invasion |
| Mateos et al. (2013) [28] | in vitro (adenocarcinoma Caco-2/TC7 cells) | HT-acetate | 5–50 μM | ↓cell proliferation Cell cycle arrest (↑p21 and CCNG2, ↓ CCNB1) Apoptosis (↑BNIP3, BNIP3L, PDCD4, ATF3 and caspase-3) ↑ carcinogen detoxification (CYP1A1 and UGT1A10) |
| Terzuoli et al. (2016) [29] | in vitro (human colorectal adenocarcinoma cells HT-29, CaCo2, and WiDr) in vivo (mice with HT-29 xenografts) | HT | in vitro100 μM in vivo 10 mg/Kg (200 μL) | ↓ tumor cell growth (↑EGFR degradation: EGFR phosphorylation at pY1045 and ↑ Cbl activity with EGFR ubiquitination) |
| Terzuoli et al. (2017) [30] | in vitro (HT-29 and WiDr cells) | HT-cetuximab combination | HT (10 μM) with cetuximab (1 μg/mL) | ↓ tumor cell growth (cell cycle blockade at G2/M phase) ↓cyclins B, D1, and E, and CDK2, CDK4, and CDK6 ↑ CDK inhibitors p21 and p27 |
| Hamdi and Castellon (2005) [31] | in vitro (TF-1a; 786-O, T-47D, RPMI-7951, and colon cancer LoVo) in vivo Swiss albino mice with spontaneous soft tissue sarcomas | Oleuropein | in vitro 0.005–0.1%: in vivo 1% in drinking water | ↓ cell proliferation, motility and invasion ↑actin filament disruption Dramatic tumor regression in mice |
| Cárdeno et al. (2013) [32] | in vitro HT-29 human colon adenocarcinoma cells | Oleuropein | 200–400 μM | ↓ cell proliferation (p53 pathway activation and ↓HIF-1α) |
| Giner et al. (2016) [33] | in vivo model of azoxymethane/Dextran sulfate sodium-induced CRC in C57BL/6 mice | Oleuropein | 50–100 mg/Kg | Chemoprevention (↓intestinal IL-6, IFN-γ, TNF-α, IL-17A, ↓COX-2, Bax, PCNA, ↓NF-κB, Wnt/β-catenin, P3IK/Akt, STAT3) Modulatory effect on the Th17 response (↓CD4+, Rorγt+, IL-17+, IFN-γ+ T-cells in the lamina propria) |
| Khanal et al. (2011) [34] | in vitro HT-29 human colon adenocarcinoma cells in vivo chorioallantoic membrane assay | Oleocanthal | 1–10 μg/mL | Antitumor effect (↑ AMPK) Apoptosis (↑ caspase-3 and poly-adenosine diphosphate-ribose polymerase, phosphorylation of p53 (Ser15)) Disruption of DNA |
| Bartolí et al. (2000) [35] | in vivo on rats with azoxymethane-induced CRC | n9 and n3 fatty acids (oleic and eicosapentaenoic acids) | n9: 57% of diet n3: 27.7% of diet equivalent to a 5% fat diet containing olive oil | Chemoprevention (modulation in colonic mucosa of arachidonic acid metabolism and prostaglandin E2 synthesis) |
| Xu et al. (2016) [36] | in vitro human CRC cell lines (SW480 and HCT15) | Apigenin | 20–40 µM | ↓cell proliferation (↓Wnt/β‑catenin signaling pathway) |
| Shao et al.(2013) [37] | in vitro human colon cancer cell lines (DLD1, HCT116, HCT8, HT29 and SW48) in vivo C.B.-17 SCID mice implanted with HCT116 cells | Apigenin | in vitro 20 mmol/L in vivo 25 mg/Kg | Synergistic effect between apigenin and ABT-263 on apoptosis (↓Mcl-1, AKT, and ERK) |
| Chen et al. (2018) [38] | in vitro LoVo human colon cancer cells in vivo BalbC nude mice inoculated with LoVo cells | Luteolin | IC50 value of 66.70 and 30.47 µmol/L at 24 and 72 h, respectively in vivo 20–40 mg/Kg | Apoptosis (↑APAF-1) Cell cycle arrest at the G2/M phase ↓tumor growth |
| Zuo et al. (2018) [39] | in vitro HCT116 and HT29 cells | Luteolin | - | ↓CRC carcinogenesis (↑Nrf2/ARE pathway) |
| Reyes-Zurita et al. (2016) [40] | in vitro Caco-2 p53-Deficient Colon Adenocarcinoma Cells | Maslinic acid | IC50 was 40.7 ± 0.4 μg/mL IC80 was 56.8 μg/mL. | Apoptosis (cleavage of caspases -8 and -3, ↑t-Bid) |
| Reyes-Zurita et al. (2009) [41] | in vitro HT29 cells | Maslinic acid | IC50 was 28.8 ± 0.9 μg/mL IC80 was 37.5 ± 0.2 μg/mL | Apoptosis (↓Bcl-2, ↑Bax, ↑ caspase-9 and -3) |
| Baskar et al. (2010) [42] | in vitro human colon cancer cell lines (COLO 320 DM) in vivo Wistar rats inoculated with 1,2-dimethylhydrazine | β-sitosterol | IC50 was 266.2 μM in vivo10–20 mg/Kg | Chemoprevention ↓Tumor growth (↓β-catenin and PCNA) |
| Miene et al. (2011) [43] | in vitro human colorectal adenoma cell line LT97 | 3,4-dihydroxyphenylacetic acid (ES) and 3-(3,4-dihydroxyphenyl)-propionic acid (PS), metabolites of quercetin and caffeic acid, respectively. | ES: 2.5–10 µM PS: 5–25 µM | Chemoprevention after degradation of polyphenols in the gut (↑GSTT2, ↓COX2) |
| Losso et al. (2004) [44] | in vitro human umbilical vein endothelial cells, normal human lung fibroblast cells HEL 299, Caco-2 colon, MCF-7 breast, Hs 578T breast, and DU 145 human prostatic cancer cells | Ellagic acid | 1–100 µmol/L | Anti-proliferative activity Apoptosis (↓ATP, pro-MMP-2, -9 and VEGF) |
| Oleuropein | Oleocanthal |
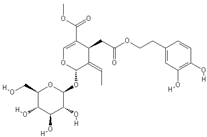 | 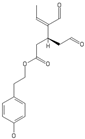 |
| Tyrosol | Hydroxytyrosol |
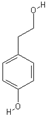 | 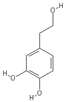 |
| Apigenin | Luteolin |
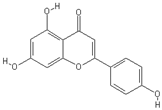 | 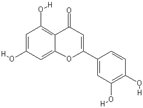 |
| Quercetin | Pinoresinol |
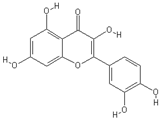 |  |
| Caffeic acid | Epigallocatechin-3-gallate |
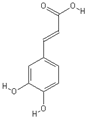 | 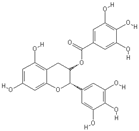 |
| Ellagic acid | |
 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive Oil Effects on Colorectal Cancer. Nutrients 2019, 11, 32. https://doi.org/10.3390/nu11010032
Borzì AM, Biondi A, Basile F, Luca S, Vicari ESD, Vacante M. Olive Oil Effects on Colorectal Cancer. Nutrients. 2019; 11(1):32. https://doi.org/10.3390/nu11010032
Chicago/Turabian StyleBorzì, Antonio Maria, Antonio Biondi, Francesco Basile, Salvatore Luca, Enzo Saretto Dante Vicari, and Marco Vacante. 2019. "Olive Oil Effects on Colorectal Cancer" Nutrients 11, no. 1: 32. https://doi.org/10.3390/nu11010032
APA StyleBorzì, A. M., Biondi, A., Basile, F., Luca, S., Vicari, E. S. D., & Vacante, M. (2019). Olive Oil Effects on Colorectal Cancer. Nutrients, 11(1), 32. https://doi.org/10.3390/nu11010032









