A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. CMI-168
2.2. Animals
2.3. Open Field Test
2.4. Novel Object Recognition (ORT) Test
2.5. Morris Water Maze (MWM) Test
2.6. Electrophysiology
2.7. Single-Neuron Labeling
2.8. Immunoblotting
2.9. Statistical Analysis
3. Results
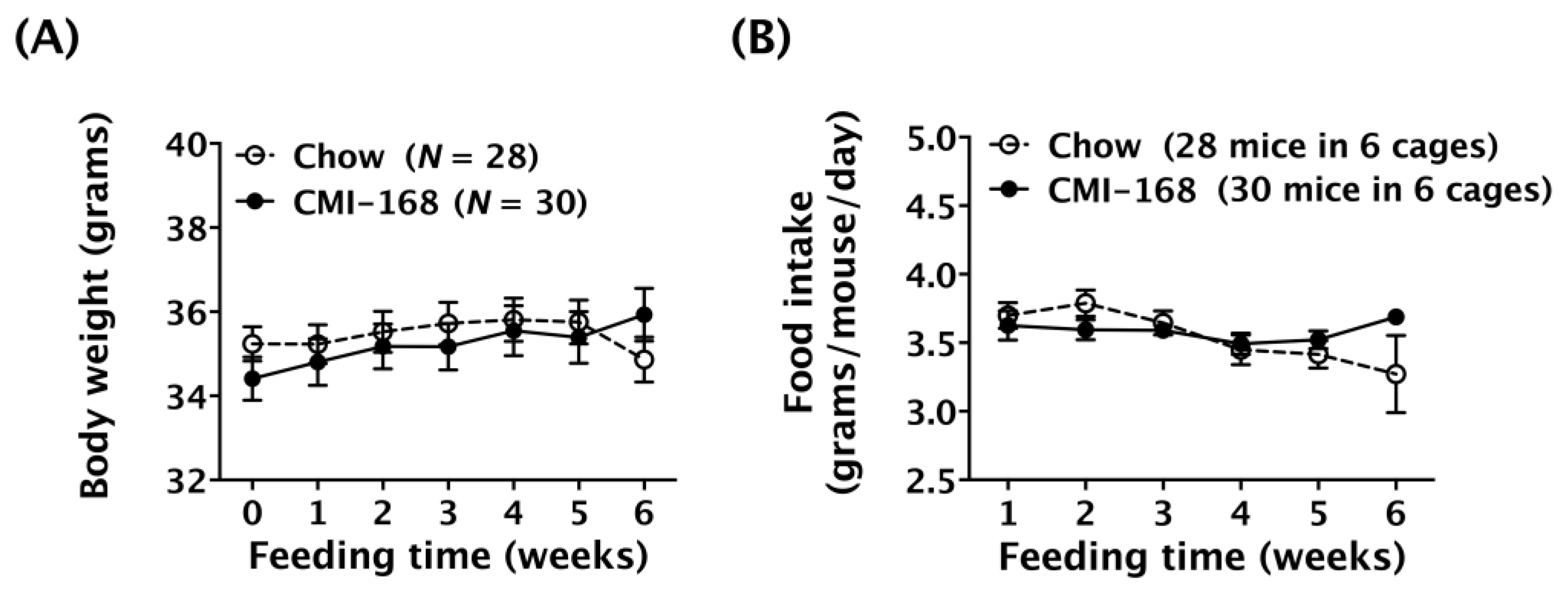
3.1. CMI-168 Did Not Affect Body Weight or Food Consumption in Middle-Aged Mice
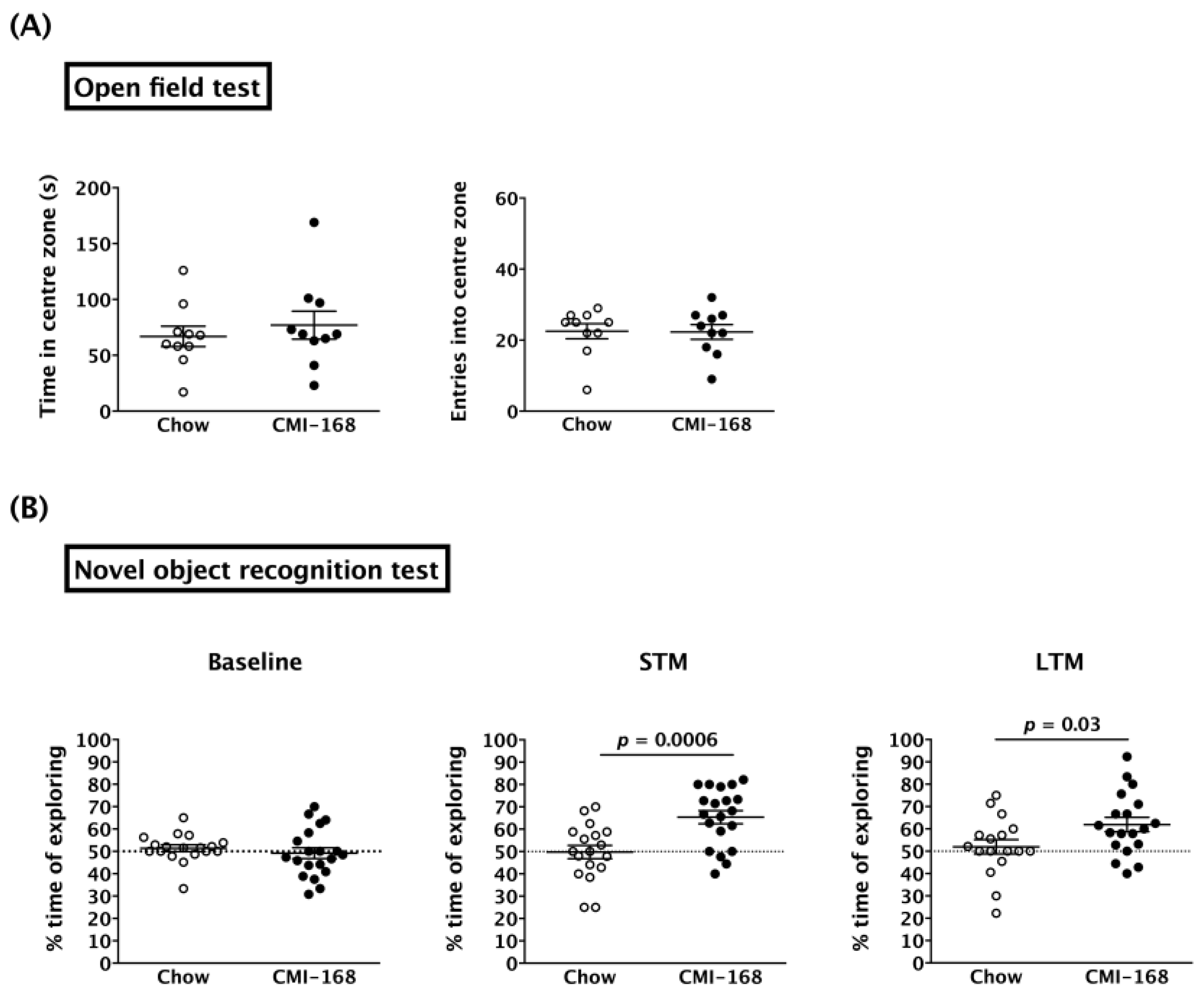
3.2. CMI-168 Enhanced the Hippocampus-Related Non-Spatial Memory in Middle-Aged Mice
3.3. CMI-168 Did Not Alter the Hippocampus-Related Spatial Memory in Middle-Aged Mice
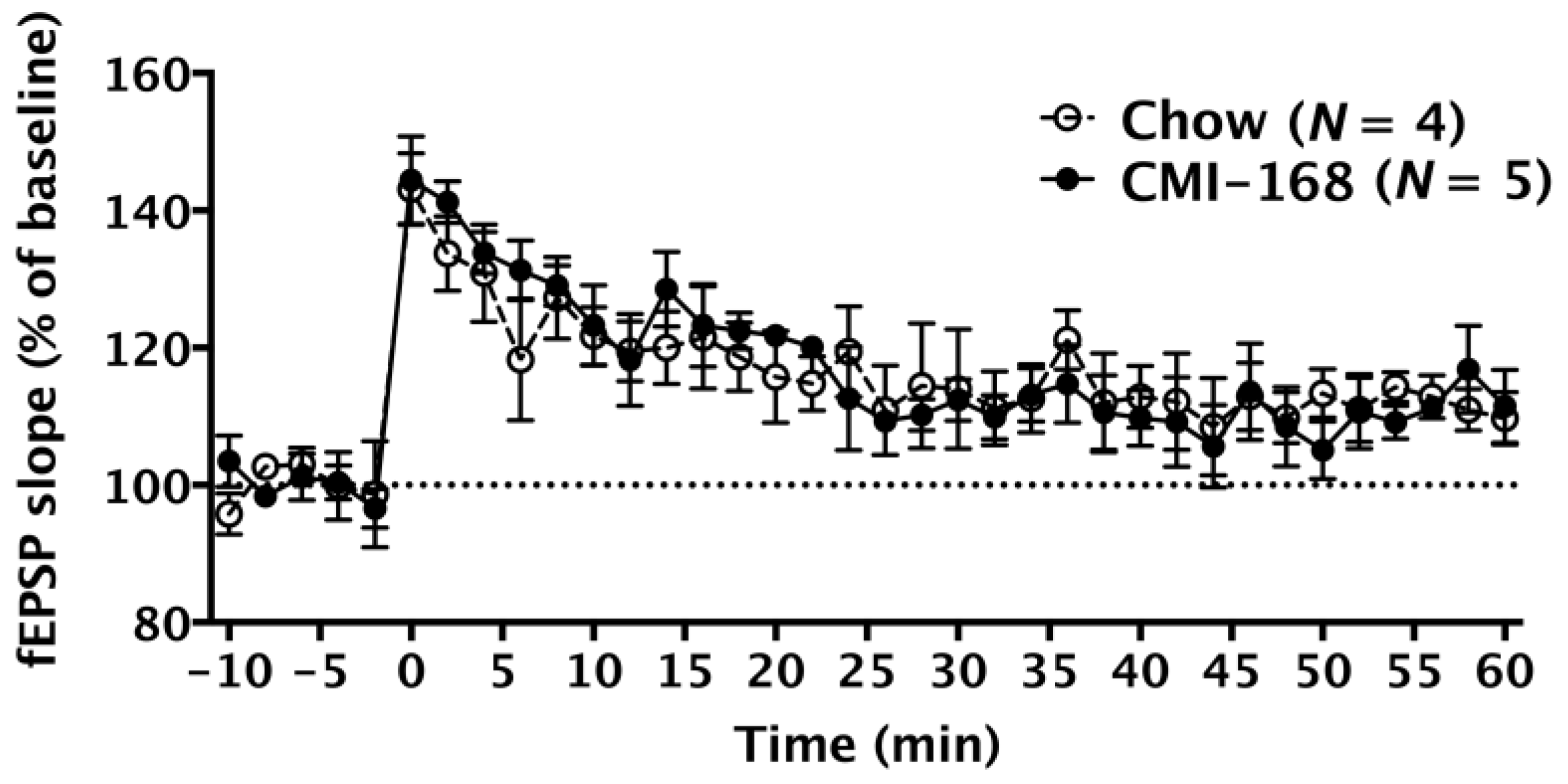
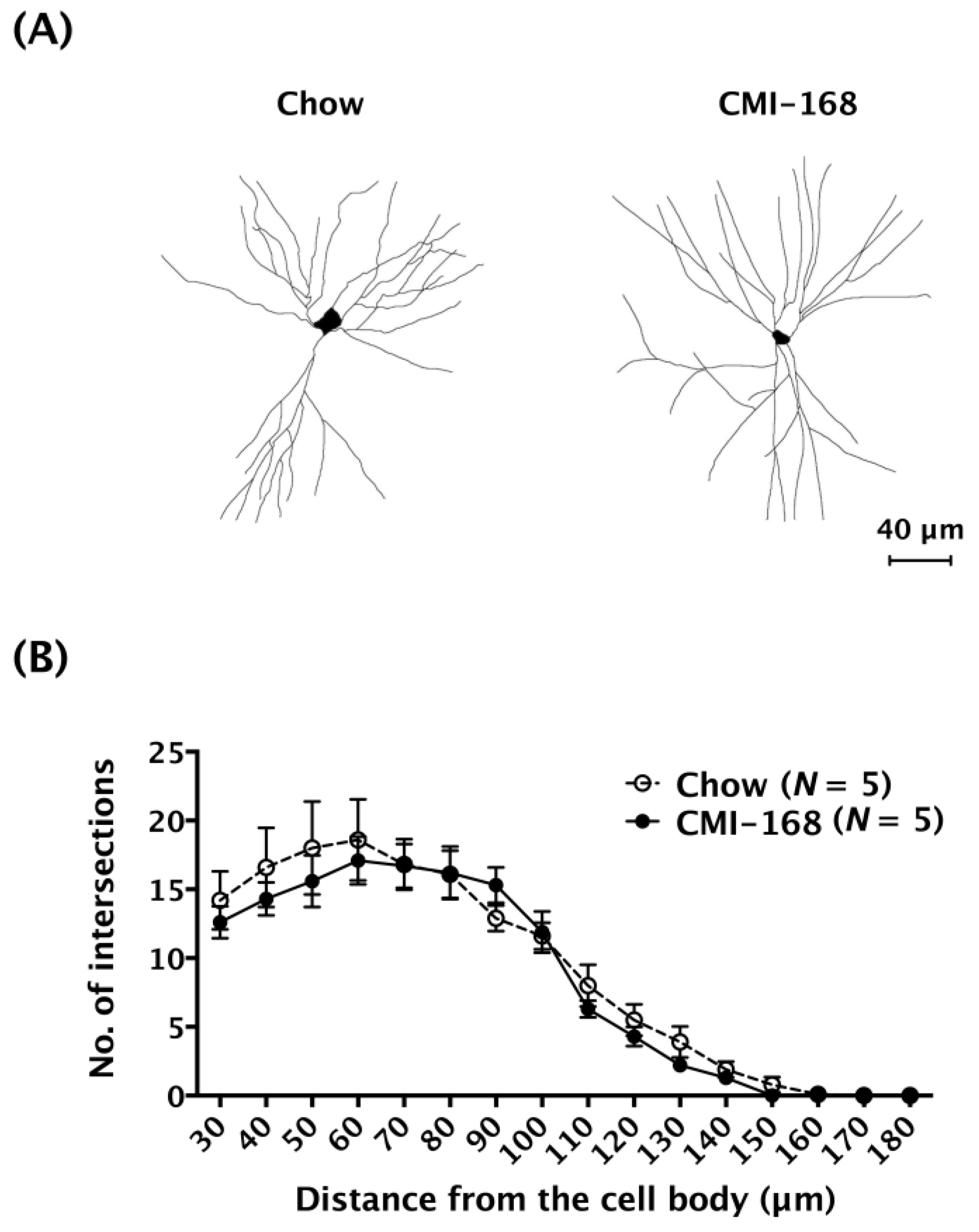
3.4. CMI-168 Did Not Alter the LTP Induction, LTP Maintenance or the Dendritic Complexity of the Hippocampal CA1 Neurons in Middle-Aged Mice
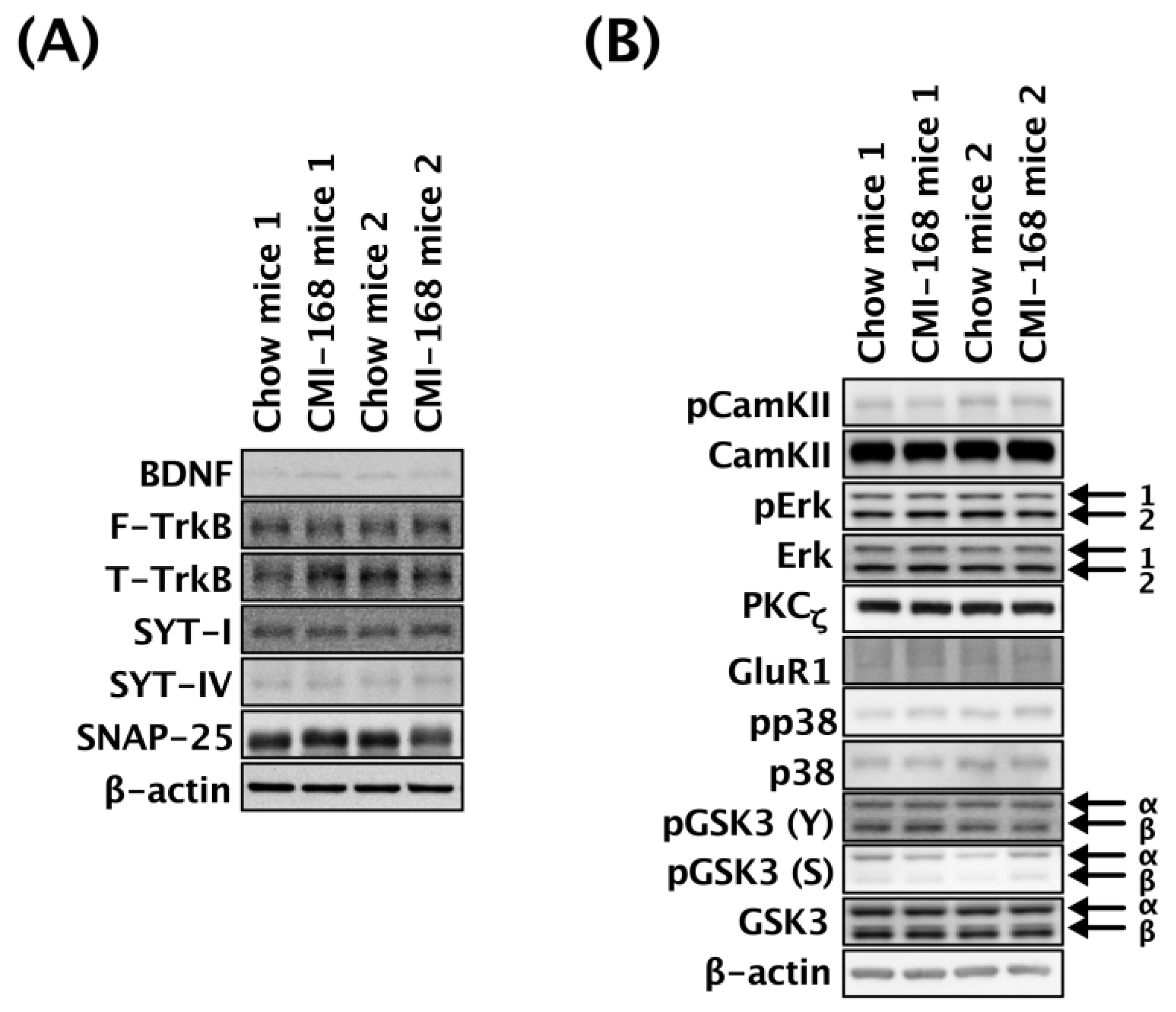
3.5. CMI-168 Did Not Alter the Hippocampal Expression of Neuroplasticity-Related Molecules in Middle-Aged Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, S.J.; Grimwood, P.D.; Morris, R.G. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 2000, 23, 649–711. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Yang, C.H.; Hsu, K.S.; Ming, G.L.; Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 2007, 54, 559–566. [Google Scholar] [CrossRef]
- Eichenbaum, H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron 2004, 44, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Voglis, G.; Tavernarakis, N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006, 7, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Azhar, Z.M.; Zubaidah, J.O.; Norjan, K.O.; Zhuang, C.Y.; Tsang, F. A pilot placebo-controlled, double-blind, and randomized study on the cognition-enhancing benefits of a proprietary chicken meat ingredient in healthy subjects. Nutr. J. 2013, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, N.J.; Gaskin, S.; Squire, L.R.; Clark, R.E. Object recognition memory and the rodent hippocampus. Learn. Mem. 2010, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry-Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2005; p. 7.
- Tatem, K.S.; Quinn, J.L.; Phadke, A.; Yu, Q.; Gordish-Dressman, H.; Nagaraju, K. Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. J. Vis. Exp. 2014, 51785. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, S.F.; Huang, S.H.; Chiang, Y.T.; Hughes, M.W.; Wu, S.Y.; Lee, C.W.; Yang, T.T.; Kuo, Y.M. Hypertension impairs hippocampus-related adult neurogenesis, ca1 neuron dendritic arborization and long-term memory. Neuroscience 2016, 322, 346–357. [Google Scholar] [CrossRef]
- Lin, T.W.; Shih, Y.H.; Chen, S.J.; Lien, C.H.; Chang, C.Y.; Huang, T.Y.; Chen, S.H.; Jen, C.J.; Kuo, Y.M. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (app/ps1) transgenic mice. Neurobiol. Learn. Mem. 2015, 118, 189–197. [Google Scholar] [CrossRef]
- Tsai, S.F.; Huang, T.Y.; Chang, C.Y.; Hsu, Y.C.; Chen, S.J.; Yu, L.; Kuo, Y.M.; Jen, C.J. Social instability stress differentially affects amygdalar neuron adaptations and memory performance in adolescent and adult rats. Front. Behav. Neurosci. 2014, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zain, A.M.; Syedsahiljamalulail, S. Effect of taking chicken essence on stress and cognition of human volunteers. Malays. J. Nutr. 2003, 9, 19–29. [Google Scholar] [PubMed]
- Wang, M.F.; Chen, Y.J.; Zhou, M.Y.; Lin, L.H.; Nakao, Y.; Lim, A.L.; Yong, S.M. Protective effects of chicken meat ingredient (CMI-168) on memory retention and brain oxidative stress in senescence-accelerated mice. in preparation.
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef]
- Solomonia, R.O.; Kotorashvili, A.; Kiguradze, T.; McCabe, B.J.; Horn, G. Ca2+/calmodulin protein kinase II and memory: Learning-related changes in a localized region of the domestic chick brain. J. Physiol. 2005, 569, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Feld, M.; Dimant, B.; Delorenzi, A.; Coso, O.; Romano, A. Phosphorylation of extra-nuclear ERK/MAPK is required for long-term memory consolidation in the crab Chasmagnathus. Behav. Brain Res. 2005, 158, 251–261. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-González, M.; Salgado-Mendialdúa, V.; Galera-López, L.; Puighermanal, E.; Martín-García, E.; Maldonado, R.; Ozaita, A. Hippocampal protein kinase C signaling mediates the short-term memory impairment induced by delta9-tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 1021–1031. [Google Scholar] [CrossRef]
- Sanderson, D.J.; Good, M.A.; Seeburg, P.H.; Sprengel, R.; Rawlins, J.N.; Bannerman, D.M. The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog. Brain Res. 2008, 169, 159–178. [Google Scholar]
- Giese, K.P.; Mizuno, K. The roles of protein kinases in learning and memory. Learn. Mem. 2013, 20, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Markevich, V.; Plattner, F.; Killick, R.; Schofield, E.; Engel, T.; Hernandez, F.; Anderton, B.; Rosenblum, K.; Bliss, T.; et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur. J. Neurosci. 2007, 25, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Ungar, D.; Hughson, F.M. SNARE protein structure and function. Annu. Rev. Cell Dev. Biol. 2003, 19, 493–517. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Chen, S.J.; Huang, T.Y.; Chang, C.Y.; Chuang, J.I.; Wu, F.S.; Kuo, Y.M.; Jen, C.J. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol. Learn. Mem. 2012, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Konagai, C.; Watanabe, H.; Abe, K.; Tsuruoka, N.; Koga, Y. Effects of essence of chicken on cognitive brain function: A near-infrared spectroscopy study. Biosci. Biotechnol. Biochem. 2013, 77, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C. Bioactive cyclic dipeptides. Peptides 1995, 16, 151–164. [Google Scholar] [CrossRef]
- Gudasheva, T.A.; Boyko, S.S.; Akparov, V.; Ostrovskaya, R.U.; Skoldinov, S.P.; Rozantsev, G.G.; Voronina, T.A.; Zherdev, V.P.; Seredenin, S.B. Identification of a novel endogenous memory facilitating cyclic dipeptide cyclo-prolylglycine in rat brain. FEBS Lett. 1996, 391, 149–152. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Beppu, Y.; Koda, H.; Doe, N.; Watanabe, H.; Abe, K. A dkp cyclo(l-phe-l-phe) found in chicken essence is a dual inhibitor of the serotonin transporter and acetylcholinesterase. PLoS ONE 2012, 7, e50824. [Google Scholar] [CrossRef]
- Faden, A.I.; Knoblach, S.M.; Movsesyan, V.A.; Cernak, I. Novel small peptides with neuroprotective and nootropic properties. J. Alzheimer’s Dis. 2004, 6, S93–S97. [Google Scholar] [CrossRef]
- Prakash, K.R.; Tang, Y.; Kozikowski, A.P.; Flippen-Anderson, J.L.; Knoblach, S.M.; Faden, A.I. Synthesis and biological activity of novel neuroprotective diketopiperazines. Bioorg. Med. Chem. 2002, 10, 3043–3048. [Google Scholar] [CrossRef]
- Yagasaki, M.; Hashimoto, S. Synthesis and application of dipeptides; current status and perspectives. Appl. Microbiol. Biotechnol. 2008, 81, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Morita-Tsuzuki, Y.; Hardebo, J.E.; Bouskela, E. Inhibition of nitric oxide synthase attenuates the cerebral blood flow response to stimulation of postganglionic parasympathetic nerves in the rat. J. Cereb. Blood Flow Metab. 1993, 13, 993–997. [Google Scholar] [CrossRef]
- Hylland, P.; Nilsson, G.E. Evidence that acetylcholine mediates increased cerebral blood flow velocity in crucian carp through a nitric oxide-dependent mechanism. J. Cereb. Blood Flow Metab. 1995, 15, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Kanetaka, H.; Hirose, D.; Sakurai, H.; Hanyu, H. Differential effects of acetylcholinesterase inhibitors on clinical responses and cerebral blood flow changes in patients with alzheimer’s disease: A 12-month, randomized, and open-label trial. Dement. Geriatr. Cogn. Dis. Extra 2015, 5, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Ahmad, S.; Madiha, S.; Khaliq, S.; Shahzad, S.; Batool, Z.; Haider, S. Impact of oral supplementation of glutamate and gaba on memory performance and neurochemical profile in hippocampus of rats. Pak. J. Pharm. Sci. 2017, 30, 1013–1021. [Google Scholar] [PubMed]
- Yi, J.; Horky, L.L.; Friedlich, A.L.; Shi, Y.; Rogers, J.T.; Huang, X. L-arginine and alzheimer’s disease. Int. J. Clin. Exp. Pathol. 2009, 2, 211–238. [Google Scholar] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Dzau, V.J. Nitric oxide synthase: Role in the genesis of vascular disease. Annu. Rev. Med. 1997, 48, 489–509. [Google Scholar] [CrossRef]
- Bohme, G.A.; Bon, C.; Lemaire, M.; Reibaud, M.; Piot, O.; Stutzmann, J.M.; Doble, A.; Blanchard, J.C. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc. Natl. Acad. Sci. USA 1993, 90, 9191–9194. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Nakaya, J. Effect of oral administration of l-arginine on senile dementia. Am. J. Med. 2000, 108, 439. [Google Scholar] [CrossRef]
- Paul, V.; Reddy, L.; Ekambaram, P. Prevention of picrotoxin convulsions-induced learning and memory impairment by nitric oxide increasing dose of l-arginine in rats. Pharmacol. Biochem. Behav. 2003, 75, 329–334. [Google Scholar] [CrossRef]
- Hosseini, M.; Anaeigoudari, A.; Beheshti, F.; Soukhtanloo, M.; Nosratabadi, R. Protective effect against brain tissues oxidative damage as a possible mechanism for beneficial effects of l-arginine on lipopolysaccharide induced memory impairment in rats. Drug Chem. Toxicol. 2018, 41, 175–181. [Google Scholar] [CrossRef]
- Anaeigoudari, A.; Shafei, M.N.; Soukhtanloo, M.; Sadeghnia, H.R.; Reisi, P.; Nosratabadi, R.; Behradnia, S.; Hosseini, M. The effects of l-arginine on spatial memory and synaptic plasticity impairments induced by lipopolysaccharide. Adv. Biomed. Res. 2015, 4, 202. [Google Scholar]
- Lubec, B.; Hayn, M.; Kitzmuller, E.; Vierhapper, H.; Lubec, G. L-arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Radic. Biol. Med. 1997, 22, 355–357. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Liu, J.; Head, E.; Gharib, A.M.; Yuan, W.; Ingersoll, R.T.; Hagen, T.M.; Cotman, C.W.; Ames, B.N. Memory loss in old rats is associated with brain mitochondrial decay and rna/DNA oxidation: Partial reversal by feeding acetyl-l-carnitine and/or r-alpha-lipoic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 2356–2361. [Google Scholar] [CrossRef]
- Kawano, H.; Motoyama, T.; Hirai, N.; Kugiyama, K.; Yasue, H.; Ogawa, H. Endothelial dysfunction in hypercholesterolemia is improved by l-arginine administration: Possible role of oxidative stress. Atherosclerosis 2002, 161, 375–380. [Google Scholar] [CrossRef]
- Miliutina, N.P.; Ananian, A.A.; Shugalei, V.S. Antiradical and antioxidant effect of arginine and its action on lipid peroxidation in hypoxia. Bull. Exp. Biol. Med. 1990, 110, 263–265. [Google Scholar]
- Wink, D.A.; Miranda, K.M.; Espey, M.G.; Pluta, R.M.; Hewett, S.J.; Colton, C.; Vitek, M.; Feelisch, M.; Grisham, M.B. Mechanisms of the antioxidant effects of nitric oxide. Antioxid. Redox Signal. 2001, 3, 203–213. [Google Scholar] [CrossRef]
- Calabrese, V.; Bates, T.E.; Stella, A.M. No synthase and no-dependent signal pathways in brain aging and neurodegenerative disorders: The role of oxidant/antioxidant balance. Neurochem. Res. 2000, 25, 1315–1341. [Google Scholar] [CrossRef]
- Thomas, J.R.; Lockwood, P.A.; Singh, A.; Deuster, P.A. Tyrosine improves working memory in a multitasking environment. Pharmacol. Biochem. Behav. 1999, 64, 495–500. [Google Scholar] [CrossRef]
- Shurtleff, D.; Thomas, J.R.; Schrot, J.; Kowalski, K.; Harford, R. Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol. Biochem. Behav. 1994, 47, 935–941. [Google Scholar] [CrossRef]
- Colzato, L.S.; Jongkees, B.J.; Sellaro, R.; Hommel, B. Working memory reloaded: Tyrosine repletes updating in the n-back task. Front. Behav. Neurosci. 2013, 7, 200. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Stillman, M.J.; Lieberman, H.R. Tyrosine administration prevents hypoxia-induced decrements in learning and memory. Physiol. Behav. 1996, 59, 867–871. [Google Scholar] [CrossRef]
- Mul, J.D.; Zheng, J.; Goodyear, L.J. Validity assessment of 5 day repeated forced-swim stress to model human depression in young-adult C57BL/6J and BALB/cJ Mice. eNeuro 2016, 3, 201–216. [Google Scholar] [CrossRef]
- Suttiwan, P.; Yuktanandana, P.; Ngamake, S. Effectiveness of essence of chicken on cognitive function improvement: A randomized controlled clinical trial. Nutrients 2018, 10, 845. [Google Scholar] [CrossRef]
- Nagai, H.; Harada, M.; Nakagawa, M.; Tanaka, T.; Gunadi, B.; Setiabudi, M.L.; Uktolseja, J.L.; Miyata, Y. Effects of chicken extract on the recovery from fatigue caused by mental workload. Appl. Hum. Sci. 1996, 15, 281–286. [Google Scholar] [CrossRef]
- Young, H.; Benton, D.; Carter, N. The effect of chicken extract on mood, cognition and heart rate variability. Nutrients 2015, 7, 887–904. [Google Scholar] [CrossRef]
- Zhai, Y.J.; He, R.R.; Tsoi, B.; Li, Y.F.; Li, X.D.; Tsuruoka, N.; Abe, K.; Kurihara, H. Protective effect of extract of chicken meat on restraint stress-induced liver damage in mice. Food Funct. 2012, 3, 662–667. [Google Scholar] [CrossRef]
- Peng, H.C.; Lin, S.H. Effects of chicken extract on antioxidative status and liver protection under oxidative stress. J. Nutr. Sci. Vitaminol. 2004, 50, 325–329. [Google Scholar] [CrossRef]
- Chan, L.; Wang, H.M.; Chen, K.Y.; Lin, Y.C.; Wu, P.J.; Hsieh, W.L.; Chen, Y.R.; Liu, C.P.; Tsai, H.Y.; Chen, Y.R.; et al. Effectiveness of essence of chicken in improving cognitive function in young people under work-related stress: A randomized double-blind trial. Medicine (Baltimore) 2016, 95, e3640. [Google Scholar] [CrossRef]

| Antibody | Species/Clonality | Source (Catalogue Number) | Dilution |
|---|---|---|---|
| Anti-BDNF | Rabbit/Polyclonal | Santa Cruz, sc-546 | 1:500 |
| Anti-TrkB | Mouse/Monoclonal | BD Bioscience, 610101 | 1:5000 |
| Anti-SYT-I | Mouse/Monoclonal | Stressgen, SYA-130 | 1:5000 |
| Anti-SYT-IV | Rabbit/Polyclonal | Santa Cruz, sc-30095 | 1:1000 |
| Anti-SNAP-25 | Rabbit/Polyclonal | Stressgen, ADI-VAM-SV012 | 1:5000 |
| Anti-PKCζ | Goat/Polyclonal | Santa Cruz, sc-216-G | 1:1000 |
| Anti-pCamKII | Rabbit/Monoclonal | Cell Signaling Tech, 12716 | 1:1000 |
| Anti-CamKII | Rabbit/Monoclonal | Cell Signaling Tech, 11945 | 1:1000 |
| Anti-pErk1/2 | Rabbit/ Monoclonal | Cell Signaling Tech, 4370 | 1:10,000 |
| Anti-Erk1/2 | Rabbit/Monoclonal | Cell Signaling Tech, 4695 | 1:10,000 |
| Anti-GluR1 | Rabbit/Monoclonal | Merck-Millipore, 04-855 | 1:5000 |
| pp-38 | Rabbit/Monoclonal | Cell Signaling Tech, 4511 | 1:1000 |
| p-38 | Rabbit/Monoclonal | Cell Signaling Tech, 8690 | 1:1000 |
| pGSK3α/β (Y) | Mouse/Monoclonal | Merck-Millipore, 05-413 | 1:1000 |
| pGSK3α/β (S) | Rabbit/Monoclonal | Cell Signaling Tech, 9327 | 1:1000 |
| GSK3α/β | Rabbit/Monoclonal | Cell Signaling Tech, 5676 | 1:1000 |
| Anti-β-actin | Mouse/Polyclonal | Sigma-Aldrich, A2228 | 1:10,000 |
| Selected Proteins | Chow Mean ± SEM (n) | CMI-168 Mean ± SEM (n) | p-value |
|---|---|---|---|
| BDNF | 1.00 ± 0.09 (8) | 0.79 ± 0.07 (10) | 0.0858 |
| F-TrkB | 1.00 ± 0.07 (8) | 1.11 ± 0.07 (10) | 0.2652 |
| T-TrkB | 1.00 ± 0.14 (8) | 1.10 ± 0.16 (10) | > 0.5 |
| SYT-I | 1.00 ± 0.03 (8) | 0.97 ± 0.03 (10) | 0.4971 |
| SYT-IV | 1.00 ± 0.05 (8) | 1.01 ± 0.06 (10) | > 0.5 |
| SNAP-25 | 1.00 ± 0.14 (8) | 0.96 ± 0.12 (10) | > 0.5 |
| pCamKII/CamKII | 1.00 ± 0.16 (7) | 0.72 ± 0.24 (7) | 0.3580 |
| pErk1/Erk1 | 1.00 ± 0.21 (7) | 1.07 ± 0.16 (7) | > 0.5 |
| pErk2/Erk2 | 1.00 ± 0.21 (7) | 1.08 ± 0.19 (7) | > 0.5 |
| PKCζ | 1.00 ± 0.20 (7) | 1.09 ± 0.30 (7) | > 0.5 |
| GluR1 | 1.00 ± 0.10 (7) | 1.06 ± 0.16 (7) | > 0.5 |
| pp38/p38 | 1.00 ± 0.24 (7) | 0.89 ± 0.23 (7) | > 0.5 |
| pGSK3α (Y)/GSK3α | 1.00 ± 0.07 (7) | 1.03 ± 0.09 (7) | > 0.5 |
| pGSK3α (S)/GSK3α | 1.00 ± 0.16 (7) | 0.77 ± 0.11 (7) | 0.2752 |
| pGSK3β (Y)/GSK3β | 1.00 ± 0.07 (7) | 1.03 ± 0.09 (7) | > 0.5 |
| pGSK3β (S)/GSK3β | 1.00 ± 0.18 (7) | 0.91 ± 0.17 (7) | > 0.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-F.; Chang, C.-Y.; Yong, S.-M.; Lim, A.-L.; Nakao, Y.; Chen, S.-J.; Kuo, Y.-M. A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice. Nutrients 2019, 11, 27. https://doi.org/10.3390/nu11010027
Tsai S-F, Chang C-Y, Yong S-M, Lim A-L, Nakao Y, Chen S-J, Kuo Y-M. A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice. Nutrients. 2019; 11(1):27. https://doi.org/10.3390/nu11010027
Chicago/Turabian StyleTsai, Sheng-Feng, Chia-Yuan Chang, Shan-May Yong, Ai-Lin Lim, Yoshihiro Nakao, Shean-Jen Chen, and Yu-Min Kuo. 2019. "A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice" Nutrients 11, no. 1: 27. https://doi.org/10.3390/nu11010027
APA StyleTsai, S.-F., Chang, C.-Y., Yong, S.-M., Lim, A.-L., Nakao, Y., Chen, S.-J., & Kuo, Y.-M. (2019). A Hydrolyzed Chicken Extract CMI-168 Enhances Learning and Memory in Middle-Aged Mice. Nutrients, 11(1), 27. https://doi.org/10.3390/nu11010027





