A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates
Abstract
1. Introduction
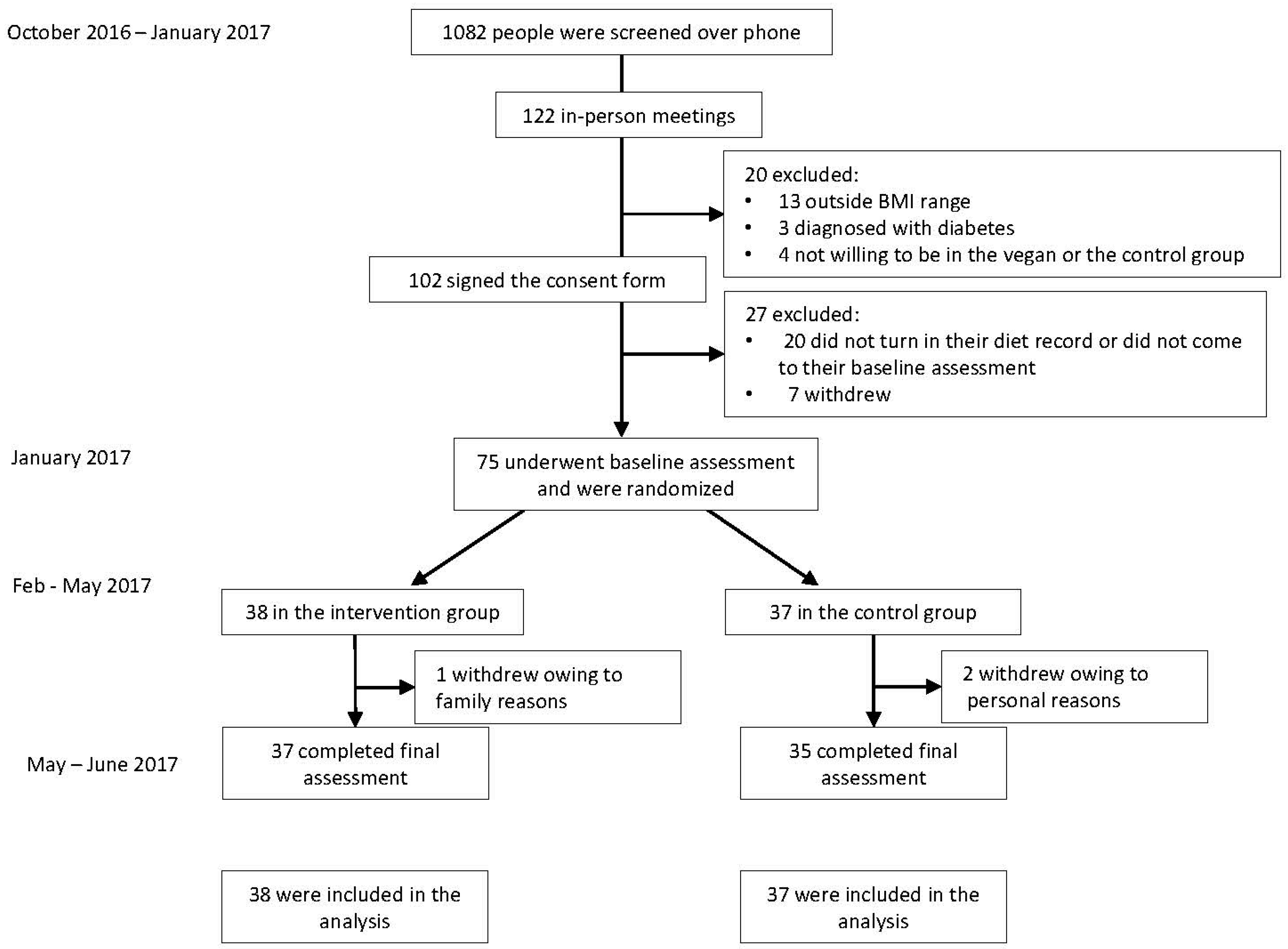
2. Materials and Methods
2.1. Study Design
2.2. Randomization and Study Groups
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Dietary Intake and Physical Activity
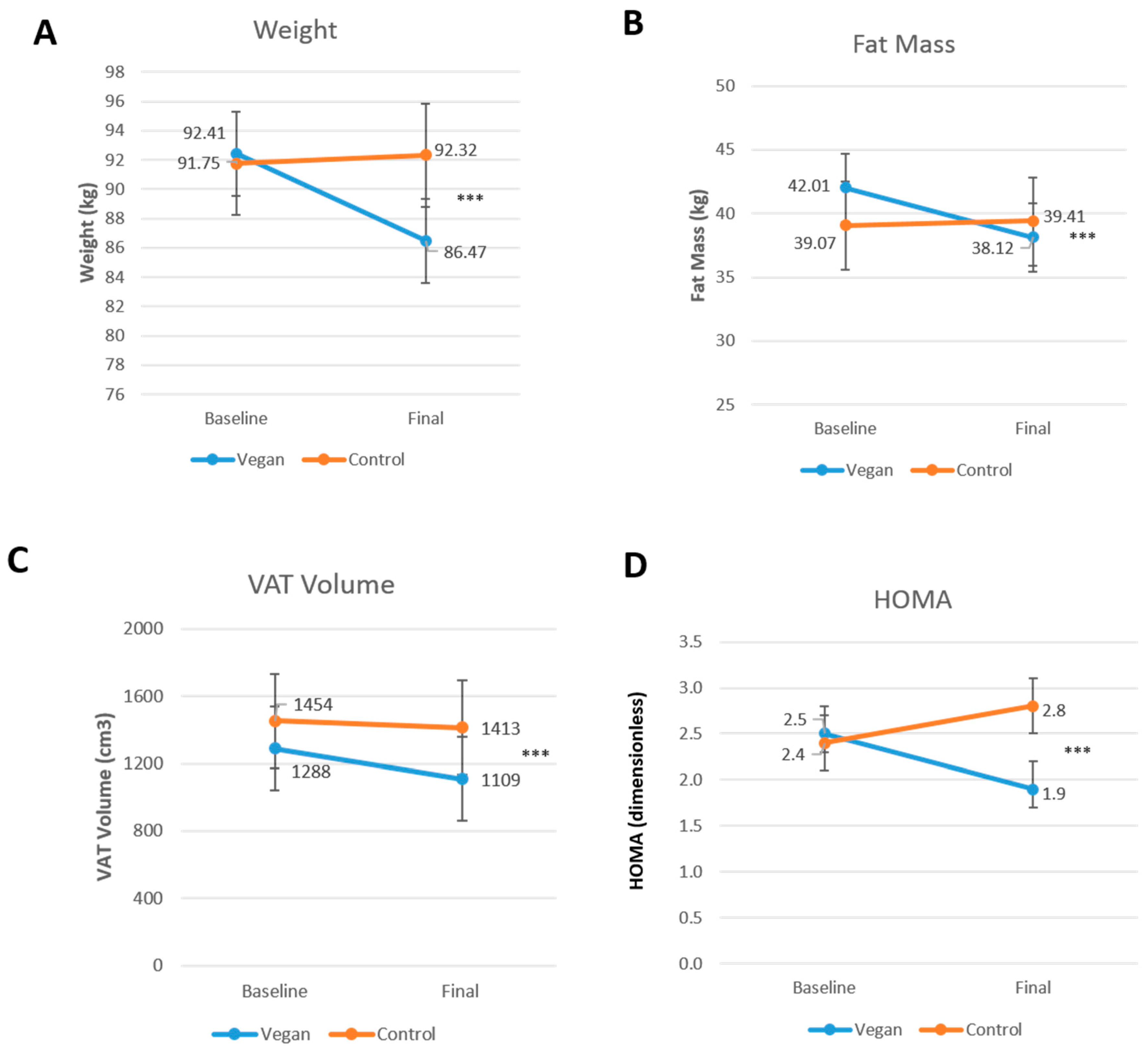
3.3. Body Composition and Insulin Resistance
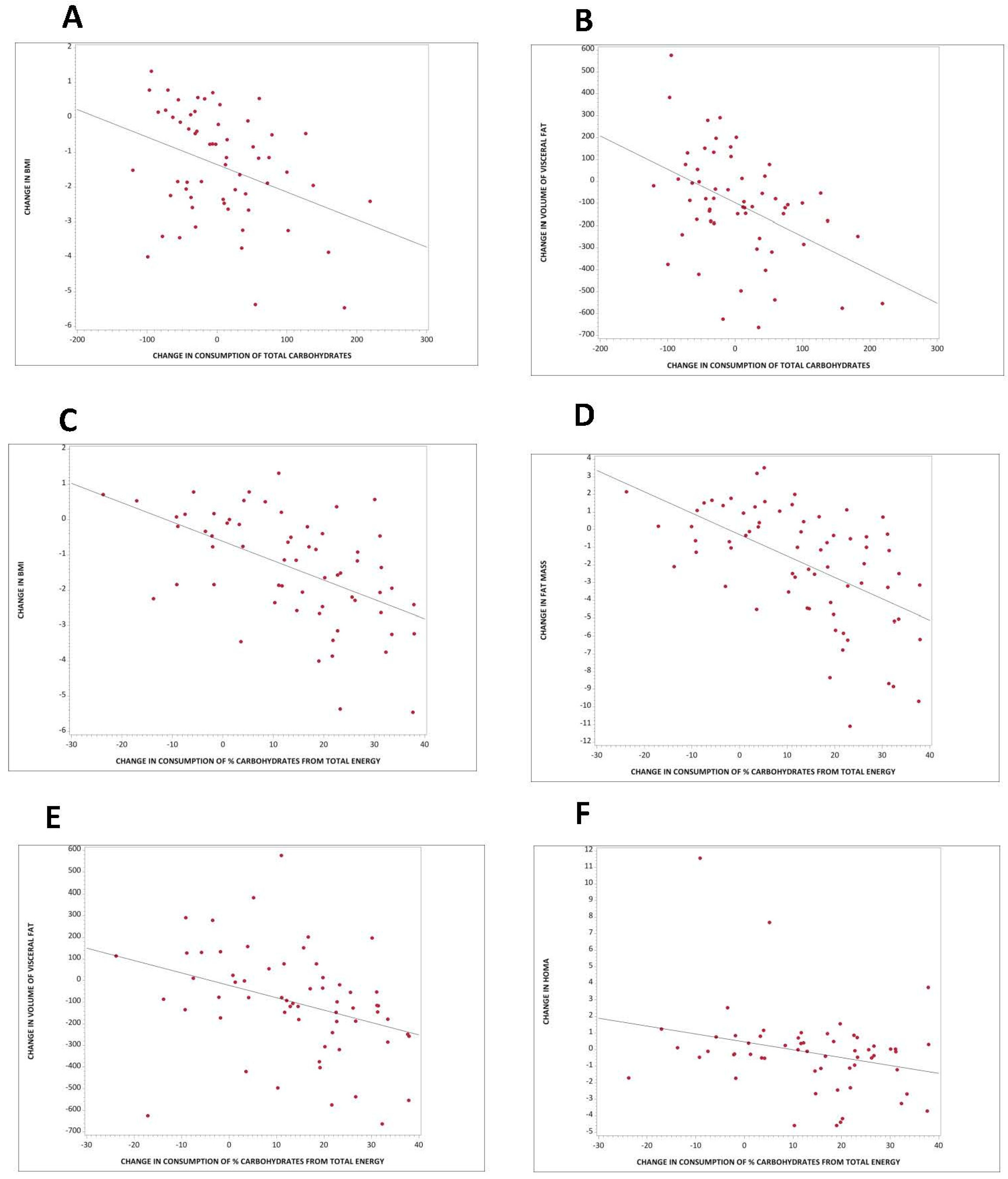
3.4. Association between Carbohydrate Quantity and Body Composition and Insulin Resistance
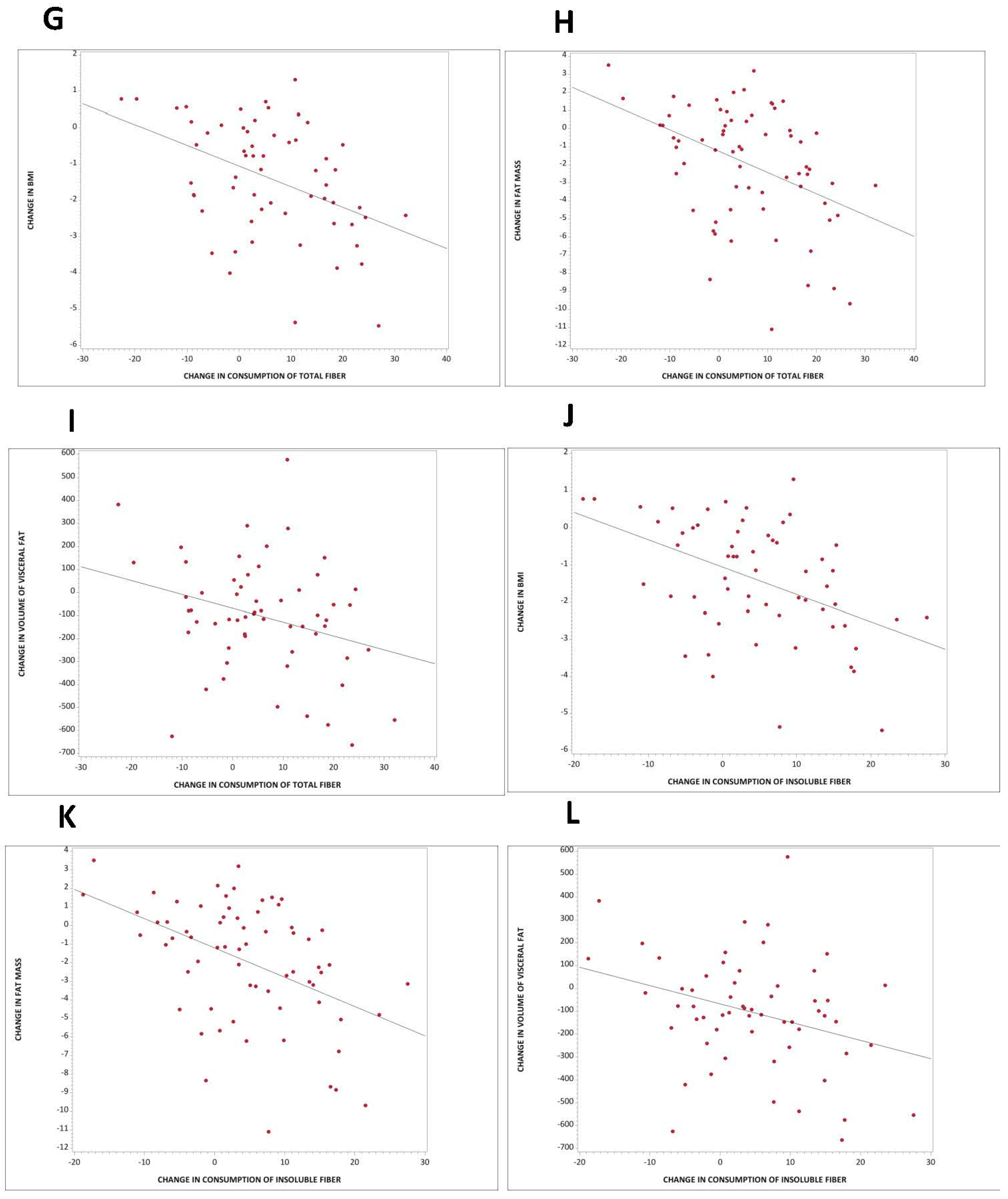
3.5. Association between Carbohydrate Quality and Body Composition and Insulin Resistance
4. Discussion
4.1. Main Findings
4.2. Carbohydrate Quantity in Weight Regulation, Body Composition, and Insulin Resistance
4.3. Dietary Fiber in Weight Regulation, Body Composition, and Insulin Resistance
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. Burden of Disease Collaborators The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013, 113, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E. Vegetarian diets: What do we know of their effects on common chronic diseases? Am. J. Clin. Nutr. 2009, 89, 1607S–1612S. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the academy of nutrition and dietetics: Vegetarian diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Pol, K.; Christensen, R.; Bartels, E.M.; Raben, A.; Tetens, I.; Kristensen, M. Whole grain and body weight changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2013, 98, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Harland, J.I.; Garton, L.E. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr. 2008, 11, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; de Souza, R.J.; Choo, V.L.; Ha, V.; Cozma, A.I.; Chiavaroli, L.; Mirrahimi, A.; Blanco Mejia, S.; Di Buono, M.; Bernstein, A.M.; et al. Effects of dietary pulse consumption on body weight: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2016, 103, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Mukamal, K.J.; Cahill, L.E.; Hou, T.; Ludwig, D.S.; Mozaffarian, D.; Willett, W.C.; Hu, F.B.; Rimm, E.B. Changes in intake of fruits and vegetables and weight change in United States men and women followed for up to 24 years: Analysis from three prospective cohort studies. PLoS Med. 2015, 12, e1001878. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Batterham, M.J.; Thorne, R.L.; O’Shea, J.E.; Grafenauer, S.J.; Probst, Y.C. Weight loss effects from vegetable intake: A 12-month randomised controlled trial. Eur. J. Clin. Nutr. 2014, 68, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.E.; Elliott, E.J.; Baur, L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst. Rev. 2007, CD005105. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Schakel, S.F.; Sievert, Y.A.; Buzzard, I.M. Sources of data for developing and maintaining a nutrient database. J. Am. Diet. Assoc. 1988, 88, 1268–1271. [Google Scholar] [PubMed]
- Barnard, N.D.; Levin, S.M.; Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet. 2015, 115, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Scialli, A.R.; Turner-McGrievy, G.; Lanou, A.J.; Glass, J. The effects of a low-fat, plant-based dietary intervention on body weight, metabolism, and insulin sensitivity. Am. J. Med. 2005, 118, 991–997. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Thomas, L.E.; McDougall, C.; Moloney, G.; Saul, B.; Finnell, J.S.; Richardson, K.; Petersen, K.M. Effects of 7 days on an ad libitum low-fat vegan diet: The McDougall Program cohort. Nutr. J. 2014, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Ryan, L.; Grunwald, G.K.; Storgaard, M.; Saris, W.; Melanson, E.; Hill, J.O. The role of dietary fat in body fatness: Evidence from a preliminary meta-analysis of ad libitum low-fat dietary intervention studies. Br. J. Nutr. 2000, 83, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef] [PubMed]
- Toubro, S.; Astrup, A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: Ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997, 314, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Cunningham, W.M.; Quintana, R.; Kasim-Karakas, S.E. An ad libitum, very low-fat diet results in weight loss and changes in nutrient intakes in postmenopausal women. J. Am. Diet. Assoc. 2003, 103, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Jakše, B.; Pinter, S.; Jakše, B.; Bučar Pajek, M.; Pajek, J. Effects of an Ad Libitum Consumed Low-Fat Plant-Based Diet Supplemented with Plant-Based Meal Replacements on Body Composition Indices. BioMed Res. Int. 2017, 2017, 9626390. [Google Scholar] [CrossRef] [PubMed]
- Gaesser, G.A. Carbohydrate quantity and quality in relation to body mass index. J. Am. Diet. Assoc. 2007, 107, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Faerch, K.; Glümer, C.; Tetens, I.; Pedersen, O.; Carstensen, B.; Jørgensen, T.; Borch-Johnsen, K. Inter99 study Dietary glycemic index, glycemic load, fiber, simple sugars, and insulin resistance: The Inter99 study. Diabetes Care 2005, 28, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Willett, W.C.; Manson, J.E.; Hu, F.B.; Rosner, B.; Colditz, G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am. J. Clin. Nutr. 2003, 78, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Koh-Banerjee, P.; Franz, M.; Sampson, L.; Liu, S.; Jacobs, D.R.; Spiegelman, D.; Willett, W.; Rimm, E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am. J. Clin. Nutr. 2004, 80, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Samra, R.A.; Anderson, G.H. Insoluble cereal fiber reduces appetite and short-term food intake and glycemic response to food consumed 75 min later by healthy men. Am. J. Clin. Nutr. 2007, 86, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Raben, A.; Macdonald, I.; Astrup, A. Replacement of dietary fat by sucrose or starch: Effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1997, 21, 846–859. [Google Scholar] [CrossRef]
- Saris, W.H.; Astrup, A.; Prentice, A.M.; Zunft, H.J.; Formiguera, X.; Verboeket-van de Venne, W.P.; Raben, A.; Poppitt, S.D.; Seppelt, B.; Johnston, S.; et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The CARMEN study. The Carbohydrate Ratio Management in European National diets. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2000, 24, 1310–1318. [Google Scholar] [CrossRef]
- Scagliusi, F.B.; Ferriolli, E.; Pfrimer, K.; Laureano, C.; Cunha, C.S.F.; Gualano, B.; Lourenço, B.H.; Lancha, A.H. Characteristics of women who frequently under report their energy intake: A doubly labelled water study. Eur. J. Clin. Nutr. 2009, 63, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
| n = 75 | |
|---|---|
| Age (years) | 53.2 ± 12.6 |
| Sex (number, %) | |
| Male | 8 (11%) |
| Female | 67 (89%) |
| Race (number, %) | |
| White | 34 (45%) |
| Black | 34 (45%) |
| Asian, Pacific Islander | 4 (5%) |
| Native American | 2 (3%) |
| N/A—did not disclose | 1 (1%) |
| Ethnicity (number, %) | |
| Non-hispanic | 64 (85%) |
| Hispanic | 6 (8%) |
| N/A—did not disclose | 5 (7%) |
| Education | |
| College | 37 (49%) |
| Graduate degree | 37 (49%) |
| NA | 1 (1%) |
| Medications | |
| Lipid-lowering therapy (%) | 9 (12%) |
| Antihypertensive therapy (%) | 18 (24%) |
| Thyroid medications (%) | 9 (12%) |
| Activity and Diet | Control Group | Vegan Group | Treatment Effect | p-Value | ||
|---|---|---|---|---|---|---|
| Baseline | Week 16 | Baseline | Week 16 | |||
| Physical activity (METs) | 2642 (1476–3809) | 2575 (1169–3980) | 2207 (1444–2969) | 2490 (1586–3395) | +351 (−1143 to +1846) | 0.46 |
| Caloric intake (kcal.day−1) | 1923 (1627–2219) | 1582 (1368–1795) ** | 1851 (1695–2007) | 1450 (1249–1652) *** | −60 (−352 to +233) | 0.69 |
| Carbohydrates (% of daily energy) | 45.5 (42.6–48.4) | 46.6 (42.9–50.4) | 46.1 (43.5–48.8) | 69.6 (67.3–71.8) *** | +22.3 (+17.7 to +26.9) | <0.001 |
| Fats (% of daily energy) | 35.6 (32.3–38.9) | 35.0 (31.5–38.4) | 36.1 (34.0–38.1) | 17.5 (15.5–19.4) *** | −17.9 (−22.3 to -13.6) | <0.001 |
| Proteins (% of daily energy) | 16.0 (14.94–17.07) | 16.99 (15.45–18.52) | 16.77 (15.36–18.19) | 12.26 (11.26–13.25) *** | −5.50 (−7.90 to -3.11) | <0.001 |
| Cholesterol (mg/day) | 290 (220–360) | 212 (149–275) | 264 (213–315) | 6.5 (2.5–10.5) *** | −180 (−278 to −82) | <0.001 |
| Total carbohydrates (g/day) | 215 (186–244) | 187 (161–212) | 217 (198–236) | 260 (222–298) * | +70.6 (+28.8 to +115) | 0.001 |
| Total fiber (g/day) | 25.2 (20.9–29.6) | 23.5 (19.6–27.4) | 24.2 (21.0–27.4) | 37.8 (31.4–44.1) *** | +15.3 (+8.0 to +22.6) | <0.001 |
| Soluble fiber (g/day) | 6.42 (5.52–7.31) | 6.80 (5.81–7.79) | 7.02 (6.09–7.96) | 9.87 (7.34–12.4) * | +2.46 (−0.13 to +5.05) | 0.06 |
| Insoluble fiber (g/day) | 18.5 (14.7–22.3) | 16.7 (13.5–19.8) | 17.1 (14.6–19.6) | 27.7 (23.6–31.7) *** | +12.5 (+7.16 to +17.7) | <0.001 |
| Glycemic index | 58.1 (56.2–59.9) | 57.4 (55.3–59.5) * | 57.7 (55.5–59.9) | 54.4 (53.4–55.5) ** | −2.6 (−5.7 to +0.5) | 0.10 |
| Starch (g/day) | 91.6 (78.6–105) | 70.8 (59.2–82.4) ** | 95.6 (82.7–109) | 125 (102–147) ** | +49.6 (+24.9 to +74.2) | <0.001 |
| Sucrose (g/day) | 36.8 (28.6–44.9) | 32.9 (25.5–40.3) | 36.8 (28.2–45.4) | 30.4 (25.9–34.9) | −2.58 (−14.6 to +9.48) | 0.67 |
| Glucose (g/day) | 18.2 (13.5–22.9) | 17.2 (13.1–21.3) | 15.7 (13.4–18.0) | 20.6 (17.3–23.9) * | +5.86 (+0.53 to +11.2) | 0.032 |
| Fructose (g/day) | 18.1 (13.0–23.3) | 17.5 (13.2–21.8) | 17.5 (14.4–20.6) | 22.8 (19.4–26.1) * | +5.94 (−0.49 to +12.4) | 0.07 |
| Lactose (g/day) | 5.99 (3.99–8.00) | 6.17 (3.66–8.68) | 7.18 (4.93–9.43) | 0.56 (0.14–0.98) *** | −6.80 (−10.1 to −3.55) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 10, 1302. https://doi.org/10.3390/nu10091302
Kahleova H, Dort S, Holubkov R, Barnard ND. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients. 2018; 10(9):1302. https://doi.org/10.3390/nu10091302
Chicago/Turabian StyleKahleova, Hana, Sara Dort, Richard Holubkov, and Neal D. Barnard. 2018. "A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates" Nutrients 10, no. 9: 1302. https://doi.org/10.3390/nu10091302
APA StyleKahleova, H., Dort, S., Holubkov, R., & Barnard, N. D. (2018). A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients, 10(9), 1302. https://doi.org/10.3390/nu10091302





