Preventive Effect of Flavonol Derivatives Abundant Sanglan Tea on Long-Term High-Fat-Diet-Induced Obesity Complications in C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ultrahigh-Performance Liquid Chromatography (UHPLC)-Orbitrap-MS Analysis of SLT
2.2. Preparation of Water Extract of SLT
2.3. Experimental Animals and Treatment
2.3.1. Glucose and Insulin Tolerance Test
2.3.2. Collection of Serum and Tissue Samples
2.4. Biochemical Analysis
2.5. Hematoxylin–Eosin (HE) Staining
2.6. Real-Time PCR
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Profiling of SLT
3.2. Quercetin and Kaempferol Derivatives
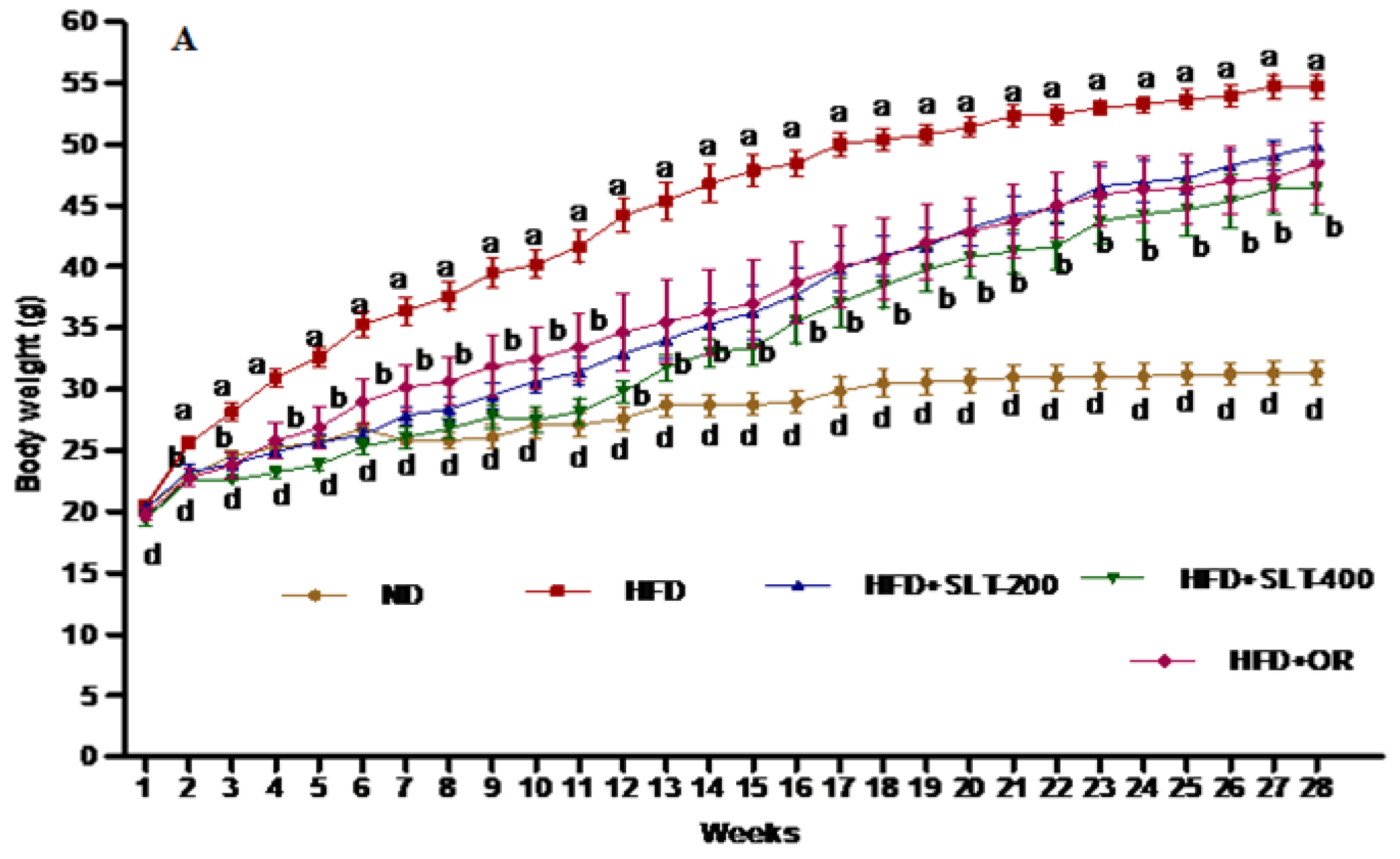
3.3. SLT Prevented Body Weight Gain and Fatty Liver in HFD-Induced C57BL/6 Mice
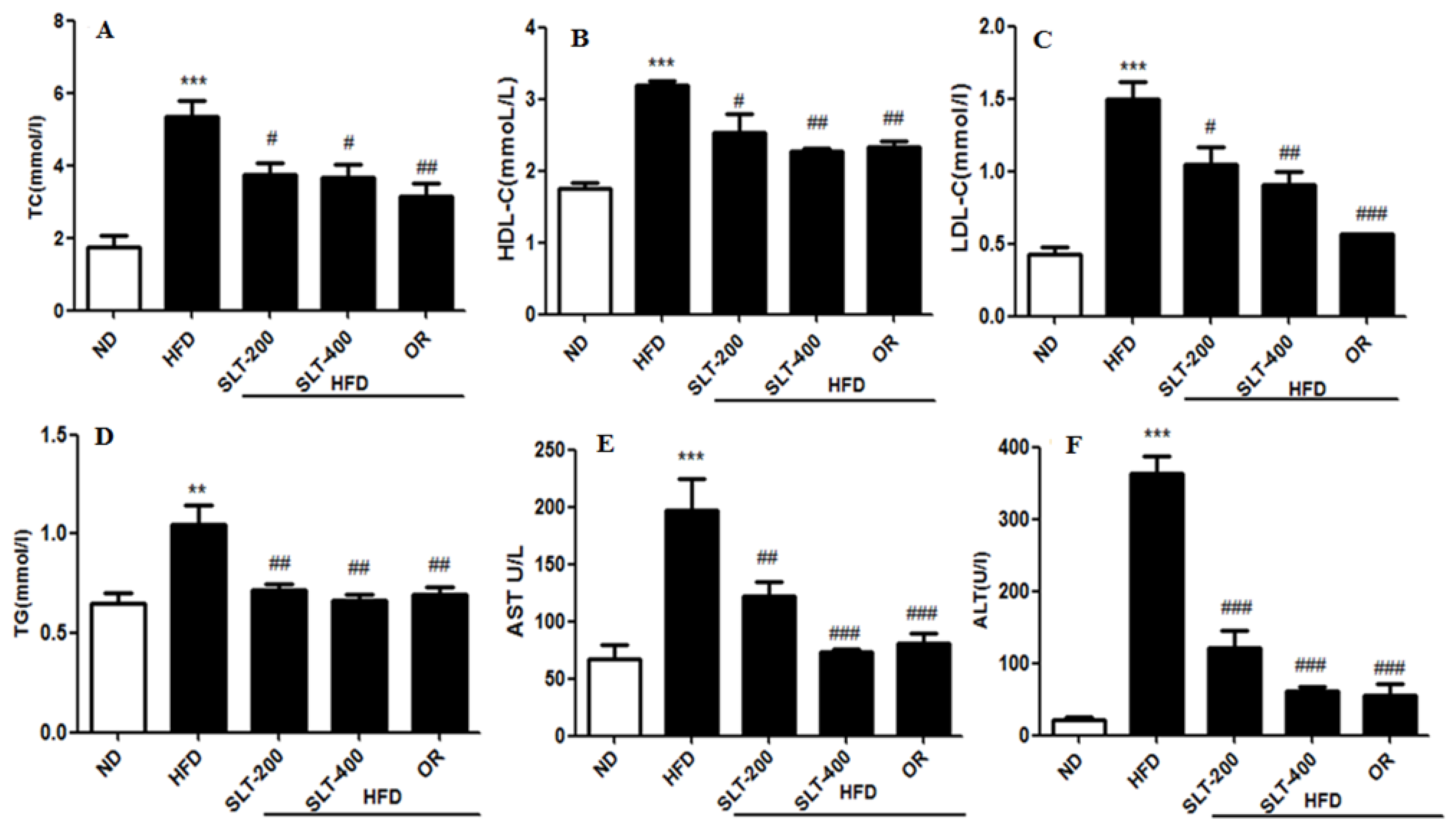
3.4. SLT Prevented Serum Lipid Increase and Hepatic Abnormality in HFD Mice
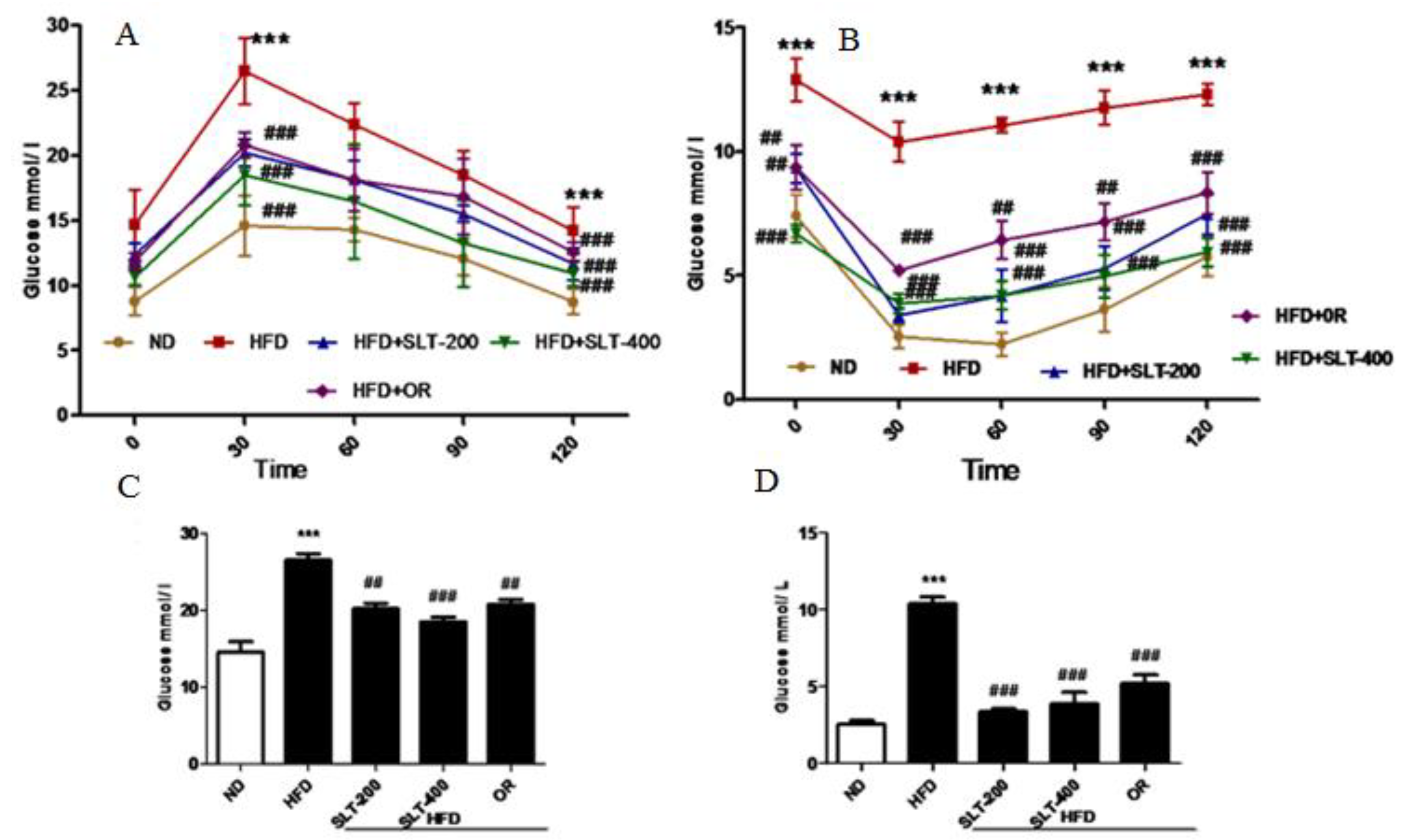
3.5. SLT Ameliorated Elevated Blood Glucose and Insulin Insensitivity in HFD Mice
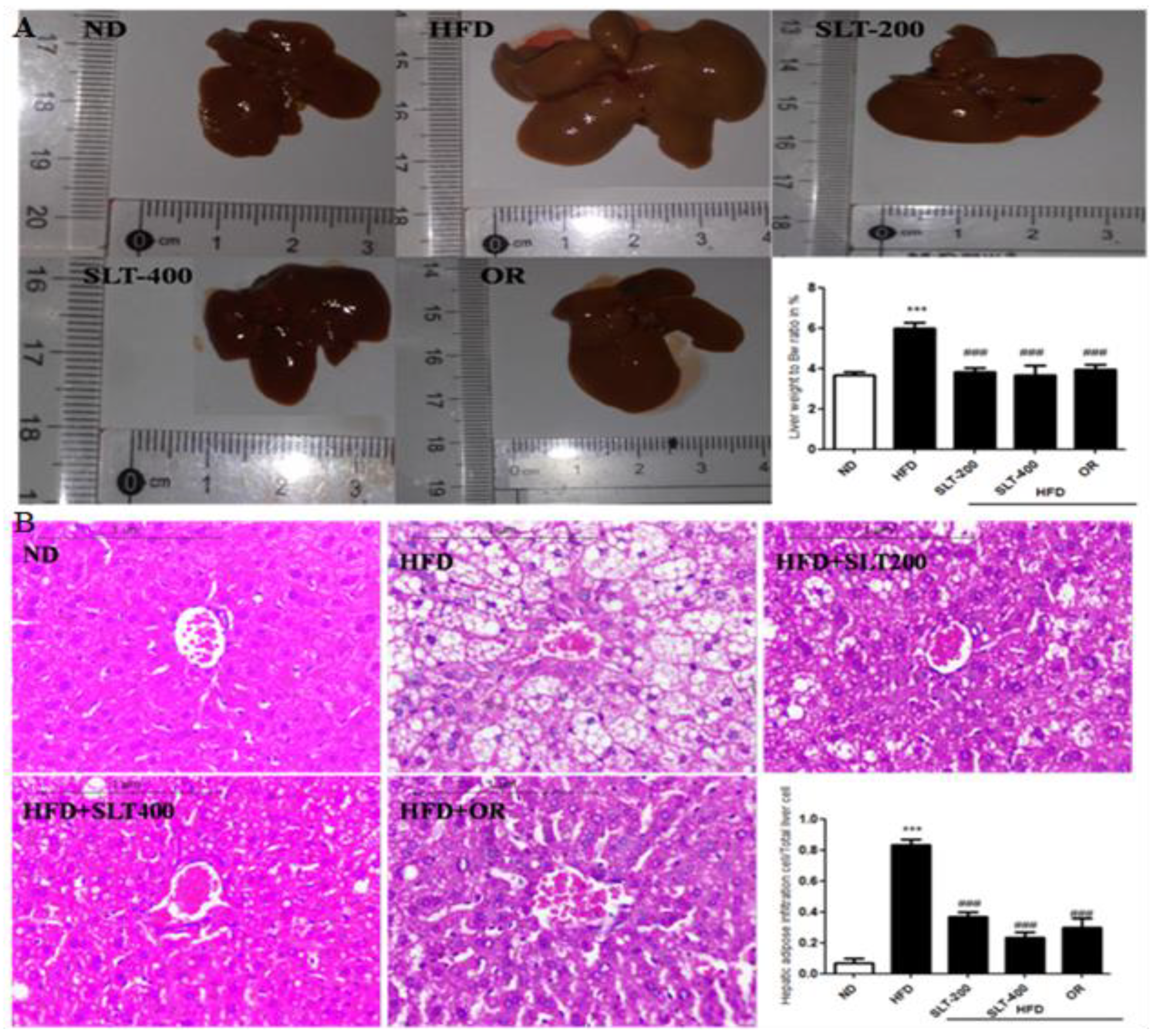
3.6. SLT Protected Fatty Liver Formation in HFD Mice
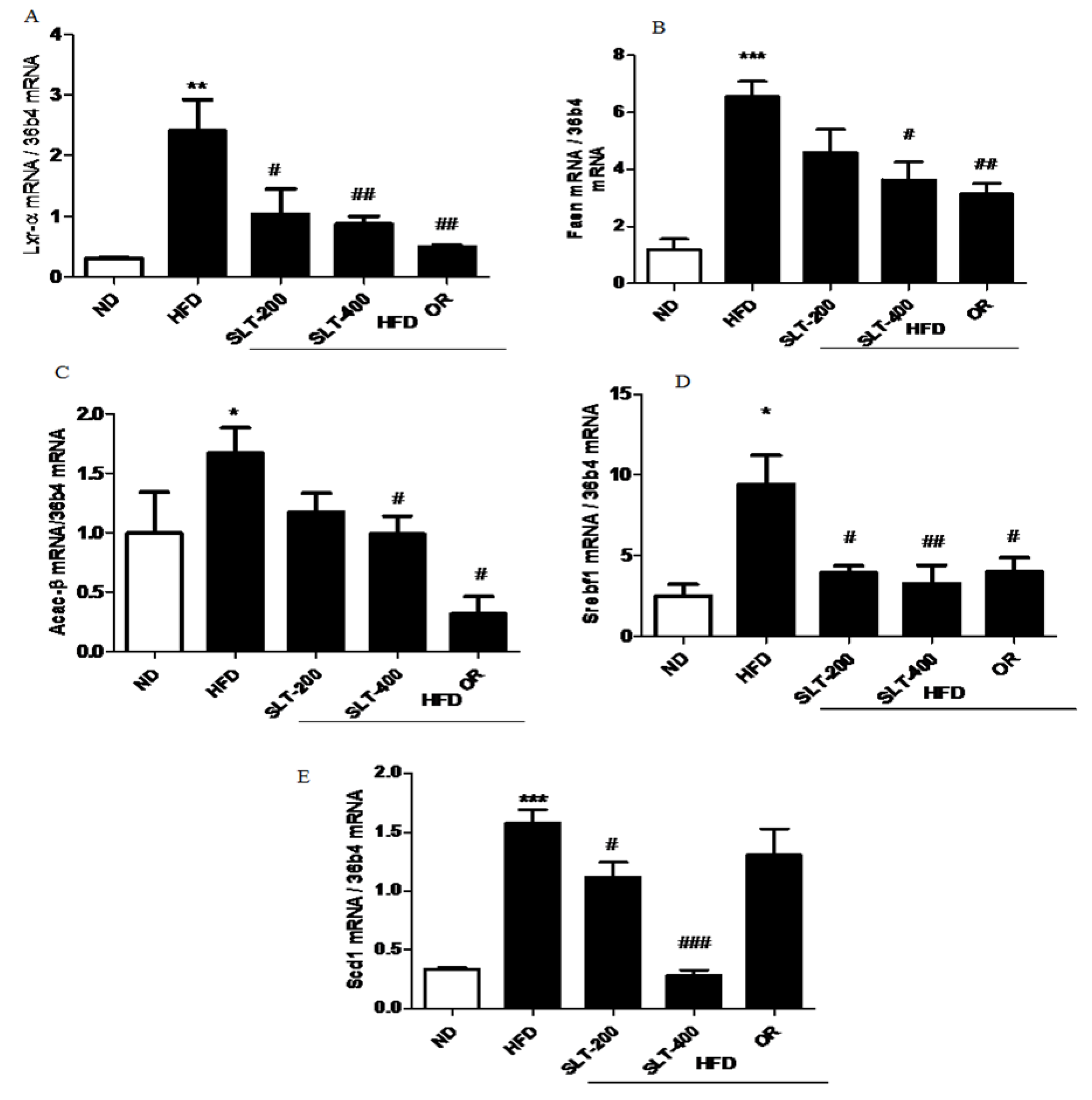
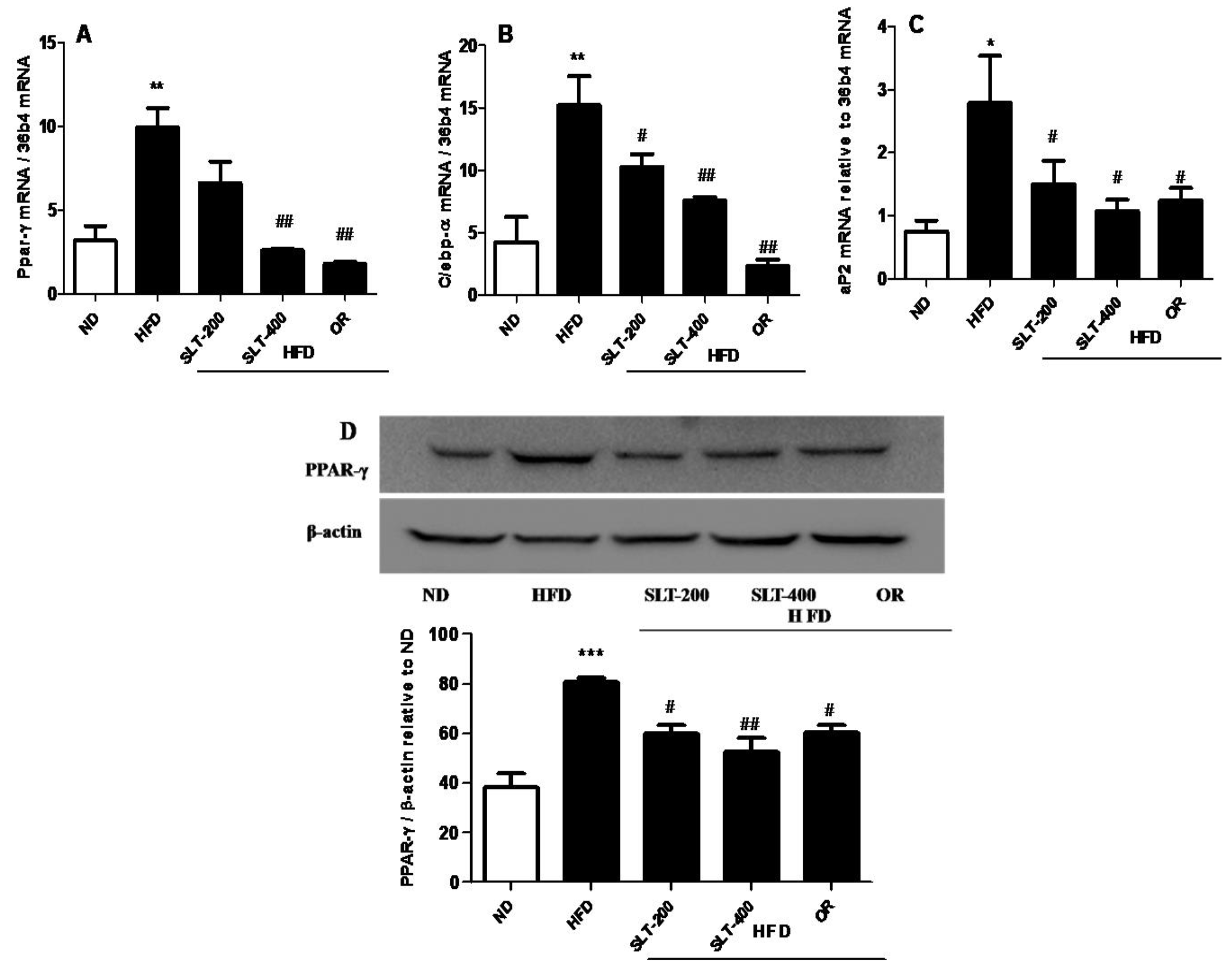
3.7. SLT Prevented Lipogensis and Adipogenesis in the Liver of HFD Mice
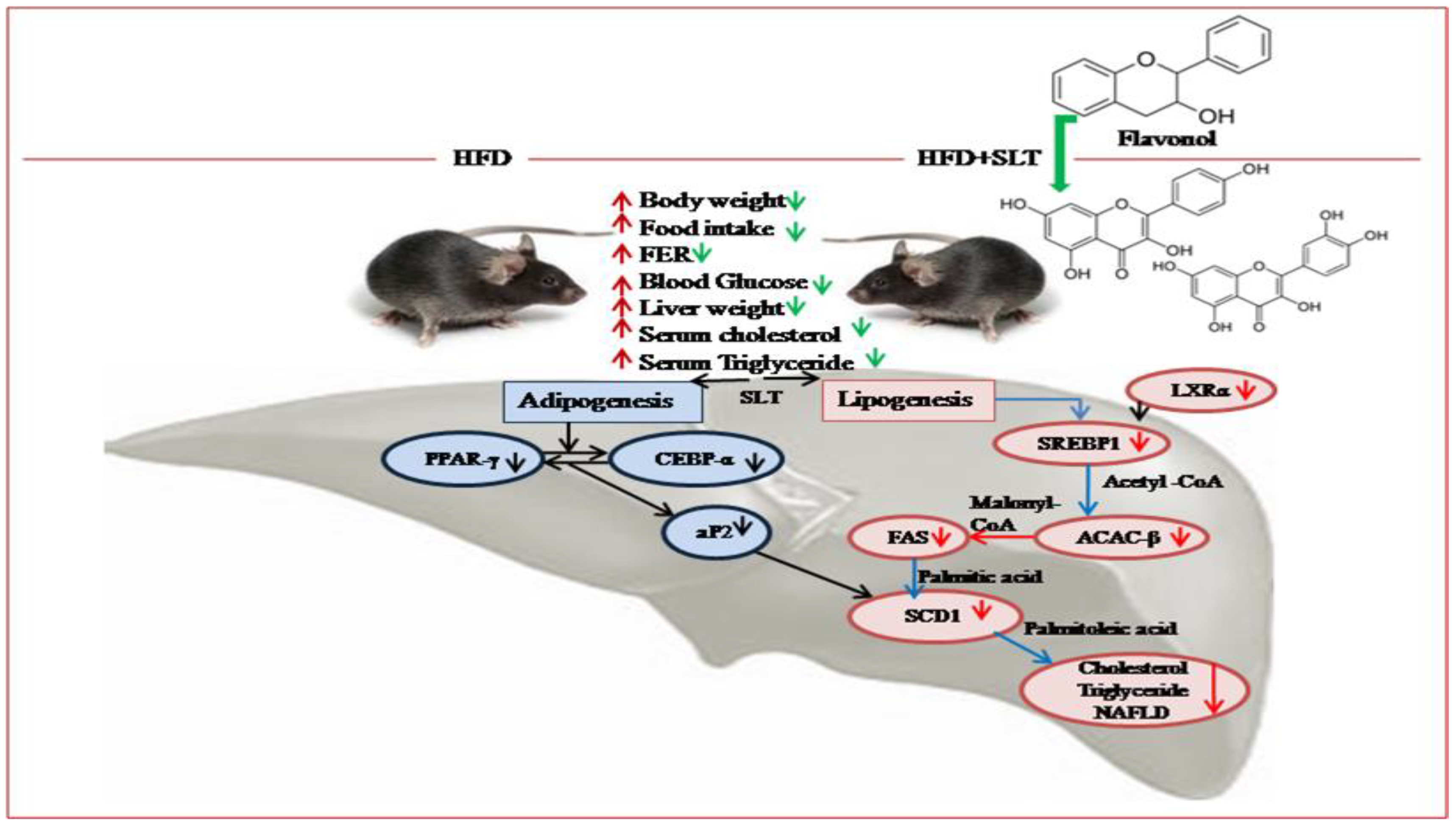
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 14, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Zobel, E.H.; Hansen, T.W.; Rossing, P.; von Scholten, B.J. Global Changes in Food Supply and the Obesity Epidemic. Curr. Obes. Rep. 2016, 5, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systemic review and analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Yadav, M.K.; Bose, S.; Wang, J.H.; Lim, D.; Song, Y.K.; Ko, S.G.; Kim, H. Daesiho-Tang Is an Effective Herbal Formulation in Attenuation of Obesity in Mice through Alteration of Gene Expression and Modulation of Intestinal Microbiota. PLoS ONE 2016, 11, e0165483. [Google Scholar] [CrossRef] [PubMed]
- Razmovski-Naumovski, V.; Huang, T.H.W.; Tran, V.H.; Li, G.Q.; Duke, C.C. Chemistry and pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 14, 197–219. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Xu, B.; Zeng, G.; Tan, J.; He, X.; Hu, C.; Zhou, Y. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia 2014, 98, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, G.; He, Y.; Xu, B.; Mi, X.; Wang, H.; Wang, Z. Pronuciferine and nuciferine inhibit lipogenesis in 3T3-L1 adipocytes by activating the AMPK signaling pathway. Life Sci. 2015, 1, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.W.; Lee, C.; Jin, Q.; Lee, M.K.; Lee, C.K.; Lee, M.K.; Hwang, B.Y. Flavonol glycosides from the aerial parts of Gynostemma pentaphyllum and their antioxidant activity. Arch. Pharm. Res. 2016, 39, 1232–1236. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Tran, V.H.; Roufogalis, B.D.; Li, Y. Gypenoside XLIX, a naturally occurring PPAR-alpha activator, inhibits cytokine-induced vascular cell adhesion molecule-1 expression and activity in human endothelial cells. Eur. J. Clin. Pharmacol. 2007, 565, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kim, J.H.; Jang, Y.S.; Kim, C.H.; Lee, J.Y.; Lim, S.S. Anti-obesity effect of Solidago virgaurea var. gigantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mice (C57BL/6N). Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Cai, Z.; Song, L.; Liu, Y.; Wang, Q.; Feng, X. Gynostemma pentaphyllum attenuates the progression of nonalcoholic fatty liver disease in mice: A biomedical investigation integrated with in silico assay. Evid. Based Complement. Altern. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.F.; Liu, L.H.; Zu, M.L.; Ding, X.F.; Cui, W.Y.; Chang, T.; Piao, X.L. the inhibitory effect of gypenoside stereoisomers, gypenoside L and gypenoside LI, isolated from Gynostemma pentaphyllum on the growth of human lung cancer A549 cells. J. Ethnopharmacol. 2018, 219, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Zong, B.; Yuan, H.; Wang, Z.; Wei, Y.; Wang, X.; Liu, G.; Zhang, J.; Li, S.; et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J. Cell. Mol. Med. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kwon, M.; Choi, J.S.; Jeong, H.O.; Chung, H.Y.; Kim, H.R. Kaempferol isolated from Nelumbo nucifera inhibits lipid accumulation and increases fatty acid oxidation signaling in adipocytes. J. Med. Food 2015, 18, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through PPARα/PGC1α pathway. Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)-phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Tassotti, M.; Del Rio, D.; Hernández, F.; Martínez, J.J.; Mena, P. (Poly) phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC–MS approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Liktor-Busa, E.; Márki, A.; Martins, A.; Jedlinszki, N.; Hsieh, T.J.; Bathori, M.; Hohmann, J.; Zupko, I. Metabolic effects of mulberry leaves: Exploring potential benefits in type 2 diabetes and hyperuricemia. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, D.; Huang, B.; Chen, Y.X.; Lu, X.C.; Wang, Y.W. Inhibition of pancreatic lipase, -glucosidase, -amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J. Ethnopharmacol. 2013, 149, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.I.; Ha, T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hwang, S.H.; Kim, J.H.; Lim, S.S. Anti-Obesity Effect of the Above-Ground Part of Valeriana dageletiana Nakai ex F. Maek Extract in High-Fat Diet-Induced Obese C57BL/6N Mice. Nutrients 2017, 2, 689. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.A.; Hoang, M.H.; Jia, Y.; Lee, J.H.; Jun, H.J.; Lee, D.H.; Lee, H.J.; Lee, C.; Lee, M.K.; Hwang, B.Y.; et al. Ombuin-3-O-β-D-glucopyranoside from Gynostemma pentaphyllum is a dual agonistic ligand of peroxisome proliferator-activated receptors α and δ/β. Biochem. Biophys. Res. Commun. 2013, 430, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Flaczyk, E.; Jeszka, J.; Krejpcio, Z.; Król, E.; Buchowski, M.S. Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (STZ)-induced diabetic rats fed high-fat diet. J. Funct. Foods 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Ono, Y.; Hattori, E.; Fukaya, Y.; Imai, S.; Ohizumi, Y. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J. Ethnopharmacol. 2006, 106, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Horžić, D.; Jambrak, A.R.; Belščak-Cvitanović, A.; Draženka, K.; Vesna, L. Comparison of Conventional and Ultrasound Assisted Extraction Techniques of Yellow Tea and Bioactive Composition of Obtained Extracts. Food Bioprocess Technol. 2012, 5, 2858–2870. [Google Scholar] [CrossRef]
- Tian, L.; Zeng, K.; Shao, W.; Yang, B.B.; Fantus, I.G.; Weng, J.; Jin, T. Short-Term Curcumin Gavage Sensitizes Insulin Signaling in Dexamethasone-Treated C57BL/6 mice. J. Nutr. 2015, 145, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Koh, Y.; Han, J.; Kim, D.; Kim, T.; Kang, H. Adiponectin mediates the additive effects of combining daily exercise with caloric restriction for treatment of non-alcoholic fatty liver. Int. J. Obes. 2016, 40, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Gong, M.C.; Su, W.; Turk, J.; Guo, Z. Group VIA phospholipase A2 (iPLA2β) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J. Biol. Chem. 2007, 282, 25278–25289. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, D.; Liu, S.; Calderon, L.; Zhao, G.; Turk, J.; Guo, Z. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key Cis-regulatory element for RGS2 transcriptional regulation by angiontensin II in cultured vascular smooth muscles. J. Biol. Chem. 2011, 286, 44646–44658. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Su, W.; Guo, Z.; Pang, H.; Post, S.R.; Gong, M.C. Up-regulation of CPI-17 phosphorylation in diabetic vasculature and high glucose cultured vascular smooth muscle cells. Cardiovasc. Res. 2006, 69, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Su, W.; Liu, S.; Zhao, G.; Esser, K.; Schroder, E.A.; Lefta, M.; Stauss, H.M.; Guo, Z.; Gong, M.C. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J. Clin. Investig. 2015, 125, 324–336. [Google Scholar] [CrossRef] [PubMed]
- HMDB (The Human Metabolome Database). Available online: http://www.hmdb.ca/ (accessed on 23 October 2017).
- GNPS (The Global Natural Product Social Molecular Networking). Available online: http://gnps.ucsd.edu (accessed on 23 October 2017).
- TCMSP (Traditional Chinese Medicine Systems Pharmacology and analysis platform. Available online: http://sm.nwsuaf.edu.cn/lsp/tcmsp.php (accessed on 23 October 2017).
- Jiménez-López, J.; Ruiz-Medina, A.; Ortega-Barrales, P.; Llorent-Martínez, E.J. Phytochemical profile and antioxidant activity of caper berries (Capparis spinosa L.): Evaluation of the influence of the fermentation process. Food Chem. 2017, 250, 54–59. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Qi, J.; Yu, B. Simultaneous qualitative and quantitative analysis of flavonoids and alkaloids from the leaves of Nelumbo nucifera Gaertn. using high-performanceliquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, K.; Haluzik, M.; Lambert, G.; Yim, S.H.; Gavrilova, O.; Ward, J.M.; Brewer, B. Jr.; Reitman, M.L.; Gonzalez, F.J. Liver-specific disruption of PPAR_in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J. Clin. Investig. 2003, 111, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Matsusue, K.; Kashireddy, P.; Cao, W.Q.; Yeldandi, V.; Yeldandi, A.V.; Rao, M.S.; Gonzalez, F.J.; Reddy, J.K. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor -1 (PPAR-1) over expression. J. Biol. Chem. 2003, 278, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Pang, Z.R.; Pan, M.R.; Zhang, W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 Mice. Pharm. Biol. 2017, 55, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Ten, Y.; Li, D.; Guruvaiah, P.; Xu, N.; Xie, Z. Dietary Supplement of Large Yellow Tea Ameliorates Metabolic Syndrome and Attenuates Hepatic Steatosis in db/db Mice. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Chen, H.L.; Tung, Y.T.; Tsai, C.L.; Lai, C.W.; Lai, Z.L.; Tsai, H.C.; Lin, Y.L.; Wang, C.H.; Chen, C.M. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int. J. Obes. 2014, 38, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Hauptman, J. Orlistat: Selective inhibition of caloric absorption can affect long-term body weight. Endocrine 2000, 13, 201–206. [Google Scholar] [CrossRef]
- Drew, B.S.; Dixon, A.F.; Dixon, J.B. Obesity management: Update on orlistat. Vasc. Health Risk Manag. 2007, 3, 817–821. [Google Scholar] [PubMed]

| Gene Name | Primer Forward (5′–3′) | Primer Reverse (5′–3′) |
|---|---|---|
| 36b4 | CCCTGAAGTGCTCGACATCA | TGCGGACACCCTCCAGAA |
| aP2 | AACACCGAGATTTCCTTCAA | TCACGCCTTTCATAACACAT |
| Acac-β | AGACACTGCAAATCCCAACCTTAC | CTTCGTCCACATCCTTCACACA |
| Cebp-α | AGACATCAAGCGCCTACATCG | TGTAGGTGCATGGTGGTCTG |
| Fasn | CGTGTGACCGCCATCTATATCG | TGAGGTTGCTGTCGTCTGTAGTCTT |
| Lxr-α | CGACAGAGCTTCGTCCACAA | CGTTCCCCAGCATTTTGG |
| Ppar-γ | GAAAGACAACGGACAAATCACCAT | CGGCTTCTACGGATCGAAACTG |
| Scd1 | TCCTCCTTGGATTGTGTAGAAACTT | AATGTCAGAAGAAATCAGGTGGGTA |
| Srebf1 | AGTCCAGCCTTTGAGGATAGCC | CCGTAGCATCAGAGGGAGTGAG |
| ID | tR (min) | a MS (M + H)+/(M − H)− | UPLC-ESI MS Ion Fragments (m/z) | Predicted Formula | Assigned Identification | Substance class |
|---|---|---|---|---|---|---|
| 1 | 3.47 | 272.12/270.11 | 256, 273, 226, 162, 155, 108/162 | C16H17NO3 | Norcoclaurine | Benzylisoquinoline alkaloid |
| 2 | 3.77 | 611.15/609.14 | 303, 85/300, 271 | C26H26 O17 | Quercetin-3-O-arabinose-glucuronide | Flavonoid |
| 3 | 3.77 | 613.54/610.52 | 303/301, 300 | C27H32O16 | Quercetin-3-O-rhamnoside-glucoside | Flavonoid |
| 4 | 3.85 | 479.08/477.06 | 303/301 | C21H18O13 | Quercetin-3-O-glucuronide | Flavonoid |
| 5 | 3.85 | 465.10/463.08 | 303/301 | C21H20O12 | Quercetin-3-O-galactoside | Flavonoid |
| 6 | 3.85 | 303.18/301.03 | 303.18, 237.10, 171.01/301 | C15H10O7 | Quercetin | Flavonoid |
| b MS (M + H)+ | ||||||
| 7 | 0.87 | 148.98 | 148.98, 130.97, 1125.96 | C5H9NO4 | Glutamine | Amino acid |
| 8 | 1.37 | 276.14 | 259, 230 | C17H9NO3 | Liriodenine | Aporphine alkaloid |
| 9 | 3.69 | 611.31 | 611.31, 489.24, 206.12 | C27H30O16 | Rutin | Flavonoid |
| 10 | 3.77 | 328.15 | 329, 314, 313, 298, | C20H25NO3 | O-Methylarmepavine | Benzylisoquinoline alkaloid |
| 11 | 3.82 | 314.17 | 314, 283, 268 | C19H24NO3 | Lotusine | Benzylisoquinoline alkaloid |
| 12 | 4.17 | 266.11 | 249,219 | C17H15NO2 | Anonaine | Aporphine alkaloid |
| 13 | 4.23 | 296.16 | 265, 250, 235 | C19H21NO2 | Nuciferine | Aporphine alkaloid |
| 14 | 5.44 | 331.19 | 331.19 | C17H14O7 | Rhamnazin | Flavonoid |
| c MS (M − H) | ||||||
| 15 | 3.50 | 577.13 | 289, 175 | C30H26O12 | Procyanidin B | Flavonoid |
| 16 | 3.72 | 353.19 | 191, 173, 179 | C16H18O9 | 4-Caffeoylquinic acid | Cryptochlorogenic acid |
| 17 | 3.75 | 595.13 | 301 | C26H28O16 | Quercetin-3-O-arabinose-galactoside | Flavonoid |
| 18 | 3.77 | 609.14 | 300, 271, 255, 301 | C27H30O16 | Quercetinrutinoside isomer | Flavonoid |
| 19 | 3.77 | 607.50 | 300, 284, 271, 255 | C27H28O16 | Kaempferol-3-O-rhamnoside-galacturonide | Flavonoid |
| 20 | 3.78 | 133.01 | 115, 87, 71 | C4H6O5 | L-Malic acid | Hydroxy acids and derivatives |
| 21 | 3.82 | 593.15 | 284, 255, 227 | C27H30O15 | Kaempferol-3-O-rhamnopyranosyl-(1→6)-glucopyranoside | Flavonoid |
| 22 | 3.85 | 463.08 | 301 | C21H20O12 | Hirsutrin | Flavonoid |
| 23 | 3.85 | 463.08 | 301, 300, 271, 256, 255 | C21H20O12 | Quercetin-3-O-glucopyranoside (isoquercetin) | Flavonoid |
| 24 | 3.93 | 447.13 | 285, 257, 255, 229, 245, 227 | C21H20O11 | Kaempferol 3-O-glucoside (astragalin) | Flavonoid |
| GROUPS | ND | HFD | HFD + SLT 200 | HFD + SLT 400 | HFD + OR |
|---|---|---|---|---|---|
| Body weight gain (g) | 11.05 ± 2.20 a | 34.25 ± 2.41 b | 25.2 ± 3.56 c | 19.52 ± 3.30 d | 23.14 ± 2.043 c |
| Food intake (Kcal/day) | 12.6 ± 0.03 a | 15.0 ± 0.08 b | 14.04 ± 0.1 b | 14.56 ± 0.2 b | 14.56 ± 0.12 b |
| FER | 3.15 ± 0.72 a | 11.81 ± 0.86 b | 9.33 ± 2.43 c | 6.97 ± 3.01 d | 8.26 ± 1.67 c |
| Fasting Glucose (mmol L−1) | 7.6 ± 0.93 a | 13.4 ± 1.42 b | 9.8 ± 0.91 c | 9.2 ± 0.86 c | 10.1 ± 1.04 c |
| Subcutaneous fat mass relative to BW (%) | 2.4 ± 0.55 a | 3.9 ± 0.80 b | 4.0 ± 0.61 b | 4.1 ± 0.54 b | 4.1 ± 0.56 b |
| Liver weight | 1.14 ± 0.13 a | 3.31 ± 0.47 b | 1.94 ± 0.31 a | 1.56 ± 0.26 a | 1.76 ± 0.42 a |
| Liver weight relative to BW (%) | 3.6 ± 0.48 a | 5.9 ± 0.58 b | 3.8 ± 0.47 a | 3.6 ± 0.80 a | 3.9 ± 0.62 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guruvaiah, P.; Guo, H.; Li, D.; Xie, Z. Preventive Effect of Flavonol Derivatives Abundant Sanglan Tea on Long-Term High-Fat-Diet-Induced Obesity Complications in C57BL/6 Mice. Nutrients 2018, 10, 1276. https://doi.org/10.3390/nu10091276
Guruvaiah P, Guo H, Li D, Xie Z. Preventive Effect of Flavonol Derivatives Abundant Sanglan Tea on Long-Term High-Fat-Diet-Induced Obesity Complications in C57BL/6 Mice. Nutrients. 2018; 10(9):1276. https://doi.org/10.3390/nu10091276
Chicago/Turabian StyleGuruvaiah, Ponmari, Huimin Guo, Daxiang Li, and Zhongwen Xie. 2018. "Preventive Effect of Flavonol Derivatives Abundant Sanglan Tea on Long-Term High-Fat-Diet-Induced Obesity Complications in C57BL/6 Mice" Nutrients 10, no. 9: 1276. https://doi.org/10.3390/nu10091276
APA StyleGuruvaiah, P., Guo, H., Li, D., & Xie, Z. (2018). Preventive Effect of Flavonol Derivatives Abundant Sanglan Tea on Long-Term High-Fat-Diet-Induced Obesity Complications in C57BL/6 Mice. Nutrients, 10(9), 1276. https://doi.org/10.3390/nu10091276





