Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation
Abstract
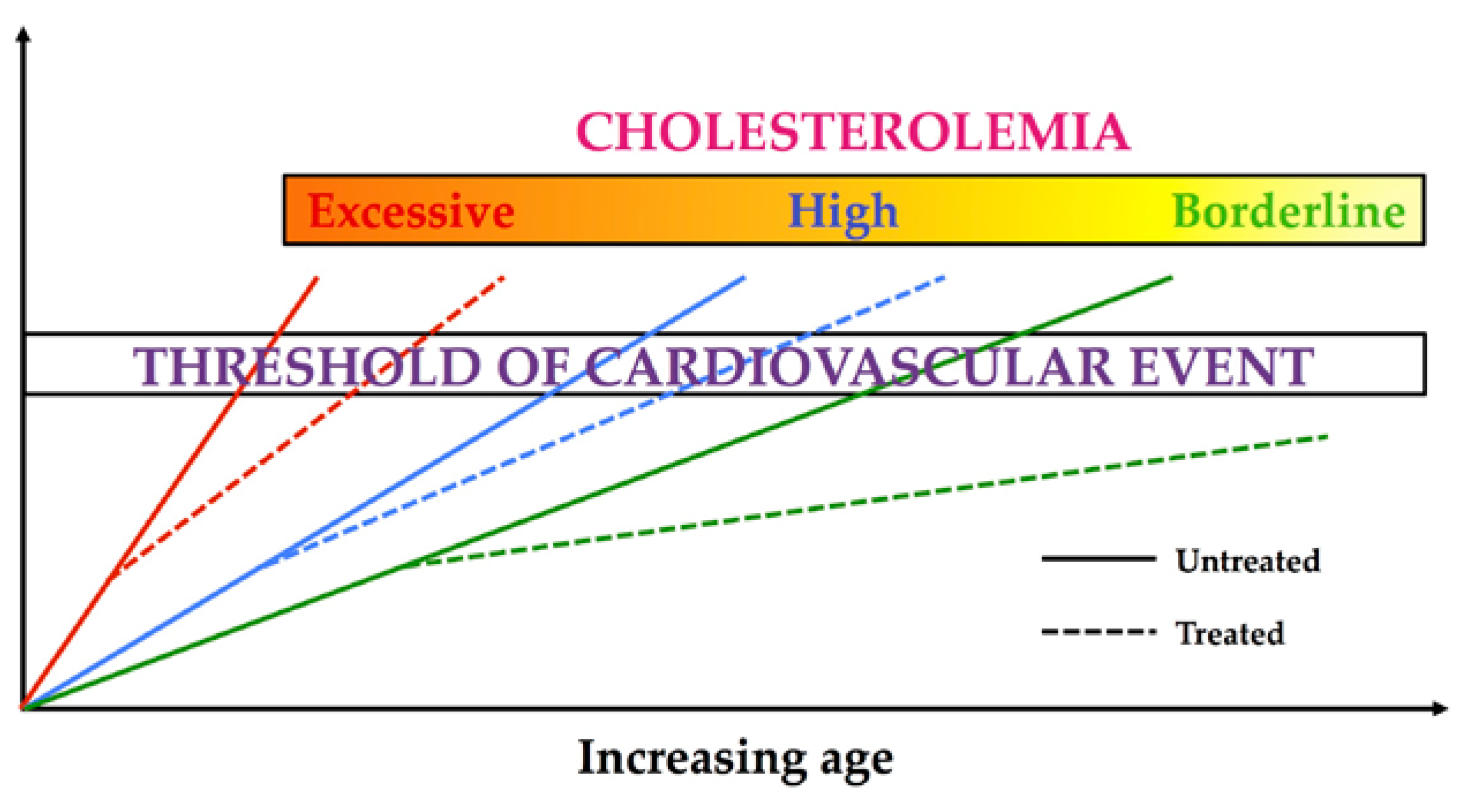
:1. Introduction
2. Plasma Cholesterol Control: From Soy to Hempseed
2.1. Glycine Max
2.1.1. Experimental Evidences
2.1.2. Clinical Studies
2.2. Lupinus
2.2.1. Experimental Evidences
2.2.2. Clinical Studies
2.3. Pisum Sativum L.
2.3.1. Experimental Evidences
2.3.2. Clinical Studies
2.4. Cannabis sativa L.
2.4.1. Experimental Evidences
2.4.2. Clinical Studies
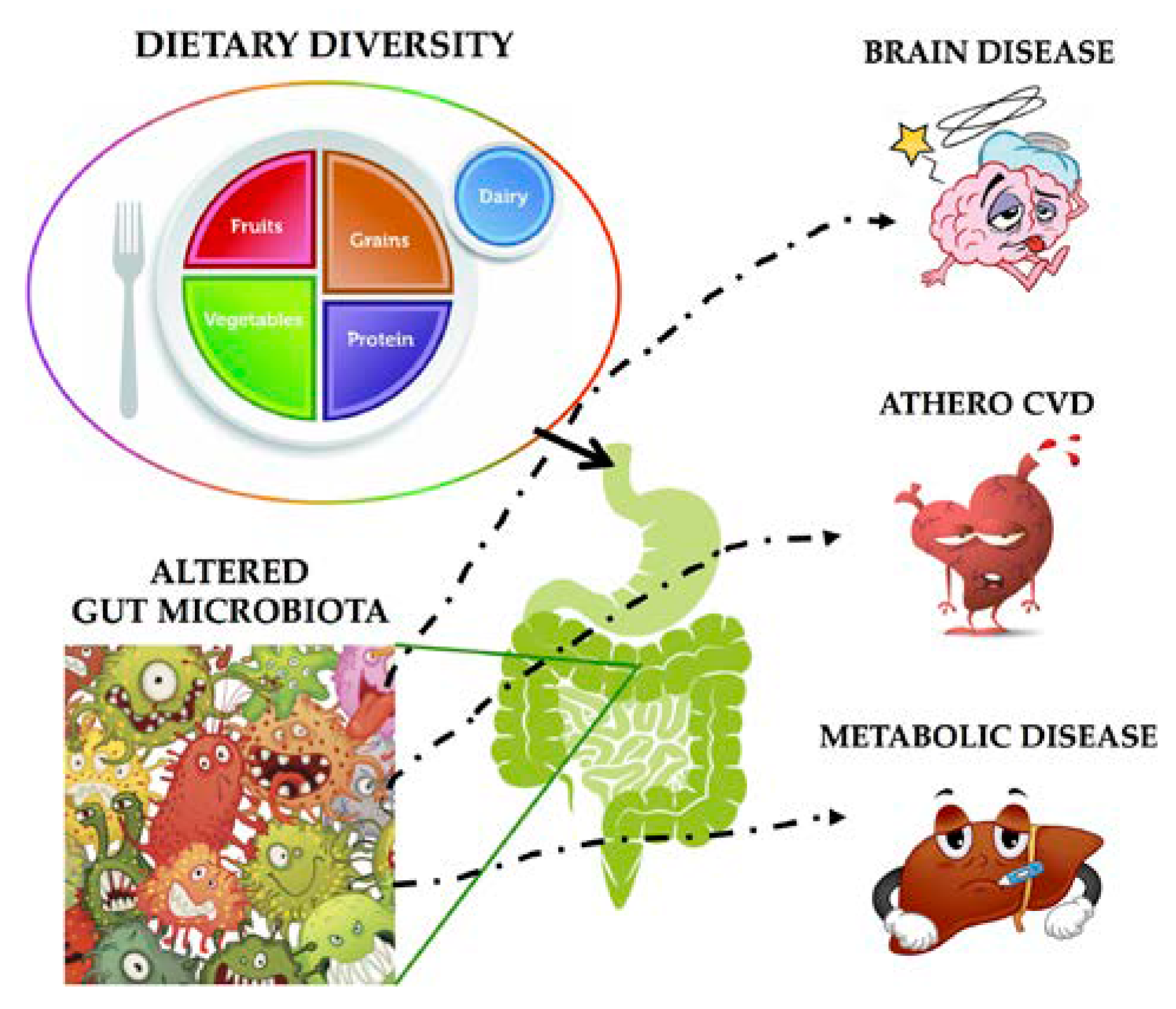
3. Gut Microbioma Modulation: From Soy to Hempseed
3.1. Glycine Max
3.2. Pisum Sativum L.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorresteijn, J.A.; Kaasenbrood, L.; Cook, N.R.; van Kruijsdijk, R.C.; van der Graaf, Y.; Visseren, F.L.; Ridker, P.M. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016, 352, i1548. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.; Robson, J.; Brindle, P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: Cohort study using QResearch database. BMJ 2010, 341, c6624. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. American College of Cardiology/American Heart Association Task Force on Practice, G., 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. JACC 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, N.E.M.; Visseren, F.L.J.; Numans, M.E.; Smulders, Y.M.; van Loenen Martinet, F.A.; van der Graaf, Y.; Dorresteijn, J.A.N. Variation in minimum desired cardiovascular disease-free longevity benefit from statin and antihypertensive medications: A cross-sectional study of patient and primary care physician perspectives. BMJ Open 2018, 8, e021309. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Chiesa, G.; Zhu, Y.; Forte, T.; Caligari, S.; Gianazza, E.; Sacco, M.G.; Sirtori, C.R.; Rubin, E.M. Targeted replacement of mouse apolipoprotein A-I with human ApoA-I or the mutant ApoA-IMilano. Evidence of APOA-IM impaired hepatic secretion. J. Biol. Chem. 2003, 278, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.; Parolini, C.; Valetti, C.; Mangione, P.; Obici, L.; Giorgetti, S.; Raimondi, S.; Donadei, S.; Gregorini, G.; Merlini, G.; et al. The intracellular quality control system down-regulates the secretion of amyloidogenic apolipoprotein A-I variants: A possible impact on the natural history of the disease. Biochim. Biophys. Acta 2011, 1812, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wait, R.; Chiesa, G.; Parolini, C.; Miller, I.; Begum, S.; Brambilla, D.; Galluccio, L.; Ballerio, R.; Eberini, I.; Gianazza, E. Reference maps of mouse serum acute-phase proteins: Changes with LPS-induced inflammation and apolipoprotein A-I and A-II transgenes. Proteomics 2005, 5, 4245–4253. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Chiesa, G.; Gong, E.; Caligari, S.; Cortese, M.M.; Koga, T.; Forte, T.M.; Rubin, E.M. Apolipoprotein A-I and the molecular variant apoA-I(Milano): Evaluation of the antiatherogenic effects in knock-in mouse model. Atherosclerosis 2005, 183, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Marchesi, M.; Lorenzon, P.; Castano, M.; Balconi, E.; Miragoli, L.; Chaabane, L.; Morisetti, A.; Lorusso, V.; Martin, B.J.; et al. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: In vivo assessment by intravascular ultrasound and magnetic resonance imaging. JACC 2008, 51, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Parolini, C.; Sirtori, C.R. Acute effects of high-density lipoproteins: Biochemical basis and clinical findings. Curr. Opin. Cardiol. 2008, 23, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Marchesi, M.; Chiesa, G. HDL therapy for the treatment of cardiovascular diseases. Curr. Vasc. Pharmacol 2009, 7, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Butt, M.S.; Qayyum, M.M.; Suleria, H.A. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, G.; Busnelli, M.; Manzini, S.; Parolini, C. Nutraceuticals and Bioactive Components from Fish for Dyslipidemia and Cardiovascular Risk Reduction. Mar. Drugs 2016, 14. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.S.F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Cinquetti, R.; Badi, I.; Campione, M.; Bortoletto, E.; Chiesa, G.; Parolini, C.; Camesasca, C.; Russo, A.; Taramelli, R.; Acquati, F. Transcriptional deregulation and a missense mutation define ANKRD1 as a candidate gene for total anomalous pulmonary venous return. Hum. Mutat. 2008, 29, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Cinquetti, R.; Frascoli, M.; Parolini, C.; Chiesa, G.; Taramelli, R.; Acquati, F. Intracellular ANKRD1 protein levels are regulated by 26S proteasome-mediated degradation. FEBS Lett. 2009, 583, 2486–2492. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes 2017, 8, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Pierre, J.F.; Chang, E.B. The Gut Microbiota: The Gateway to Improved Metabolism. Gastroenterol. Clin. N. Am. 2016, 45, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.J.; Rees, K.; Ward, K.; Burke, M.; Thorogood, M. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2007, CD002128. [Google Scholar] [CrossRef]
- Rees, K.; Dyakova, M.; Wilson, N.; Ward, K.; Thorogood, M.; Brunner, E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013, CD002128. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Sawler, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The Genetic Structure of Marijuana and Hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef] [PubMed]
- Ramdath, D.D.; Padhi, E.M.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Torre-Villalvazo, I.; Tovar, A.R. Regulation of lipid metabolism by soy protein and its implication in diseases mediated by lipid disorders. J. Nutr. Biochem. 2006, 17, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnaboldi, F.; Busnelli, M.; Cornaghi, L.; Manzini, S.; Parolini, C.; Dellera, F.; Ganzetti, G.S.; Sirtori, C.R.; Donetti, E.; Chiesa, G. High-density lipoprotein deficiency in genetically modified mice deeply affects skin morphology: A structural and ultrastructural study. Exp. Cell Res. 2015, 338, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Galli, G.; Lovati, M.R.; Carrara, P.; Bosisio, E.; Kienle, M.G. Effects of dietary proteins on the regulation of liver lipoprotein receptors in rats. J. Nutr. 1984, 114, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Soma, M.R.; Parolini, C.; Donetti, E.; Fumagalli, R.; Paoletti, R. Inhibition of isoprenoid biosynthesis and arterial smooth-muscle cell proliferation. J. Cardiovasc. Pharmacol. 1995, 25 Suppl 4, S20–S24. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Arnoldi, A.; Kurowska, E.; Carroll, K.K.; Sirtori, C.R. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J. Nutr. 2000, 130, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Butteiger, D.N.; Rains, T.M.; Lawless, A.; Reeves, M.S.; Schasteen, C.; Krul, E.S. Effects of soy protein on lipoprotein lipids and fecal bile acid excretion in men and women with moderate hypercholesterolemia. J. Clin. Lipidol. 2010, 4, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Dellera, F.; Ganzetti, G.S.; Froio, A.; Manzini, S.; Busnelli, M.; Meinitzer, A.; Sirtori, C.R.; Chiesa, G.; Parolini, C. L-homoarginine administration reduces neointimal hyperplasia in balloon-injured rat carotids. J. Thromb. Haemost. 2016, 116, 400–402. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Agradi, E.; Conti, F.; Mantero, O.; Gatti, E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet 1977, 1, 275–277. [Google Scholar] [CrossRef]
- Jokinen, P.; Bruck, A.; Aalto, S.; Forsback, S.; Parkkola, R.; Rinne, J.O. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat. Disord. 2009, 15, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Summary of Health Canada’s Assessment of a Health Claim about Soy Protein and Cholesterol Lowering. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/summary-assessment-health-claim-about-protein-cholesterol-lowering.html (accessed on 11 March 2015).
- Scientific Opinion on the Substantiation of a Health Claim Related to Isolated Soy Protein and Reduction of Blood LDL-Cholesterol Concentrations Pursuant to Article 14 of Regulation (EC) No 1924/2006. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1688 (accessed on 11 March 2015).
- Caligari, S.; Chiesa, G.; Johnson, S.K.; Camisassi, D.; Gilio, D.; Marchesi, M.; Parolini, C.; Rubio, L.A.; Sirtori, C.R. Lupin (Lupinus albus) protein isolate (L-ISO) has adequate nutritional value and reduces large intestinal weight in rats after restricted and ad libitum feeding. Ann. Nutr. Metab. 2006, 50, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Rigamonti, E.; Marchesi, M.; Busnelli, M.; Cinquanta, P.; Manzini, S.; Sirtori, C.R.; Chiesa, G. Cholesterol-lowering effect of dietary Lupinus angustifolius proteins in adult rats through regulation of genes involved in cholesterol homeostasis. Food Chem. 2012, 132, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Manzini, S.; Pinna, C.; Busnelli, M.; Cinquanta, P.; Rigamonti, E.; Ganzetti, G.S.; Dellera, F.; Sala, A.; Calabresi, L.; Franceschini, G.; et al. Beta2-adrenergic activity modulates vascular tone regulation in lecithin:cholesterol acyltransferase knockout mice. Vascul. Pharmacol. 2015, 74, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soma, M.R.; Donetti, E.; Parolini, C.; Barberi, L.; Paoletti, R.; Fumagalli, R.; Catapano, A.L. Effect of lacidipine on the carotid intimal hyperplasia induced by cuff injury. J. Cardiovasc. Pharmacol. 1994, 23 Suppl 5, S71–S74. [Google Scholar] [CrossRef]
- Chiesa, G.; Rigamonti, E.; Monteggia, E.; Parolini, C.; Marchesi, M.; Miragoli, L.; Grotti, A.; Maggioni, F.; Lorusso, V.; Sirtori, C.R. Evaluation of a soft atherosclerotic lesion in the rabbit aorta by an invasive IVUS method versus a non-invasive MRI technology. Atherosclerosis 2004, 174, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.; Parolini, C.; Diani, E.; Rigamonti, E.; Cornelli, L.; Arnoldi, A.; Sirtori, C.R.; Chiesa, G. Hypolipidaemic and anti-atherosclerotic effects of lupin proteins in a rabbit model. Br. J. Nutr. 2008, 100, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Bettzieche, A.; Brandsch, C.; Eder, K.; Stangl, G.I. Lupin protein acts hypocholesterolemic and increases milk fat content in lactating rats by influencing the expression of genes involved in cholesterol homeostasis and triglyceride synthesis. Mol. Nutr. Food Res. 2009, 53, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Vik, R.; Busnelli, M.; Parolini, C.; Bjorndal, B.; Holm, S.; Bohov, P.; Halvorsen, B.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; et al. An immunomodulating fatty acid analogue targeting mitochondria exerts anti-atherosclerotic effect beyond plasma cholesterol-lowering activity in apoe(-/-) mice. PLoS ONE 2013, 8, e81963. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Vik, R.; Busnelli, M.; Bjorndal, B.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Halvorsen, B.; et al. A salmon protein hydrolysate exerts lipid-independent anti-atherosclerotic activity in ApoE-deficient mice. PLoS ONE 2014, 9, e97598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, C.; Busnelli, M.; Ganzetti, G.S.; Dellera, F.; Manzini, S.; Scanziani, E.; Johnson, J.L.; Sirtori, C.R.; Chiesa, G. Magnetic resonance imaging visualization of vulnerable atherosclerotic plaques at the brachiocephalic artery of apolipoprotein E knockout mice by the blood-pool contrast agent B22956/1. Mol. Imaging 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.; Schutkowski, A.; Brandsch, C.; Hirche, F.; Hasenkopf, K.; Stangl, G.I. Isolated Conglutin gamma from Lupin, but not Phytate, Lowers Serum Cholesterol Without Influencing Vascular Lesion Development in the ApoE-deficient Mouse Model. Plant Foods Hum. Nutr. 2015, 70, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C.; et al. Hypocholesterolaemic effects of lupin protein and pea protein/fibre combinations in moderately hypercholesterolaemic individuals. Br. J. Nutr. 2012, 107, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Flament, M.P.; Leterme, P.; Bizi, M.; Baudet, G.; Gayot, A. Study of talcs as antisticking agents in the production of tablets. Eur. J. Pharm. Sci. 2002, 17, 239–245. [Google Scholar] [CrossRef]
- Vander Pol, M.; Hristov, A.N.; Zaman, S.; Delano, N. Peas can replace soybean meal and corn grain in dairy cow diets. J. Dairy Sci. 2008, 91, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, A.S. Bioavailability of minerals in legumes. Br. J. Nutr. 2002, 88, S281–S285. [Google Scholar] [CrossRef] [PubMed]
- Jacques, H.; Deshaies, Y.; Savoie, L. Relationship between dietary proteins, their in vitro digestion products, and serum cholesterol in rats. Atherosclerosis 1986, 61, 89–98. [Google Scholar] [CrossRef]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic effect of dietary pea proteins: Impact on genes regulating hepatic lipid metabolism. Mol. Nutr. Food Res. 2010, 54 (Suppl. 1), S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Caligari, S.; Gilio, D.; Manzini, S.; Busnelli, M.; Montagnani, M.; Locatelli, M.; Diani, E.; Giavarini, F.; Caruso, D.; et al. Reduced biliary sterol output with no change in total faecal excretion in mice expressing a human apolipoprotein A-I variant. Liver Int. 2012, 32, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Manzini, S.; Busnelli, M.; Rigamonti, E.; Marchesi, M.; Diani, E.; Sirtori, C.R.; Chiesa, G. Effect of the combinations between pea proteins and soluble fibres on cholesterolaemia and cholesterol metabolism in rats. Br. J. Nutr. 2013, 110, 1394–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, M.; Manzini, S.; Hilvo, M.; Parolini, C.; Ganzetti, G.S.; Dellera, F.; Ekroos, K.; Janis, M.; Escalante-Alcalde, D.; Sirtori, C.R.; et al. Liver-specific deletion of the Plpp3 gene alters plasma lipid composition and worsens atherosclerosis in apoE(-/-) mice. Sci. Rep. 2017, 7, 44503. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch. Intern. Med. 2001, 161, 2573–2758. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Parra, D.; Martinez, J.A. Legume-, fish-, or high-protein-based hypocaloric diets: Effects on weight loss and mitochondrial oxidation in obese men. J. Med. Food 2009, 12, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.; Zulet, M.A.; Abete, I.; Martinez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Jayalath, V.H.; Mirrahimi, A.; Agarwal, A.; Chiavaroli, L.; Mejia, S.B.; Sacks, F.M.; Di Buono, M.; et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. CMAJ 2014, 186, E252–E262. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic characterization of hempseed (Cannabis sativa L.). J. Proteomics 2016, 147, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Hempseed Peptides Exert Hypocholesterolemic Effects with a Statin-Like Mechanism. J. Agric. Food Chem. 2017, 65, 8829–8838. [Google Scholar] [CrossRef] [PubMed]
- Soma, M.R.; Donetti, E.; Parolini, C.; Mazzini, G.; Ferrari, C.; Fumagalli, R.; Paoletti, R. HMG CoA reductase inhibitors. In vivo effects on carotid intimal thickening in normocholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 1993, 13, 571–578. [Google Scholar] [CrossRef]
- Marchesi, M.; Parolini, C.; Caligari, S.; Gilio, D.; Manzini, S.; Busnelli, M.; Cinquanta, P.; Camera, M.; Brambilla, M.; Sirtori, C.R.; et al. Rosuvastatin does not affect human apolipoprotein A-I expression in genetically modified mice: A clue to the disputed effect of statins on HDL. Br. J. Pharmacol. 2011, 164, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of Potentially Bioactive Peptides Generated from the Enzymatic Hydrolysis of Hempseed Proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Raudales, D.; Hoeflinger, J.L.; Bringe, N.A.; Cox, S.B.; Dowd, S.E.; Miller, M.J.; Gonzalez de Mejia, E. Consumption of different soymilk formulations differentially affects the gut microbiomes of overweight and obese men. Gut Microbes 2012, 3, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butteiger, D.N.; Hibberd, A.A.; McGraw, N.J.; Napawan, N.; Hall-Porter, J.M.; Krul, E.S. Soy Protein Compared with Milk Protein in a Western Diet Increases Gut Microbial Diversity and Reduces Serum Lipids in Golden Syrian Hamsters. J. Nutr. 2016, 146, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Soulage, C.O.; Koppe, L.; Fouque, D. Protein-bound uremic toxins new targets to prevent insulin resistance and dysmetabolism in patients with chronic kidney disease. J. Ren. Nutr. 2013, 23, 464–466. [Google Scholar] [CrossRef] [PubMed]
- McAllan, L.; Skuse, P.; Cotter, P.D.; O’Connor, P.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.; Roche, H.M.; Nilaweera, K.N. Protein quality and the protein to carbohydrate ratio within a high fat diet influences energy balance and the gut microbiota in C57BL/6J mice. PLoS ONE 2014, 9, e88904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, C.; Kuda, T.; Yazaki, T.; Takahashi, H.; Kimura, B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl. Microbiol. Biotechnol. 2014, 98, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Rist, V.T.; Weiss, E.; Sauer, N.; Mosenthin, R.; Eklund, M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe 2014, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Swiatecka, D.; Narbad, A.; Ridgway, K.P.; Kostyra, H. The study on the impact of glycated pea proteins on human intestinal bacteria. Int. J. Food Microbiol. 2011, 145, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Swiatecka, D.; Iwan, M.; Swiatecki, M.; Kstyra, H.; Kstyra, E. The impact of glycated pea proteins on bacterial adhesion. Food Res. Int. 2010, 43. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busnelli, M.; Manzini, S.; Sirtori, C.R.; Chiesa, G.; Parolini, C. Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation. Nutrients 2018, 10, 1249. https://doi.org/10.3390/nu10091249
Busnelli M, Manzini S, Sirtori CR, Chiesa G, Parolini C. Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation. Nutrients. 2018; 10(9):1249. https://doi.org/10.3390/nu10091249
Chicago/Turabian StyleBusnelli, Marco, Stefano Manzini, Cesare R. Sirtori, Giulia Chiesa, and Cinzia Parolini. 2018. "Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation" Nutrients 10, no. 9: 1249. https://doi.org/10.3390/nu10091249
APA StyleBusnelli, M., Manzini, S., Sirtori, C. R., Chiesa, G., & Parolini, C. (2018). Effects of Vegetable Proteins on Hypercholesterolemia and Gut Microbiota Modulation. Nutrients, 10(9), 1249. https://doi.org/10.3390/nu10091249





