Screening of Cytotoxicity and Anti-Inflammatory Properties of Feijoa Extracts Using Genetically Modified Cell Models Targeting TLR2, TLR4 and NOD2 Pathways, and the Implication for Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Feijoa Fruits and Extracts
2.2. Cell Line and Culture Media
2.3. Cell Viability Screening of Feijoa Extracts
2.4. Anti-Inflammatory Assay
2.5. Data Analysis
3. Results
3.1. Feijoa Peel Induced Higher Cytotoxicity than Flesh and Whole Fruit Extracts
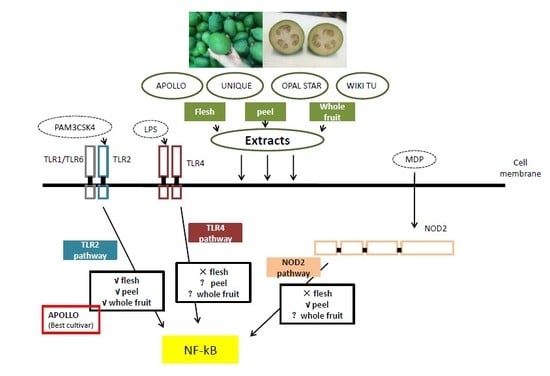
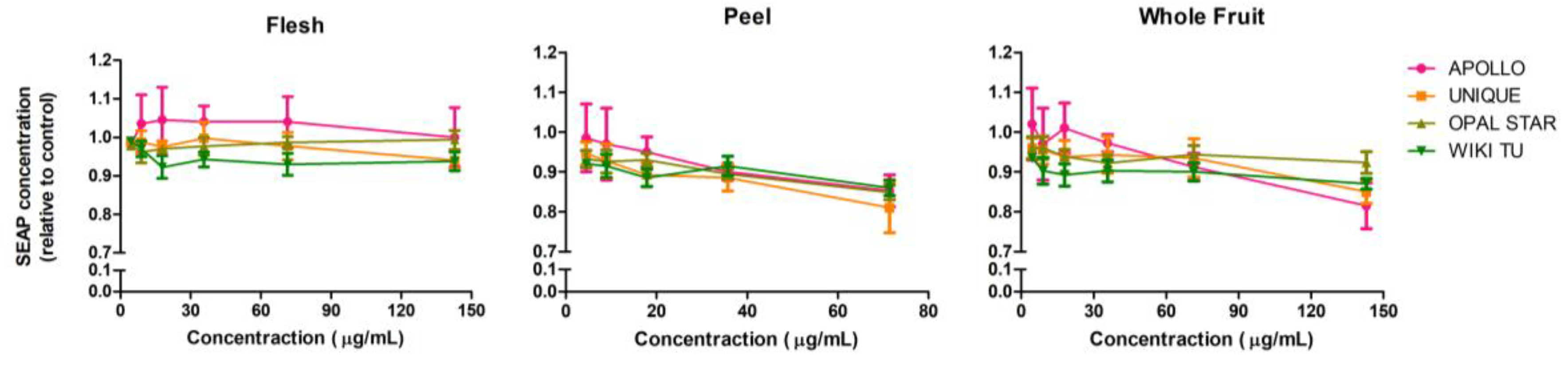
3.2. Potential Anti-Inflammatory Activity of Feijoa through TLR2 and TLR4 Pathways
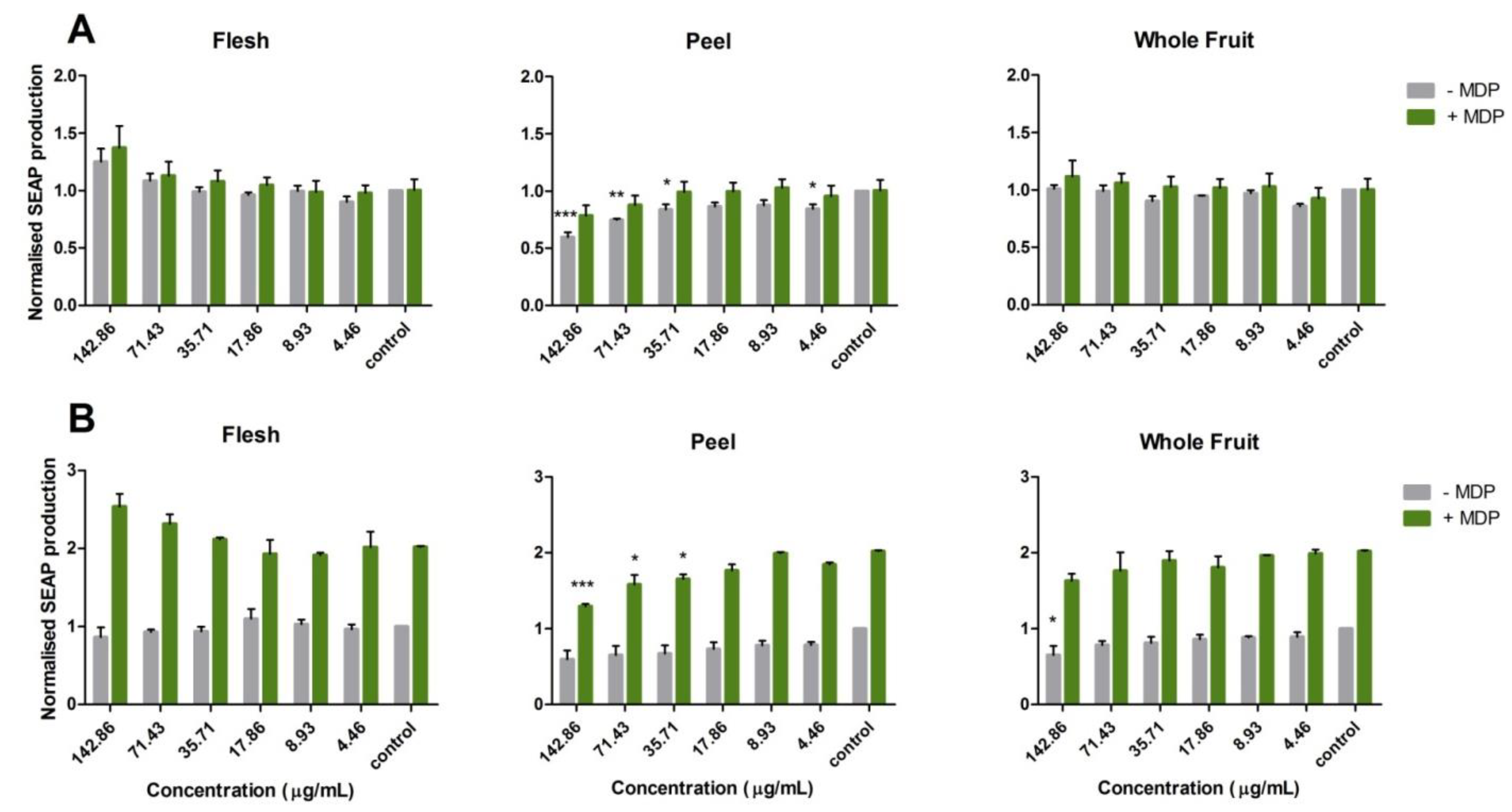
3.3. Activation of the Nucleotide-Binding Oligomerization Domain-Containing Protein 2 (NOD2) Anti-Inflammation Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weston, R.J. Bioactive products from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chem. 2010, 121, 923–926. [Google Scholar] [CrossRef]
- Sharpe, R.H.; Sherman, W.B.; Miller, E.P. Feijoa history and improvement. Proc. Fla. State Hortic. Soc. 1993, 106, 134–139. [Google Scholar]
- Basile, A.; Conte, B.; Rigano, D.; Senatore, F.; Sorbo, S. Antibacterial and antifungal properties of acetonic extract of Feijoa sellowiana fruits and its effect on Helicobacter pylori growth. J. Med. Food 2010, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Keles, H.; Ince, S.; Küçükkurt, I.; Tatli, I.I.; Akkol, E.K.; Kahraman, C.; Demirel, H.H. The effects of Feijoa sellowiana fruits on the antioxidant defense system, lipid peroxidation, and tissue morphology in rats. Pharm. Biol. 2012, 50, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Murray, P.; Marlow, G.; Ferguson, L.R. Anti-inflammatory activity of fruit fractions in vitro, mediated through toll-like receptor 4 and 2 in the context of inflammatory bowel disease. Nutrients 2014, 6, 5265–5279. [Google Scholar] [CrossRef] [PubMed]
- Turco, F.; Palumbo, I.; Andreozzi, P.; Sarnelli, G.; De Ruberto, F.; Esposito, G.; Basile, A.; Cuomo, R. Acetonic extract from the feijoa sellowiana Berg. fruit exerts antioxidant properties and modulates disaccharidases activities in human intestinal epithelial cells. Phytother. Res. 2016, 30, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, W.; Abbès, F.; Mansour, R.B.; Blecker, C.; Attia, H.; Besbes, S. Phenolic profile, antibacterial and cytotoxic properties of second grade date extract from Tunisian cultivars (Phoenix dactylifera L.). Food Chem. 2016, 194, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial activity of pomegranate peel extracts as affected by cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, K.L.; Peroni, N.; Guries, R.P.; Nodari, R.O. Traditional knowledge and management of Feijoa (Acca sellowiana) in Southern Brazil. Econ. Bot. 2009, 63, 204–214. [Google Scholar] [CrossRef]
- Siegel, C. Review article: Explaining risks of inflammatory bowel disease therapy to patients. Aliment. Pharmacol. Ther. 2011, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Lewis, D.A.; Tharib, S.M.; Veitch, G.B.A. The anti-inflammatory activity of celery Apium graveolens L. (Fam. Umbelliferae). Int. J. Crude Drug Res. 1985, 23, 27–32. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Powell, P.; Marlow, G.; Wileman, T.; Ferguson, L.R. Extracts of Feijoa inhibit Toll-like receptor 2 signaling and Activate Autophagy Implicating a role in dietary control of IBD. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Lai, W.; Rivest, S.; Hart, R.P.; Satoskar, A.R.; Popovich, P.G. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J. Neurochem. 2007, 102, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Baré, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel signal transduction pathway utilized by extracellular HSP70 role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, B.; Veres, G.; Dezsofi, A.; Rusai, K.; Vannay, A.; Mraz, M.; Majorova, E.; Arato, A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin. Exp. Immunol. 2008, 151, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Borzutzky, A.; Fried, A.; Chou, J.; Bonilla, F.A.; Kim, S.; Dedeoglu, F. NOD2-associated diseases: Bridging innate immunity and autoinflammation. Clin. Immunol. 2010, 134, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Hedley, L.E. Feijoa Variety Named Opal Star. U.S. Patent 8,825, 12 July 1994. [Google Scholar]
- Thorp, G.; Bieleski, R.L. Feijoas: Origins, Cultivation and Uses; HortResearch: Auckland, New Zealand, 2002. [Google Scholar]
- Waimea Nurseries Ltd. Feijoa Varieties for Fruit Growers-Commercial-Waimea Nurseries. Available online: http://www.webcitation.org/7203R3ror (accessed on 28 August 2018).
- Folkard, D.L.; Marlow, G.; Mithen, R.F.; Ferguson, L.R. Effect of Sulforaphane on NOD2 via NF-κB: Implications for Crohn’s disease. J. Inflamm. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Philpott, M.; Mackay, L.; Ferguson, L.R.; Forbes, D.; Skinner, M. Cell culture models in developing nutrigenomics foods for inflammatory bowel disease. Mutat. Res. 2007, 622, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gheewala, N.; Suthar, A.; Shah, A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009, 1, 38–46. [Google Scholar]
- Klymenko, T.; Brandenburg, M.; Morrow, C.J.; Dive, C.; Makin, G. The novel Bcl-2 inhibitor ABT-737 is more effective in hypoxia, and is able to reverse hypoxia-induced drug resistance in neuroblastoma cells. Mol. Cancer Ther. 2011. [Google Scholar] [CrossRef] [PubMed]
- Ranke, J.; Mölter, K.; Stock, F.; Bottin-Weber, U.; Poczobutt, J.; Hoffmann, J.; Ondruschka, B.; Filser, J.; Jastorff, B. Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol. Environ. Saf. 2004, 58, 396–404. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica 1996, 4, 14–19. [Google Scholar]
- Nedel, F.; Soki, F.N.; Conde, M.C.M.; Zeitlin, B.D.; Tarquinio, S.B.C.; Nör, J.E.; Seixas, F.K.; Demarco, F.F. Comparative analysis of two colorimetric assays in dental pulp cell density. Int. Endod. J. 2011, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, M.P.; Kennedy, D.R.; Kennedy, J.S.; Washabau, R.J.; Henthorn, P.S.; Moore, P.F.; Carding, S.R.; Felsburg, P.J. Expression and function of TLR2, TLR4, and Nod2 in primary canine colonic epithelial cells. Vet. Immunol. Immunopathol. 2006, 114, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, A.; Borkosky, S.; Willink, E.; Bardon, A. Toxic effects of lemon peel constituents on Ceratitis capitata. J. Chem. Ecol. 2004, 30, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kuganesan, A.; Thiripuranathar, G.; Navaratne, A.; Paranagama, P. Antioxidant and anti-inflammatory activities of peels, pulps and seed kernels of three common mango (Mangifera indical L.) varieties in Sri Lanka. Int. J. Pharm. Sci. Res. 2017, 8, 70–78. [Google Scholar]
- Dabbou, S.; Maatallah, S.; Castagna, A.; Guizani, M.; Sghaeir, W.; Hajlaoui, H.; Ranieri, A. Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Foods Hum. Nutr. 2017, 72, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Lecat, A.; Piette, J.; Legrand-Poels, S. The protein Nod2: An innate receptor more complex than previously assumed. Biochem. Pharmacol. 2010, 80, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef] [PubMed]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Bouic, P.J. The role of phytosterols and phytosterolins in immune modulation: A review of the past 10 years. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Elmastaş, M.; Gedikli, F. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). J. Med. Plant. Res. 2010, 4, 1065–1072. [Google Scholar]
- Tuncel, N.B.; Yılmaz, N. Optimizing the extraction of phenolics and antioxidants from feijoa (Feijoa sellowiana, Myrtaceae). J. Food Sci. Technol. 2015, 52, 141–150. [Google Scholar] [CrossRef]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Takegawa, D.; Hirao, K.; Takahashi, K.; Yumoto, H.; Matsuo, T. Roles of dental pulp fibroblasts in the recognition of bacterium-related factors and subsequent development of pulpitis. Jpn. Dent. Sci. Rev. 2011, 47, 161–166. [Google Scholar] [CrossRef]
- Natsuka, M.; Uehara, A.; Yang, S.; Echigo, S.; Takada, H. A polymer-type water-soluble peptidoglycan exhibited both Toll-like receptor 2-and NOD2-agonistic activities, resulting in synergistic activation of human monocytic cells. Innate Immun. 2008, 14, 298–308. [Google Scholar] [CrossRef] [PubMed]
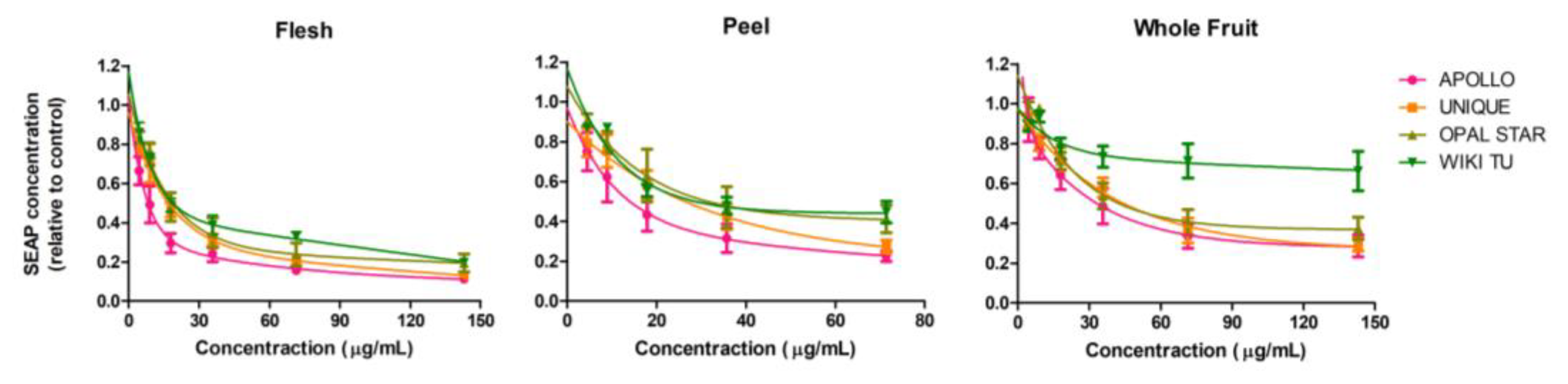
| Cell Line | Flesh (μg/mL) | Peel (μg/mL) | Whole Fruit (μg/mL) |
|---|---|---|---|
| HEK-Blue™ hTLR2 | 419.50 | 77.19 | 252.80 |
| HEK-Blue™ hTLR4 | 360.60 | 79.17 | 257.20 |
| NOD2-WT | 190.50 | 83.57 | 200.00 |
| NOD2-G908R | 232.90 | 100.40 | 247.10 |
| Mean (SD) | 300.88 ± 92.79 | 85.08 ± 9.14 | 239.28 ± 22.96 |
| Conc. (μg/mL) | HEK-Blue™ hTLR2 | HEK-Blue™ hTLR4 | NOD2-WT | NOD2-G908R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | WF | F | P | WF | F | P | WF | F | P | WF | |
| APOLLLO | ||||||||||||
| 142.86 | 0.63 ± 0.06 ab/BC * | 0.54 ± 0.09 a/AB * | 0.66 ± 0.07 a/BC * | 0.66 ± 0.10 ab/BC | 0.41 ± 0.07 a/A * | 0.64 ± 0.05 a/BC * | 0.72 ± 0.13 ab/BC | 0.57 ± 0.12 abc/BC | 0.69 ± 0.13 ab/BC | 0.71 ± 0.04 ab/BC | 0.63 ± 0.05 abc/BC | 0.73 ± 0.08 abc/C |
| 71.43 | 0.80 ± 0.05 cdef/BC | 0.60 ± 0.06 a/A * | 0.83 ± 0.04 bcd/BC | 0.78 ± 0.07 bcdef/BC | 0.57 ± 0.07 b/A * | 0.80 ± 0.05 cde/BC | 0.88 ± 0.06 ab/C | 0.68 ± 0.12 abcde/AB | 0.85 ± 0.14 ab/BC | 0.79 ± 0.04 abcd/BC | 0.76 ± 0.12 abcdefg/BC | 0.88 ± 0.16 abcde/BC |
| 35.71 | 0.86 ± 0.08 defg/A | 0.86 ± 0.04 cde/A | 0.90 ± 0.02 de/A | 0.81 ± 0.06 cdefg/A | 0.78 ± 0.08 defg/A | 0.87 ± 0.04 efgh/A | 0.94 ± 0.10 ab/A | 0.78 ± 0.08 bcdef/A | 0.94 ± 0.11 b/A | 0.87 ± 0.06 bcd/A | 0.85 ± 0.08 cdefghi/A | 0.91 ± 0.10 abcde/A |
| 17.86 | 0.90 ± 0.06 efg/A | 0.91 ± 0.03 de/A | 0.92 ± 0.03 de/A | 0.88 ± 0.05 fgh/A | 0.87 ± 0.08 efgh/A | 0.90 ± 0.06 efgh/A | 0.96 ± 0.10 ab/A | 0.89 ± 0.12 def/A | 1.00 ± 0.15 b/A | 0.89 ± 0.09 bcd/A | 1.00 ± 0.23 defghi/A | 1.04 ± 0.24 abcde/A |
| 8.93 | 0.92 ± 0.06 fg/A | 0.93 ± 0.04 de/A | 0.96 ± 0.03 de/A | 0.93 ± 0.06 gh/A | 0.92 ± 0.06 hi/A | 0.94 ± 0.04 gh/A | 1.01 ± 0.11 ab/A | 0.91 ± 0.08 ef/A | 1.02 ± 0.12 b/A | 0.91 ± 0.09 bcd/A | 0.94 ± 0.09 fghi/A | 1.00 ± 0.14 cde/A |
| 4.46 | 0.94 ± 0.11 g/A | 0.96 ± 0.03 e/A | 0.97 ± 0.04 e/A | 0.98 ± 0.08 h/A | 0.99 ± 0.04 i/A | 0.98 ± 0.04 h/A | 1.04 ± 0.11 b/A | 0.96 ± 0.10 ef/A | 1.07 ± 0.15 b/A | 0.94 ± 0.08 d/A | 0.98 ± 0.08 ghi/A | 1.02 ± 0.11 de/A |
| UNIQUE | ||||||||||||
| 142.86 | 0.63 ± 0.08 ab/BCD * | 0.57 ± 0.07 a/ABC * | 0.71 ± 0.06 a/CD * | 0.72 ± 0.08 abcde/CD | 0.51 ± 0.06 a/A * | 0.65 ± 0.06 ab/BCD * | 0.69 ± 0.15 a/CD | 0.44 ± 0.12 a/A | 0.50 ± 0.12 a/AB | 0.77 ± 0.05 abcd/D | 0.56 ± 0.04 a/ABC | 0.65 ± 0.04 a/BCD |
| 71.43 | 0.79 ± 0.06 cde/CD | 0.74 ± 0.04 b/BCD * | 0.83 ± 0.04 bcd/D | 0.82 ± 0.09 cdefg/D | 0.60 ± 0.09 bc/AB * | 0.80 ± 0.06 cde/CD | 0.79 ± 0.11 ab/CD | 0.53 ± 0.14 ab/A | 0.66 ± 0.07 ab/BC | 0.83 ± 0.05 abcd/D | 0.70 ± 0.04 abcde/BCD | 0.81 ± 0.04 abcde/CD |
| 35.71 | 0.83 ± 0.03 cdefg/ABCD | 0.88 ± 0.03 cde/BCD | 0.86 ± 0.03 bcde/BCD | 0.87 ± 0.10 fgh/BCD | 0.77 ± 0.06 def/ABC | 0.84 ± 0.07 defg/BCD | 0.80 ± 0.07 ab/ABCD | 0.69 ± 0.10 abcde/A | 0.75 ± 0.07 ab/AB | 0.91 ± 0.06 bcd/CD | 0.92 ± 0.07 defghi/D | 0.92 ± 0.08 bcde/D |
| 17.86 | 0.85 ± 0.06 cdefg/AB | 0.90 ± 0.03 de/AB | 0.89 ± 0.06 cde/AB | 0.90 ± 0.08 fgh/AB | 0.85 ± 0.04 efgh/AB | 0.89 ± 0.09 efgh/AB | 0.87 ± 0.15 ab/AB | 0.89 ± 0.08 ef/AB | 0.91 ± 0.24 b/A | 0.89 ± 0.06 bcd/AB | 1.00 ± 0.07 hi/B | 0.98 ± 0.09 cde/B |
| 8.93 | 0.89 ± 0.05 efg/AB | 0.92 ± 0.06 de/AB | 0.93 ± 0.04 de/AB | 0.89 ± 0.09 gh/AB | 0.90 ± 0.04 ghi/AB | 0.90 ± 0.07 efgh/AB | 0.82 ± 0.06 ab/A | 0.94 ± 0.11 ef/AB | 0.93 ± 0.17 b/A | 0.86 ± 0.07 bcd/AB | 1.01 ± 0.10 i/B | 1.01 ± 0.07 cde/B |
| 4.46 | 0.92 ± 0.05 fg/ABCD | 0.94 ± 0.04 de/ABCD | 0.95 ± 0.05 de/BCD | 0.90 ± 0.04 h/ABC | 0.91 ± 0.03 hi/ABCD | 0.92 ± 0.04 fgh/ABCD | 0.82 ± 0.05 ab/A | 1.02 ± 0.10 f/CD | 0.93 ± 0.16 b/AB | 0.84 ± 0.03 bcd/AB | 1.03 ± 0.08 hi/D | 1.00 ± 0.05 cde/CD |
| OPAL STAR | ||||||||||||
| 142.86 | 0.62 ± 0.04 a/ABC | 0.58 ± 0.07 a/AB * | 0.70 ± 0.05 a/ABCD | 0.68 ± 0.07 abc/ABCD | 0.59 ± 0.08 bc/AB | 0.72 ± 0.08 abc/BCD | 0.69 ± 0.06 a/ABCD | 0.56 ± 0.08 abc/A | 0.66 ± 0.04 ab/ABCD | 0.77 ± 0.02 abcd/CD | 0.66 ± 0.06 abcd/ABCD | 0.78 ± 0.06 abcd/D |
| 71.43 | 0.73 ± 0.03 abc/AB | 0.78 ± 0.05 bc/AB | 0.76 ± 0.05 abc/AB | 0.71 ± 0.06 abcde/AB | 0.69 ± 0.07 cd/A | 0.79 ± 0.06 cde/AB | 0.78 ± 0.01 ab/AB | 0.68 ± 0.08 abcde/A | 0.79 ± 0.00 ab/AB | 0.85 ± 0.07 abcd/B | 0.80 ± 0.10 bcdefgh/AB | 0.86 ± 0.04 abcde/B |
| 35.71 | 0.83 ± 0.06 cdefg/AB | 0.86 ± 0.06 cde/AB | 0.84 ± 0.08 bcde/AB | 0.79 ± 0.05 bcdefg/A | 0.83 ± 0.08 efgh/AB | 0.86 ± 0.06 defg/AB | 0.84 ± 0.02 ab/AB | 0.79 ± 0.06 bcdef/A | 0.86 ± 0.04 ab/AB | 0.88 ± 0.04 bcd/AB | 0.91 ± 0.07 efghi/AB | 0.96 ± 0.10 bcde/B |
| 17.86 | 0.86 ± 0.04 defg/AB | 0.89 ± 0.04 cde/AB | 0.90 ± 0.08 de/AB | 0.81 ± 0.05 cdefg/A | 0.87 ± 0.08 efgh/AB | 0.85 ± 0.09 defg/AB | 0.90 ± 0.05 ab/AB | 0.89 ± 0.03 def/AB | 0.93 ± 0.06 b/AB | 0.89 ± 0.04 bcd/AB | 0.98 ± 0.08 ghi/B | 0.98 ± 0.05 cde/B |
| 8.93 | 0.88 ± 0.04 efg/A | 0.92 ± 0.06 de/A | 0.91 ± 0.05 de/A | 0.82 ± 0.06 cdefg/A | 0.87 ± 0.07 efgh/A | 0.88 ± 0.06 efgh/A | 0.91 ± 0.06 ab/A | 0.94 ± 0.06 ef/AB | 0.98 ± 0.12 b/A | 0.93 ± 0.05 cd/AB | 0.97 ± 0.10 ghi/AB | 1.08 ± 0.07 e/B |
| 4.46 | 0.88 ± 0.06 efg/AB | 0.94 ± 0.04 de/AB | 0.97 ± 0.05 e/AB | 0.83 ± 0.08 defg/A | 0.88 ± 0.11 fghi/AB | 0.91 ± 0.08 efgh/AB | 0.93 ± 0.08 ab/AB | 0.98 ± 0.04 ef/AB | 1.01 ± 0.07 b/AB | 0.90 ± 0.03 bcd/B | 0.99 ± 0.10 ghi/AB | 1.03 ± 0.02 de/AB |
| WIKI TU | ||||||||||||
| 142.86 | 0.74 ± 0.08 ab/BC * | 0.58 ± 0.08 a/A * | 0.76 ± 0.09 ab/C | 0.64 ± 0.04 a/ABC | 0.60 ± 0.06 bc/AB * | 0.67 ± 0.04 ab/ABC | 0.68 ± 0.05 a/ABC | 0.60 ± 0.06 abcd/AB | 0.72 ± 0.07 ab/ABC | 0.65 ± 0.03 a/ABC | 0.60 ± 0.03 ab/AB | 0.68 ± 0.02 ab/ABC |
| 71.43 | 0.85 ± 0.09 cdefg/A | 0.82 ± 0.12 bcd/A | 0.87 ± 0.10 bcde/A | 0.70 ± 0.04 abcd/A | 0.75 ± 0.06 de/A | 0.74 ± 0.04 bcd/A | 0.81 ± 0.08 ab/A | 0.72 ± 0.07 bcde/A | 0.84 ± 0.10 ab/A | 0.71 ± 0.04 ab/A | 0.72 ± 0.04 abcdef/A | 0.79 ± 0.04 abcde/A |
| 35.71 | 0.86 ± 0.10 defg/A | 0.86 ± 0.07 cde/A | 0.90 ± 0.10 de/A | 0.77 ± 0.07 abcdef/A | 0.82 ± 0.05 efgh/A | 0.79 ± 0.04 cde/A | 0.79 ± 0.05 ab/A | 0.81 ± 0.07 cdef/A | 0.85 ± 0.08 ab/A | 0.73 ± 0.06 abc/A | 0.81 ± 0.02 abcdefg/A | 0.81 ± 0.04 abcde/A |
| 17.86 | 0.91 ± 0.10 fg/AB | 0.89 ± 0.09 cde/AB | 0.93 ± 0.11 de/B | 0.78 ± 0.06 bcdef/AB | 0.84 ± 0.03 efgh/AB | 0.82 ± 0.06 cdef/AB | 0.85 ± 0.09 ab/AB | 0.88 ± 0.06 def/AB | 0.93 ± 0.07 b/B | 0.73 ± 0.06 abcd/A | 0.88 ± 0.03 defghi/AB | 0.81 ± 0.08 abcde/AB |
| 8.93 | 0.93 ± 0.09 fg/AB | 0.92 ± 0.08 de/AB | 0.97 ± 0.10 e/B | 0.86 ± 0.08 efgh/AB | 0.86 ± 0.03 efgh/AB | 0.84 ± 0.04 defg/AB | 0.91 ± 0.13 ab/AB | 0.89 ± 0.06 def/AB | 0.95 ± 0.04 b/B | 0.74 ± 0.09 abcd/A | 0.89 ± 0.04 defghi/AB | 0.84 ± 0.04 abcde/AB |
| 4.46 | 0.95 ± 0.09 g/AB | 0.94 ± 0.06 de/AB | 0.97 ± 0.06 e/AB | 0.90 ± 0.03 fgh/AB | 0.91 ± 0.02 hi/AB | 0.90 ± 0.03 efgh/AB | 0.87 ± 0.13 ab/AB | 0.97 ± 0.06 ef/AB | 1.02 ± 0.12 b/B | 0.79 ± 0.07 abcd/A | 0.82 ± 0.07 bcdefghi/AB | 0.87 ± 0.08 abcde/AB |
| APOLLO (μg/mL) | UNIQUE (μg/mL) | OPAL STAR (μg/mL) | WIKI TU (μg/mL) | IB (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | WF | F | P | WF | F | P | WF | F | P | WF | ||
| IC30 | 4.06 | 5.87 | 11.90 | 7.26 | 9.31 | 18.35 | 8.04 | 11.12 | 16.32 | 8.37 | 11.86 | 89.38 | 230.72 |
| IC50 | 7.88 | 12.81 | 30.84 | 13.70 | 26.68 | 55.80 | 16.70 | 31.74 | 48.40 | 17.97 | 41.24 | N/A | 442.90 |
| IC70 | 21.42 | 42.32 | 133.31 | 35.90 | 76.45 | N/A | 50.67 | N/A | N/A | 57.26 | N/A | N/A | N/A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Bishop, K.S.; Ferguson, L.R.; Quek, S.Y. Screening of Cytotoxicity and Anti-Inflammatory Properties of Feijoa Extracts Using Genetically Modified Cell Models Targeting TLR2, TLR4 and NOD2 Pathways, and the Implication for Inflammatory Bowel Disease. Nutrients 2018, 10, 1188. https://doi.org/10.3390/nu10091188
Peng Y, Bishop KS, Ferguson LR, Quek SY. Screening of Cytotoxicity and Anti-Inflammatory Properties of Feijoa Extracts Using Genetically Modified Cell Models Targeting TLR2, TLR4 and NOD2 Pathways, and the Implication for Inflammatory Bowel Disease. Nutrients. 2018; 10(9):1188. https://doi.org/10.3390/nu10091188
Chicago/Turabian StylePeng, Yaoyao, Karen Suzanne Bishop, Lynnette Robin Ferguson, and Siew Young Quek. 2018. "Screening of Cytotoxicity and Anti-Inflammatory Properties of Feijoa Extracts Using Genetically Modified Cell Models Targeting TLR2, TLR4 and NOD2 Pathways, and the Implication for Inflammatory Bowel Disease" Nutrients 10, no. 9: 1188. https://doi.org/10.3390/nu10091188
APA StylePeng, Y., Bishop, K. S., Ferguson, L. R., & Quek, S. Y. (2018). Screening of Cytotoxicity and Anti-Inflammatory Properties of Feijoa Extracts Using Genetically Modified Cell Models Targeting TLR2, TLR4 and NOD2 Pathways, and the Implication for Inflammatory Bowel Disease. Nutrients, 10(9), 1188. https://doi.org/10.3390/nu10091188