Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Extraction of Chinese Propolis (CP)
2.4. Liquid Chromatography−Mass Spectrometry (LC–MS) Analysis
2.5. Cell Viability Assay
2.6. Cell Apoptosis Assay
2.7. Cell Cycle Assay
2.8. Western Blotting
2.9. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Wound Healing Assay
2.11. ROS Determination
2.12. Statistical Analysis
3. Results
3.1. Components Identified in CP
3.2. CP Induces Mitochondria-Dependent Apoptosis in Human Melanoma Cell Line A375
3.3. CP Induces S-G2/M Cell-Cycle Arrest in Human Melanoma Cell Line A375
3.4. CP Prompts Apoptosis in A375 by Targeting NLRP1-Related Inflammatory Pathway
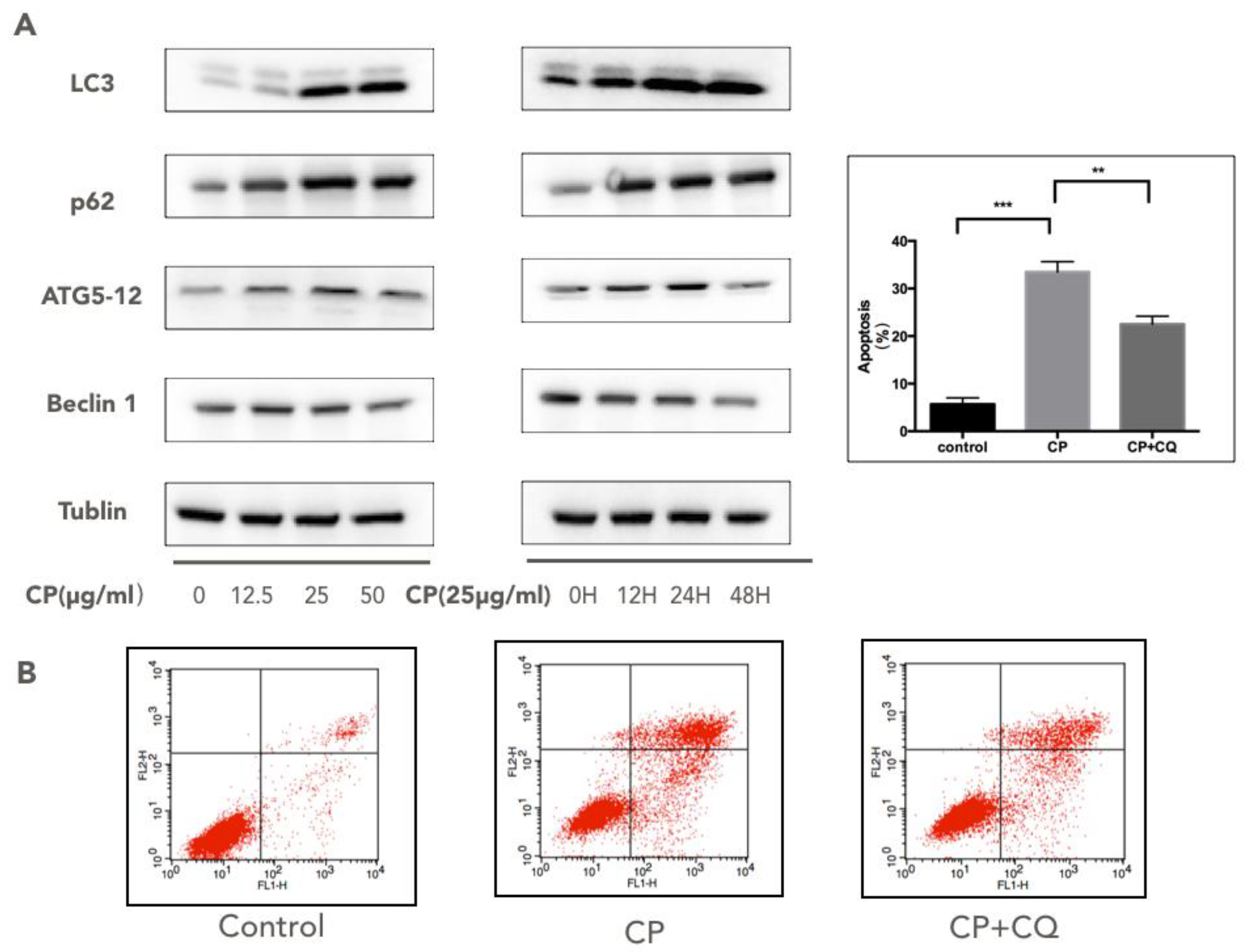
3.5. CP Triggers Autophagy in Human Melanoma, which Enhances its Cytotoxicity to A375
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eberle, J.; Fecker, L.F. Regulation of apoptosis in melanoma cells: Critical targets for therapeutic strategies. In Melanoma Development; Springer: Berlin/Heidelberg, Germany, 2017; pp. 271–287. [Google Scholar]
- Godar, D.E.; Subramanian, M.; Merrill, S.J. Cutaneous malignant melanoma incidences analyzed worldwide by skin type over advancing age of males and females: Evidence estrogen and androgenic hair are risk factors. J. Epidemiol. Res. 2016, 3, 42. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Martinon, F. Dangerous liaisons: Mitochondrial DNA meets the nlrp3 inflammasome. Immunity 2012, 36, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Awad, F.; Assrawi, E.; Louvrier, C.; Jumeau, C.; Giurgea, I.; Amselem, S.; Karabina, S.-A. Photoaging and skin cancer: Is the inflammasome the missing link? Mech Ageing Dev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. Inflammasome activation of IL-1 family mediators in response to cutaneous photodamage. Photochem. Photobiol. 2012, 88, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Sevko, A.; Ramacher, M.; Bazhin, A.V.; Falk, C.S.; Osen, W.; Borrello, I.; Kato, M.; Schadendorf, D.; Baniyash, M. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. USA 2011, 108, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, V.O.; Bar-Eli, M. Inflammation and melanoma metastasis. Pigment Cell Melanoma Res. 2009, 22, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Liu, W.; Kaur, M.; Luo, Y.; Domenico, J.; Samson, J.M.; Shellman, Y.G.; Norris, D.A.; Dinarello, C.A.; Spritz, R.A. Nlrp1 promotes tumor growth by enhancing inflammasome activation and suppressing apoptosis in metastatic melanoma. Oncogene 2017, 36, 3820. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, P.N.; Pawelec, G.P.; Mihailova, S.M.; Ivanova, M.I.; Myhailova, A.P.; Baltadjieva, D.N.; Marinova, D.I.; Ivanova, S.S.; Naumova, E.J. Association of cytokine gene polymorphisms with malignant melanoma in caucasian population. Cancer Immunol. Immunother. 2007, 56, 371. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ekmekcioglu, S.; Liu, P.; Duncan, L.M.; Lizée, G.; Poindexter, N.; Grimm, E.A. Constitutive aberrant endogenous interleukin-1 facilitates inflammation and growth in human melanoma. Mol. Cancer Res. 2011. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C. Non-inflammasome forming nlrs in inflammation and tumorigenesis. Front. Immunol. 2014, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Yuzhalin, A.E. Inherited variation in pattern recognition receptors and cancer: Dangerous liaisons? Cancer Manag. Res. 2012, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.H.; Ellis, L.Z.; Fujita, M. Inflammasomes as molecular mediators of inflammation and cancer: Potential role in melanoma. Cancer Lett. 2012, 314, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Baehrecke, E.H.; Kroemer, G. Does autophagy contribute to cell death? Autophagy 2005, 1, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.L.; Pellegrini, P.; Di Lernia, G.; Djavaheri-Mergny, M.; Brnjic, S.; Zhang, X.; Hägg, M.; Linder, S.; Fais, S.; Codogno, P. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J. Biol. Chem. 2012, 287, 30664–30676. [Google Scholar] [CrossRef] [PubMed]
- Jounai, N.; Kobiyama, K.; Shiina, M.; Ogata, K.; Ishii, K.J.; Takeshita, F. Nlrp4 negatively regulates autophagic processes through an association with beclin1. J. Immunol. 2011, 1001654. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy as an innate immunity paradigm: Expanding the scope and repertoire of pattern recognition receptors. Curr. Opin. Immunol. 2012, 24, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Fan, J.; Billiar, T.R.; Scott, M.J. Inflammasome and autophagy regulation: A two-way street. Mol. Med. 2017, 23, 188. [Google Scholar]
- Marcilla-Etxenike, A.; Martín, M.L.; Noguera-Salvà, M.A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Dey, I.; Escribá, P.V.; Busquets, X. 2-hydroxyoleic acid induces er stress and autophagy in various human glioma cell lines. PLoS ONE 2012, 7, e48235. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.-S.; Wang, W.; Chang, Y.-A.; Lin, C.-J.; Wang, J.-J.; Chen, R.-M. Honokiol induces autophagy of neuroblastoma cells through activating the pi3k/akt/mtor and endoplasmic reticular stress/erk1/2 signaling pathways and suppressing cell migration. Cancer Lett. 2016, 370, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Sun, L.; Li, B.; Gao, H.; Xie, T.; Zhang, N.; Ye, Z. Celastrol induces apoptosis and autophagy via the ros/jnk signaling pathway in human osteosarcoma cells: An in vitro and in vivo study. Cell Death Dis. 2015, 6, e1604. [Google Scholar] [CrossRef] [PubMed]
- Stewart, Z.A.; Westfall, M.D.; Pietenpol, J.A. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef]
- Pietenpol, J.; Stewart, Z. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology 2002, 181, 475–481. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Pardee, A.B. The restriction point of the cell cycle. Cell Cycle 2002, 1, 102–109. [Google Scholar] [CrossRef]
- Gismondi, A.; Trionfera, E.; Canuti, L.; Di Marco, G.; Canini, A. Royal jelly lipophilic fraction induces antiproliferative effects on sh-sy5y human neuroblastoma cells. Oncol. Rep. 2017, 38, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Sforcin, J. Propolis and the immune system: A review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.; Pascual-Maté, A.; Fernández-Muiño, M.; López-Díaz, T.; Sancho, M. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Li, Z.; Yan, H.; Sang, Q.; Wang, K.; He, Q.; Wang, Y.; Hu, F. Antitumor activity of Chinese propolis in human breast cancer mcf-7 and mda-mb-231 cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 280120. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Naoi, K.; Hashita, M.; Itoh, Y.; Suzui, M. Growth inhibitory activity of ethanol extracts of Chinese and brazilian propolis in four human colon carcinoma cell lines. Oncol. Rep. 2009, 22, 349–354. [Google Scholar] [PubMed]
- Zhao, Y.; Tian, W.; Peng, W. Anti-proliferation and insulin resistance alleviation of hepatocellular carcinoma cells hepg2 in vitro by Chinese propolis. J. Food Nutr. Res. 2014, 2, 228–235. [Google Scholar] [CrossRef]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Sha, N.; Guan, S.-H.; Lu, Z.-Q.; Chen, G.-T.; Huang, H.-L.; Xie, F.-B.; Yue, Q.-X.; Liu, X.; Guo, D.-A. Cytotoxic constituents of Chinese propolis. J. Nat. Product. 2009, 72, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, K.; Wu, Y.; Chen, Y.; Chen, X.; Hu, C.W.; Hu, F. Pinocembrin induces er stress mediated apoptosis and suppresses autophagy in melanoma cells. Cancer Lett. 2018, 431, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Spandidos, A.; Wang, H.; Seed, B. Primerbank: A pcr primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2011, 40, D1144–D1149. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-L.; Wang, K.; Li, Q.-Q.; Tian, W.-L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Antioxidant and anti-inflammatory effects of Chinese propolis during palmitic acid-induced lipotoxicity in cultured hepatocytes. J. Funct. Foods 2017, 34, 216–223. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, S.; Wei, W.; Ping, S.; Shen, X.; Li, Y.; Hu, F. Development of high-performance liquid chromatographic for quality and authenticity control of Chinese propolis. J. Food Sci. 2014, 79, C1315–C1322. [Google Scholar]
- Pichichero, E.; Cicconi, R.; Mattei, M.; Canini, A. Chrysin-induced apoptosis is mediated through p38 and bax activation in b16-f1 and a375 melanoma cells. Int. J. Oncol. 2011, 38, 473–483. [Google Scholar] [PubMed]
- Biggar, K.K.; Storey, K.B. Perspectives in cell cycle regulation: Lessons from an anoxic vertebrate. Curr. Genom. 2009, 10, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A. Nlr-containing inflammasomes: Central mediators of host defense and inflammation. Eur. J. Immunol. 2010, 40, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Lazova, R.; Klump, V.; Pawelek, J. Autophagy in cutaneous malignant melanoma. J. Cutan. Pathol. 2010, 37, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Usia, T.; Banskota, A.H.; Tezuka, Y.; Midorikawa, K.; Matsushige, K.; Kadota, S. Constituents of Chinese propolis and their antiproliferative activities. J. Nat. Prod. 2002, 65, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Xuan, H.; Wang, Y.; Li, A.; Fu, C.; Wang, Y.; Peng, W. Bioactive components of Chinese propolis water extract on antitumor activity and quality control. Evid.-Based Complement. Altern. Med. 2016, 2016, 9641965. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.; Jiang, F.; Yang, Y.; Wang, X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2015, 48, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Demestre, M.; Messerli, S.; Celli, N.; Shahhossini, M.; Kluwe, L.; Mautner, V.; Maruta, H. Cape (caffeic acid phenethyl ester)-based propolis extract (bio 30) suppresses the growth of human neurofibromatosis (nf) tumor xenografts in mice. Phytother Res. 2009, 23, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Omene, C.; Karkoszka, J.; Bosland, M.; Eckard, J.; Klein, C.B.; Frenkel, K. Caffeic acid phenethyl ester (cape), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011, 308, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Angelo, G.; Lorena, C.; Marta, G.; Antonella, C. Biochemical composition and antioxidant properties of lavandula angustifolia miller essential oil are shielded by propolis against uv radiations. Photochem. Photobiol. 2014, 90, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Taira, N.; Nguyen, B.C.Q.; Be Tu, P.T.; Tawata, S. Effect of Okinawa propolis on pak1 activity, caenorhabditis elegans longevity, melanogenesis, and growth of cancer cells. J. Agric. Food Chem. 2016, 64, 5484–5489. [Google Scholar] [CrossRef] [PubMed]
- Motomura, M.; Kwon, K.M.; Suh, S.-J.; Lee, Y.-C.; Kim, Y.-K.; Lee, I.-S.; Kim, M.-S.; Kwon, D.Y.; Suzuki, I.; Kim, C.-H. Propolis induces cell cycle arrest and apoptosis in human leukemic u937 cells through bcl-2/bax regulation. Environ. Toxicol. Pharmacol. 2008, 26, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-N.; Weng, M.-S.; Wu, C.-L.; Lin, J.-K. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. Evid.-Based Complement. Altern. Med. 2004, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Car, H.; Borawska, M.H.; Nikliński, J. The anticancer activity of propolis. Folia Histochem. Cytobiol. 2012, 50, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.-H.; Wang, Z.-J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (oe33) by causing g2/m arrest and inducing apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.; Muschel, R.J. Radiation and the g2 phase of the cell cycle. Radiat. Res. 1998, 150, S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Knibiehler, M.; Ducommun, B. P21 binding to pcna causes g1 and g2 cell cycle arrest in p53-deficient cells. Oncogene 1998, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Waldman, T.; Kinzler, K.W.; Vogelstein, B. P21 is necessary for the p53-mediated g1 arrest in human cancer cells. Cancer Res. 1995, 55, 5187–5190. [Google Scholar] [PubMed]
- Bates, S.; Ryan, K.M.; Phillips, A.C.; Vousden, K.H. Cell cycle arrest and DNA endoreduplication following p21 waf1/cip1 expression. Oncogene 1998, 17, 1691. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.; Sedivy, J.; Kinzler, K.; Vogelstein, B. Requirement for p53 and p21 to sustain g2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Ropponen, K.; Kellokoski, J.; Lipponen, P.; Pietiläinen, T.; Eskelinen, M.; Alhava, E.; Kosma, V. P21/waf1 expression in human colorectal carcinoma: Association with p53, transcription factor ap-2 and prognosis. Br. J. Cancer 1999, 81, 133. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a Tumor Promoter in Cancer Induction, Seminars in Cancer Biology; Elsevier: New York, NY, USA, 2004; pp. 433–439. [Google Scholar]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Maccalli, C.; Mattei, S.; Melani, C.; Radrizzani, M.; Parmiani, G. Expression of cytokine genes, including IL-6, in human malignant melanoma cell lines. Melanoma Res. 1992, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; Möller, A.; Algermissen, B.; Worm, M.; Sticherling, M.; Czarnetzki, B. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 1993, 151, 2667–2675. [Google Scholar] [PubMed]
- Park, H.; Byun, D.; Kim, T.S.; Kim, Y.I.; Kang, J.S.; Hahm, E.S.; Kim, S.H.; Lee, W.J.; Song, H.K.; Yoon, D.Y. Enhanced il-18 expression in common skin tumors. Immunol. Lett. 2001, 79, 215–219. [Google Scholar] [CrossRef]
- Vidal-Vanaclocha, F.; Fantuzzi, G.; Mendoza, L.; Fuentes, A.M.; Anasagasti, M.J.; Martín, J.; Carrascal, T.; Walsh, P.; Reznikov, L.L.; Kim, S.-H. Il-18 regulates il-1β-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc. Natl. Acad. Sci. USA 2000, 97, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Tőzsér, J.; Benkő, S. Natural compounds as regulators of nlrp3 inflammasome-mediated il-1β production. Mediat. Inflamm. 2016, 2016, 5460302. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.H.; Ferrero, R.L.; Philpott, D.J.; Girardin, S.E. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 2006, 7, 1250. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Reed, J.C. Card proteins as therapeutic targets in cancer. Curr. Drug Targets 2004, 5, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, Y.; Dunn, J.H.; Norris, D.A.; Dinarello, C.A.; Fujita, M. Dual role of apoptosis-associated speck-like protein containing a card (asc) in tumorigenesis of human melanoma. J. Investig. Dermatol. 2013, 133, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Lassus, P.; Opitz-Araya, X.; Lazebnik, Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 2002, 297, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Tschopp, J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Lian, S.; Khoi, P.N.; Yoon, H.J.; Joo, Y.E.; Chay, K.O.; Kim, K.K.; Do Jung, Y. Chrysin inhibits tumor promoter-induced mmp-9 expression by blocking ap-1 via suppression of erk and jnk pathways in gastric cancer cells. PLoS ONE 2015, 10, e0124007. [Google Scholar] [CrossRef] [PubMed]
- Saiki, S.; Sasazawa, Y.; Imamichi, Y.; Kawajiri, S.; Fujimaki, T.; Tanida, I.; Kobayashi, H.; Sato, F.; Sato, S.; Ishikawa, K.-I. Caffeine induces apoptosis by enhancement of autophagy via pi3k/akt/mtor/p70s6k inhibition. Autophagy 2011, 7, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Nicolson, S.; Kumar, S. Cell death by autophagy: Facts and apparent artefacts. Cell Death Differ. 2012, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.; Lotze, M.; Tang, D. The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Hartman, M.; Roche, C.; Zeng, S.G.; O’Shea, A.; Sharp, F.A.; Lambe, E.M.; Creagh, E.M.; Golenbock, D.T.; Tschopp, J. Autophagy controls il-1β secretion by targeting pro-il-1β for degradation. J. Biol. Chem. 2011, 286, 9587–9597. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting tlr4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef] [PubMed]
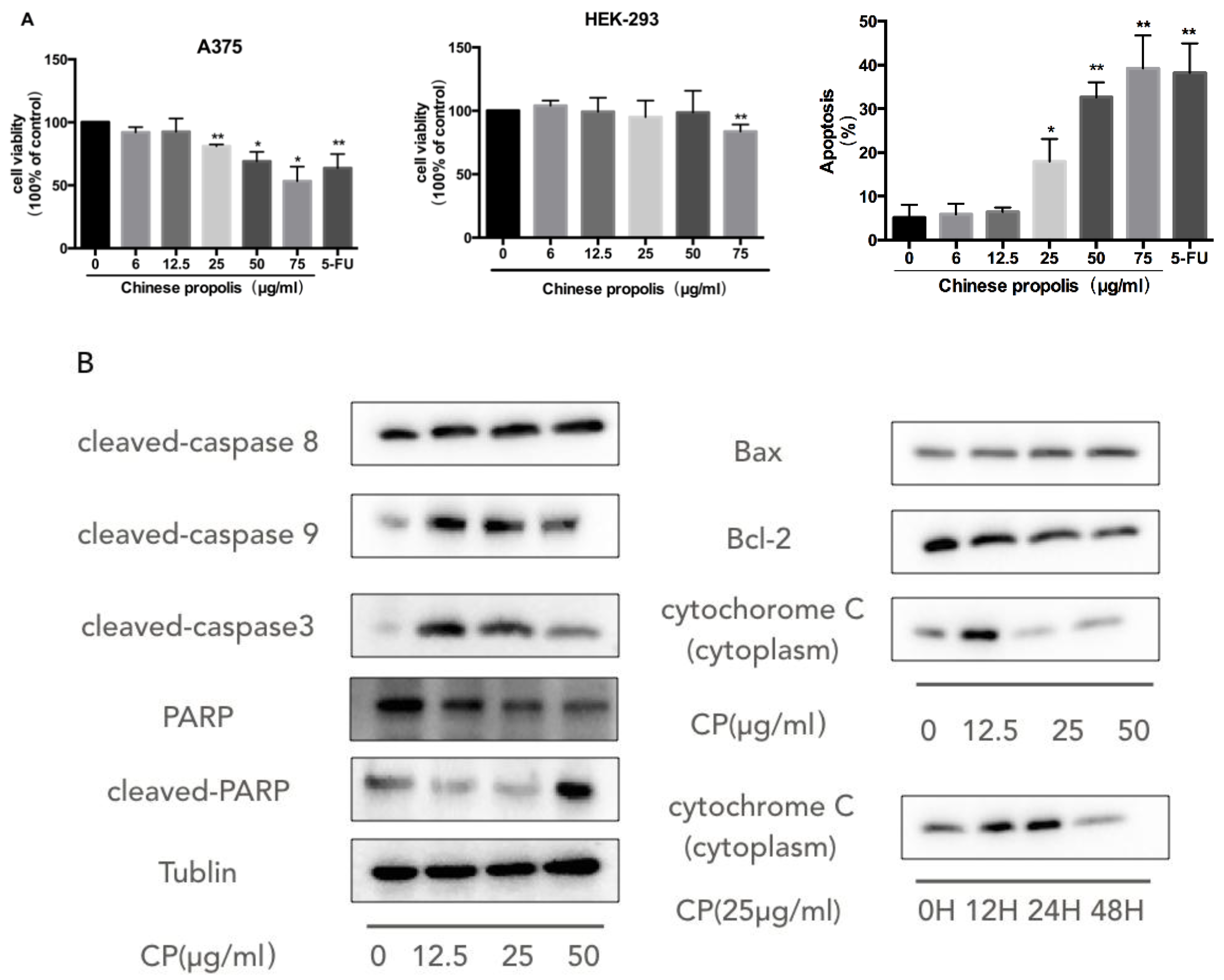
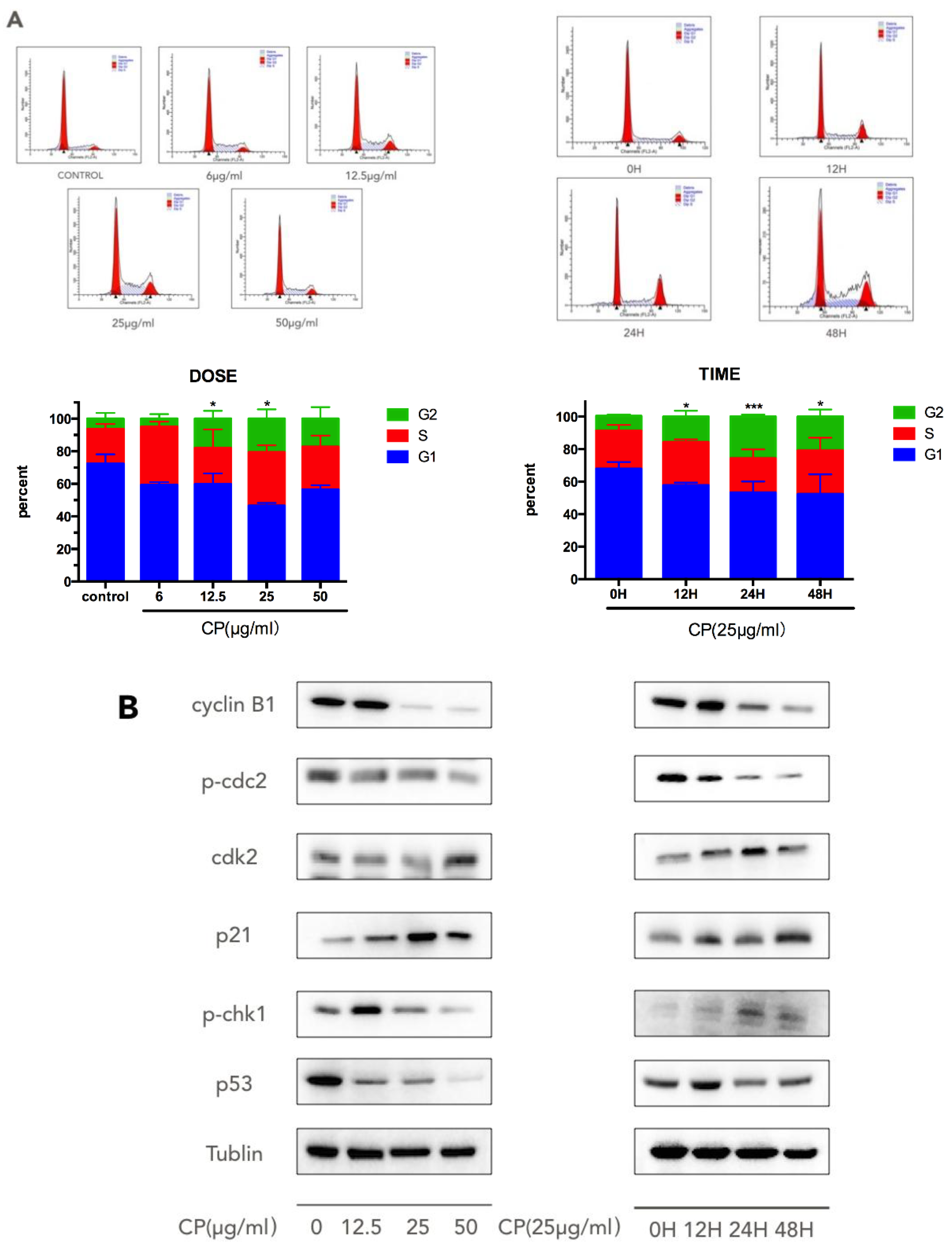
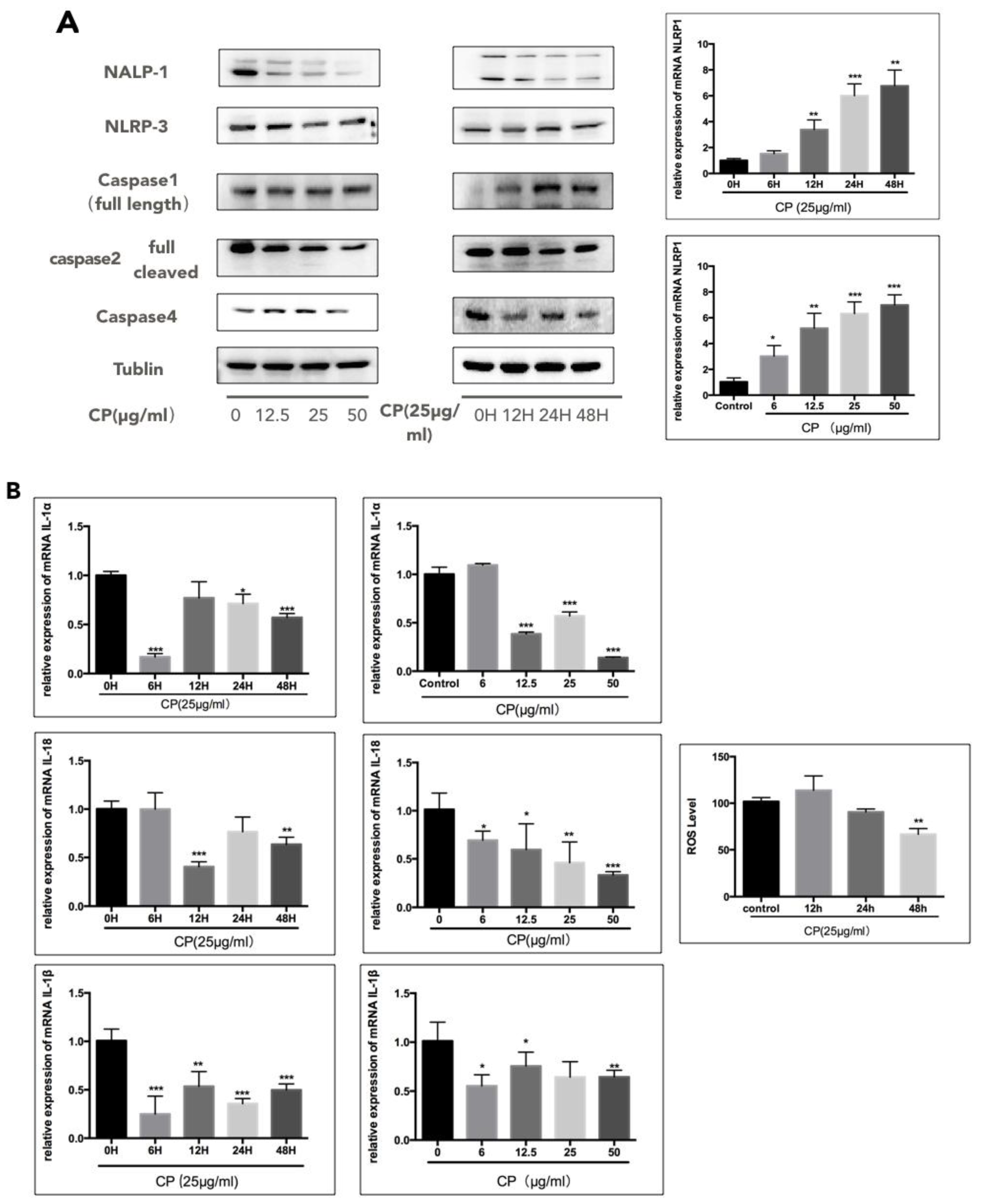
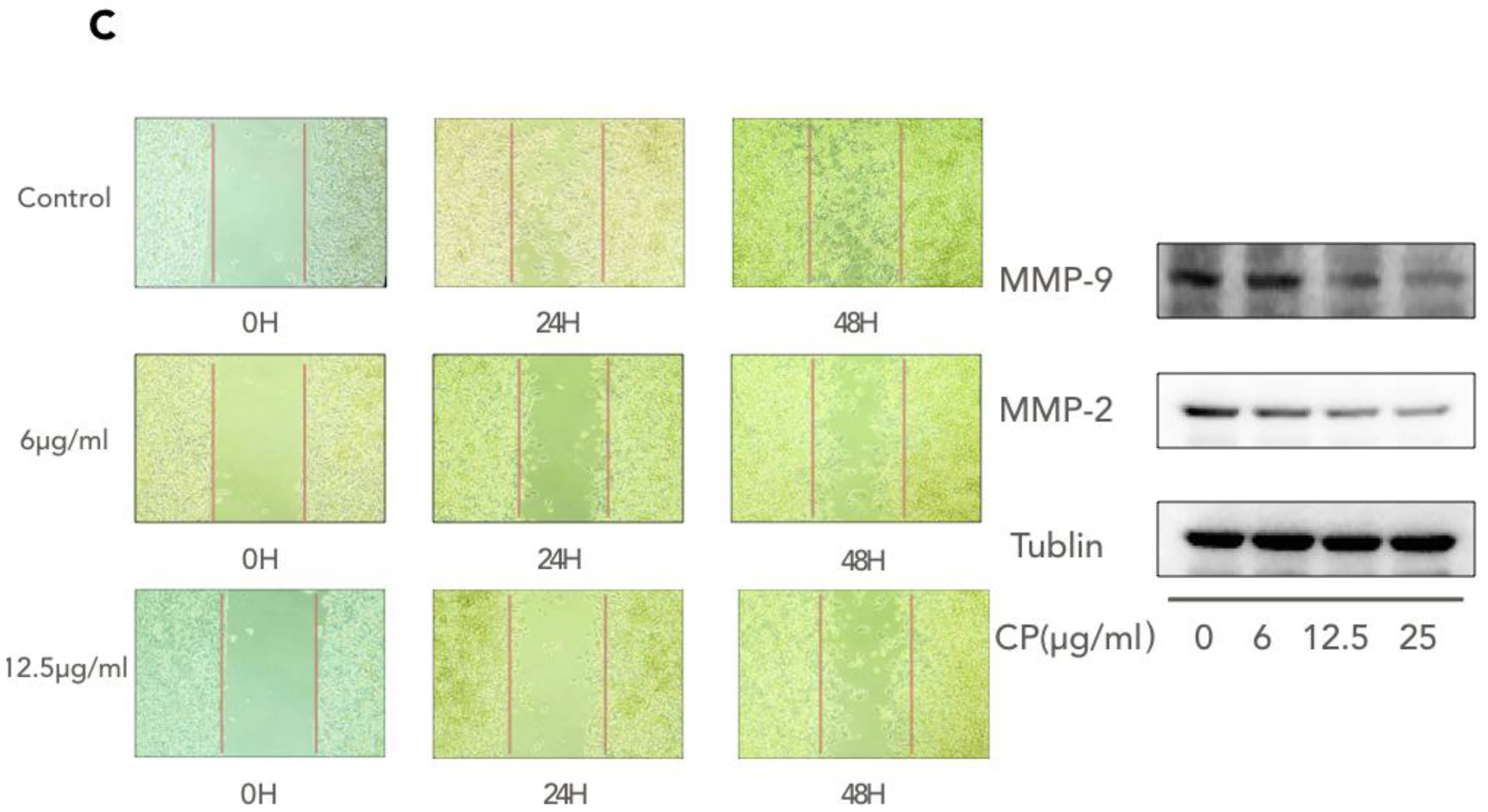
| NAME | Forward | Reverse |
|---|---|---|
| GADPH | CAGGGCTGCTTTTAACTCTGG | TGGGTGGAATCATATTGGAACA |
| NLRP1 | GCTGGACCAGACAACTCTGA | GGTTTCCGTCTGCTGAAGAT |
| IL-1α | TGGTAGTAGCAACCAACGGGA | ACTTTGATTGAGGGCGTCATTC |
| IL-1β | AGCTACGAATCTCCGACCAC | CGTTATCCCATGTGTCGAAGAA |
| IL-18 | TCTTCATTGACCAAGGAAATCGG | TCCGGGGTGCATTATCTCTAC |
| Compounds | RT(min) | [M + 1]+ | Content (mg/g) |
|---|---|---|---|
| Caffeic acid | 8.26 | 181.04226 | 8.24 |
| p-Coumaric acid | 10.36 | 165.04734 | 4.97 |
| Ferulic acid | 11.35 | 195.05791 | 2.58 |
| Isoferulic acid | 11.87 | 195.05791 | 3.16 |
| Cinnamic acid | 12.21 | 149.05243 | 0.62 |
| Vanillic acid | 15.04 | 169.04226 | |
| Kaempferol | 16.26 | 287.04774 | 1.72 |
| Myricetin | 16.38 | 319.03757 | |
| Quercetin | 16.39 | 303.04265 | 1.75 |
| 3,4-dimethoxycinnamic acid | 17.02 | 209.07356 | 9.26 |
| Apigenin | 18.41 | 271.05282 | 3.61 |
| Pinobanksin | 18.82 | 273.06847 | 35.99 |
| Luteotin | 18.90 | 287.04774 | |
| Caffeic acid benzyl ester | 23.61 | 271.08921 | |
| Chrysin | 23.66 | 255.05791 | 50.99 |
| Pinocembrin | 24.22 | 257.07356 | 43.93 |
| Galangin | 24.44 | 271.05282 | 18.24 |
| CAPE | 24.74 | 285.10486 | 15.79 |
| 3-O-Acetyl pinobanksin | 24.91 | 315.07904 | 73.81 |
| Rutin | 32.10 | 611.15339 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wu, Y.; Chen, X.; Jiang, X.; Wang, K.; Hu, F. Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy. Nutrients 2018, 10, 1170. https://doi.org/10.3390/nu10091170
Zheng Y, Wu Y, Chen X, Jiang X, Wang K, Hu F. Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy. Nutrients. 2018; 10(9):1170. https://doi.org/10.3390/nu10091170
Chicago/Turabian StyleZheng, Yufei, Yuqi Wu, Xi Chen, Xiasen Jiang, Kai Wang, and Fuliang Hu. 2018. "Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy" Nutrients 10, no. 9: 1170. https://doi.org/10.3390/nu10091170
APA StyleZheng, Y., Wu, Y., Chen, X., Jiang, X., Wang, K., & Hu, F. (2018). Chinese Propolis Exerts Anti-Proliferation Effects in Human Melanoma Cells by Targeting NLRP1 Inflammatory Pathway, Inducing Apoptosis, Cell Cycle Arrest, and Autophagy. Nutrients, 10(9), 1170. https://doi.org/10.3390/nu10091170







