Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
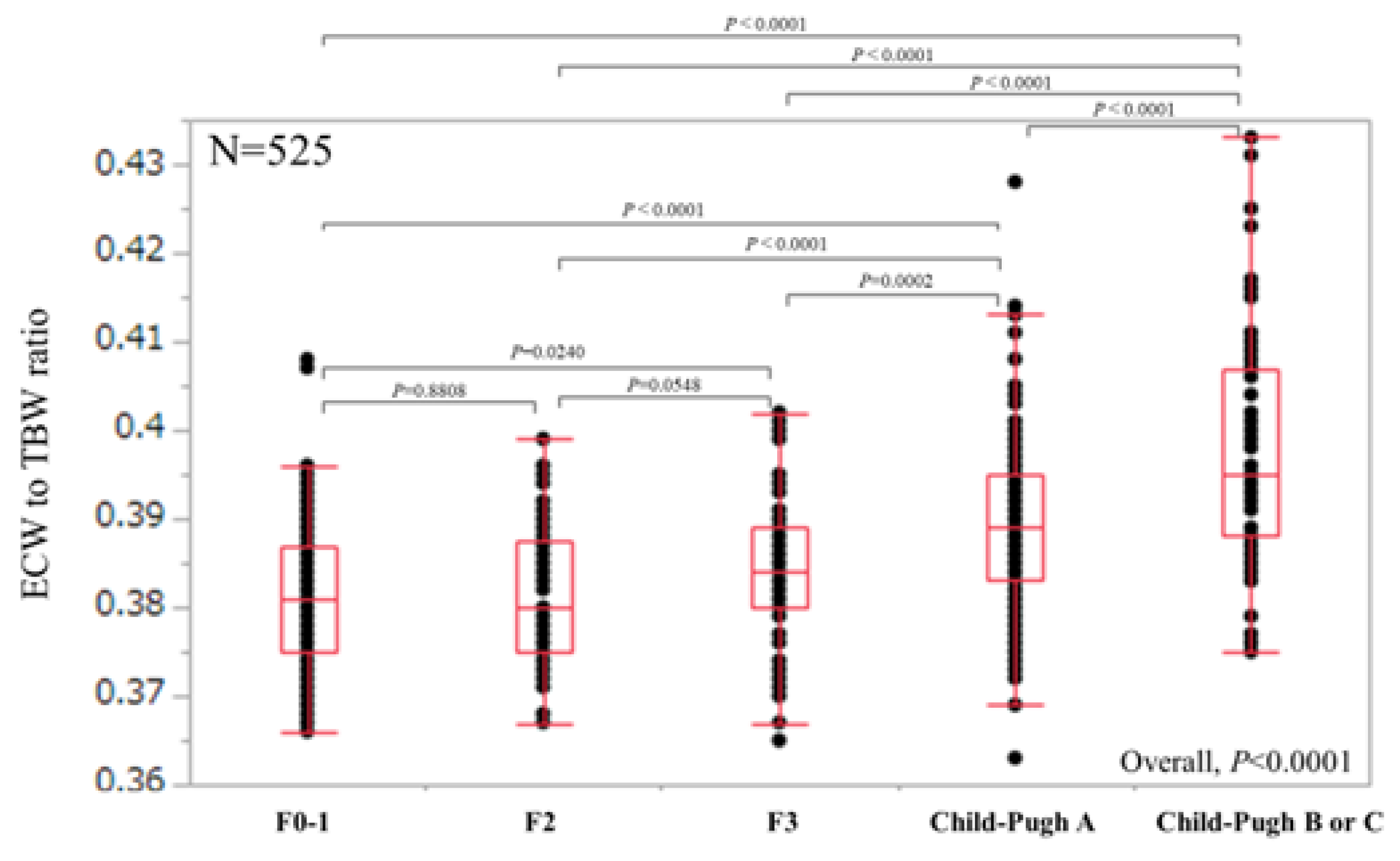
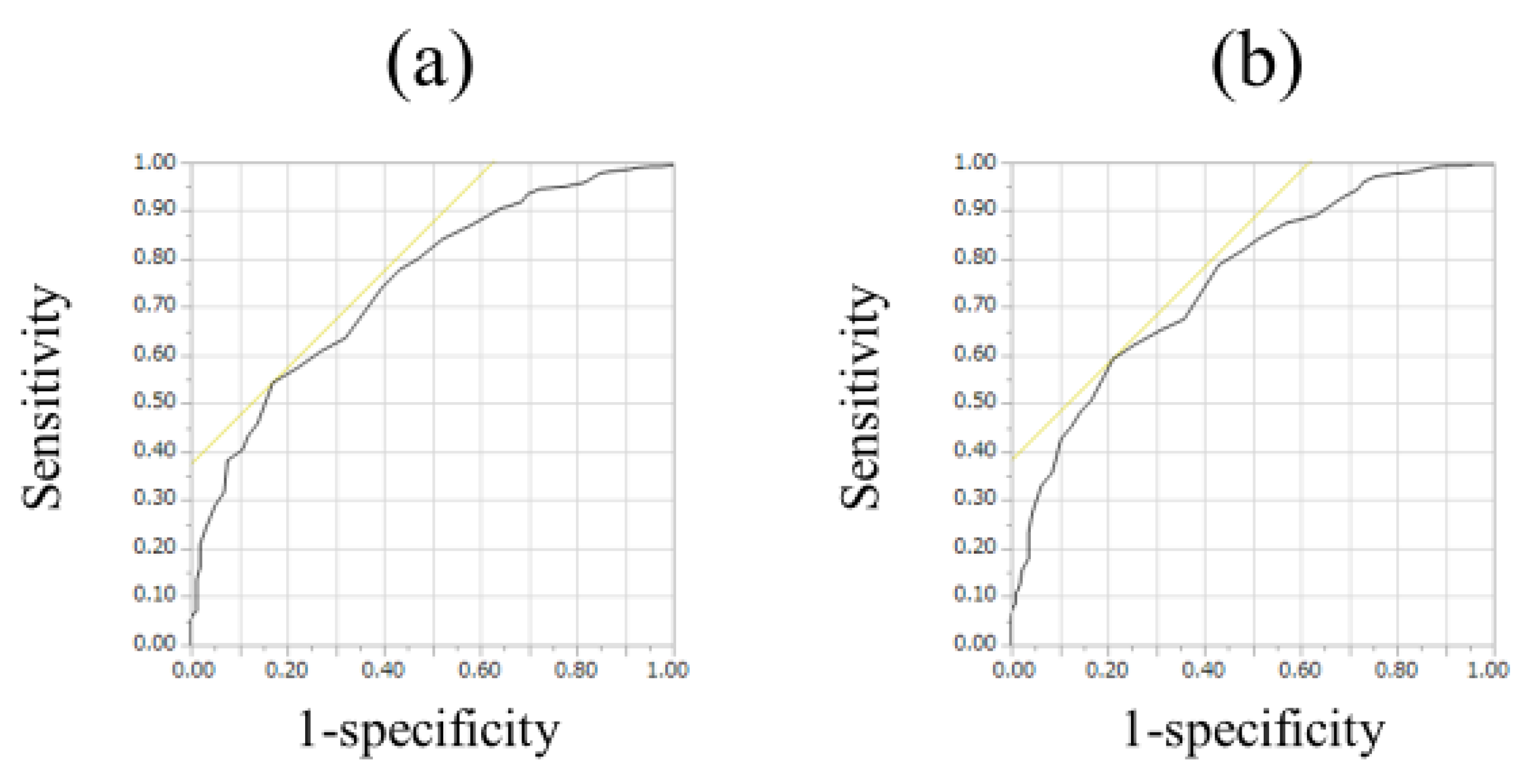
3.2. ECW/TBW According to Liver Fibrosis Stage and ROC Analyses for F3 or More and F4 in All Cases
3.3. Relationship between ECW/TBW and Other Clinical Parameters
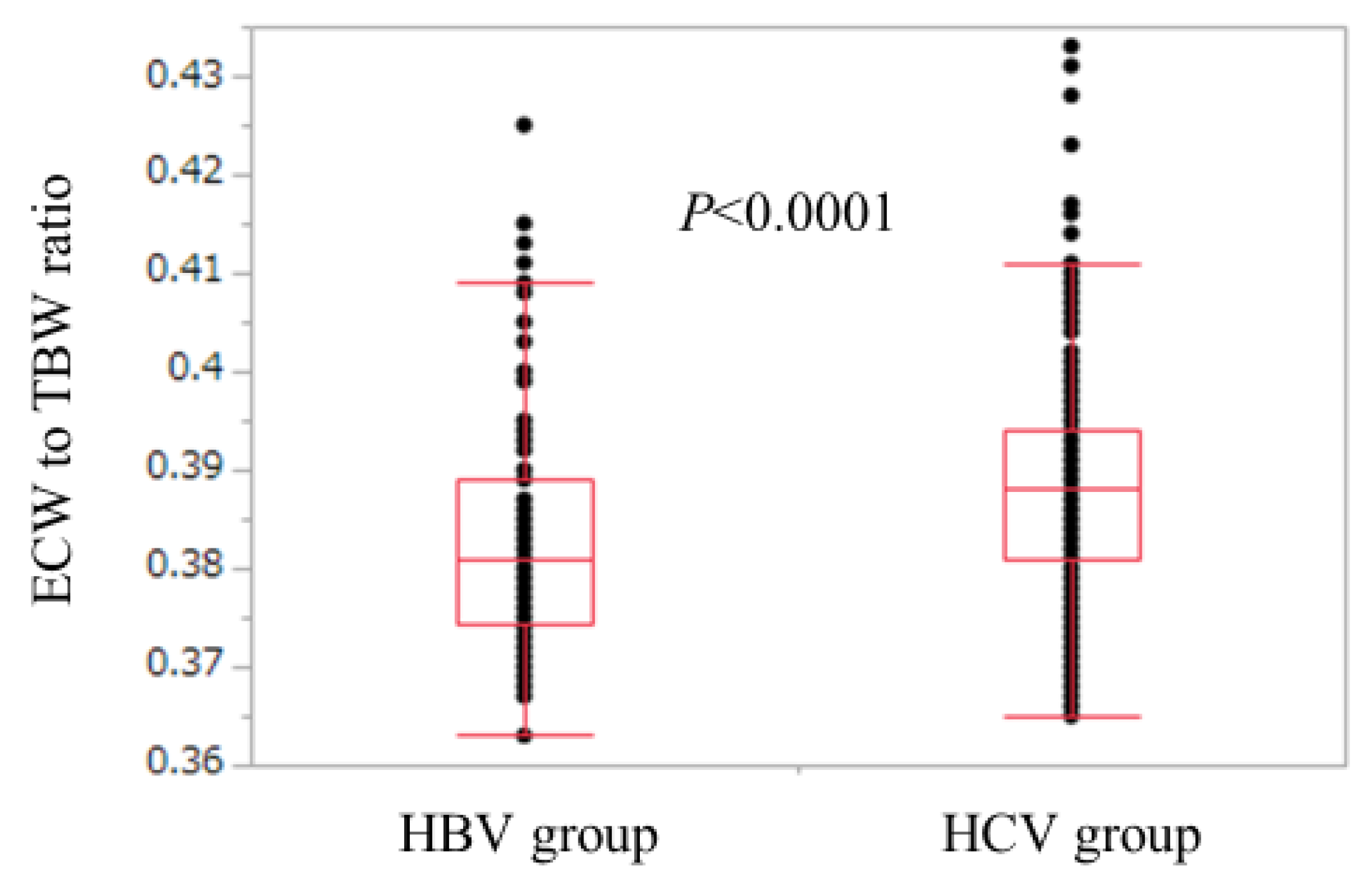
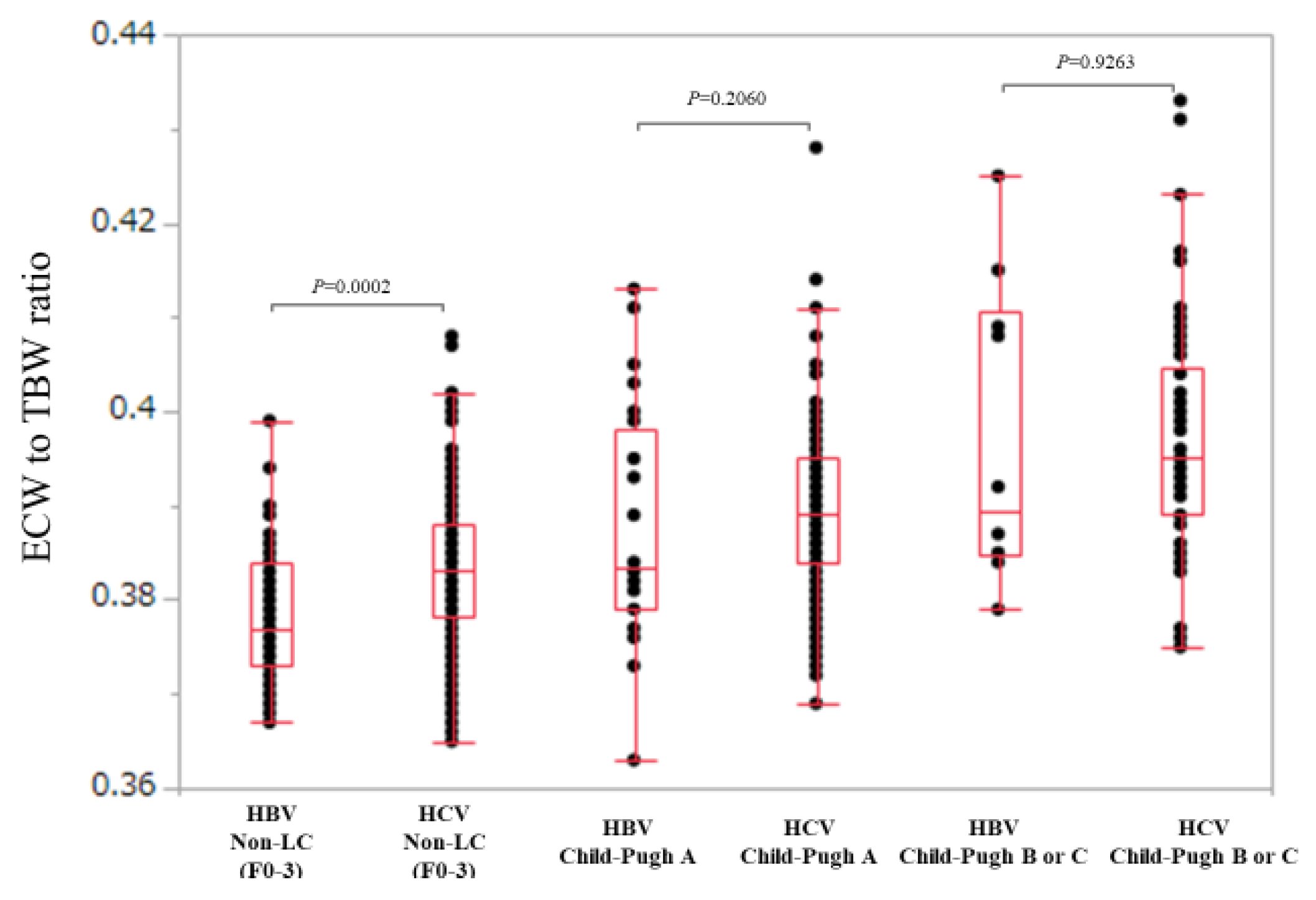
3.4. Comparison of ECW/TBW in the HBV Group and the HCV Group According to Liver Fibrosis Stage (Non-LC, Child-Pugh A and Child-Pugh B or C)
3.5. Unilabiate and Multivariate Analyses of Factors Associated with ECW/TBW ≥0.4
3.6. Comparison of ECW/TBW in the HBV Group and the HCV Group according to FIB-4 Index
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | bioimpedance analysis |
| ECF | extracellular fluid |
| ECW | extracellular water |
| TBW | total body water |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| LC | liver cirrhosis |
| BMI | body mass index |
| SMI | skeletal muscle mass index |
| ROC | receiver operating characteristics |
| AUROC | area under the ROC curve |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| BTR | branched-chain amino acid to tyrosine ratio |
| PT | prothrombin time |
References
- Malbrain, M.L.; Huygh, J.; Dabrowski, W.; De Waele, J.J.; Staelens, A.; Wauters, J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: A bench-to-bedside review. Anaesthesiol. Intensive Ther. 2014, 46, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Chumlea, W.C.; Schubert, C.M.; Sun, S.S.; Demerath, E.; Towne, B.; Siervogel, R.M. A review of body water status and the effects of age and body fatness in children and adults. J. Nutr. Health Aging 2007, 11, 111–118. [Google Scholar] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.L.; Churchwell, K.B.; Strange, K. Regulation of cell volume in health and disease. N. Engl. J. Med. 1995, 333, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Newstead, C.G. The ratio of extracellular fluid to total bodywater and technique survival in peritoneal dialysis patients. Perit. Dial. Int. 2004, 24, 353–358. [Google Scholar] [PubMed]
- Nescolarde, L.; Piccoli, A.; Roman, A.; Nunez, A.; Morales, R.; Doñate, T.; Rosell, J. Bioelectrical impedance vector analysis in haemodialysis patients: Relation between oedema and mortality. Physiol. Meas. 2004, 25, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Wang, C.H.; Huang, Y.Y.; Tung, T.H.; Lee, C.M.; Liu, P.C.; Cherng, W.J. Edema index established by a segmental multifrequency bioelectrical impedance analysis provides prognostic value in acute heart failure. J. Cardiovasc. Med. 2012, 13, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, A.; Hilsenbeck, J.; Nyga, M.; Ertle, J.; Wree, A.; Gerken, G.; Canbay, A.E. Bioelectrical impedance analysis in clinical practice: Implications for hepatitis C therapy BIA and hepatitis C. Virol. J. 2010, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Slinde, F.; Gronberg, A.; Engstrom, C.P.; Rossander-Hulthen, L.; Larsson, S. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir. Med. 2005, 99, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, A.; Beisenherz, A.; Romer, K.; Kremer, G.; Salzberger, B.; Elia, M. Phase angle from bioelectrical impedance analysis remains an independent predictive marker in HIV-infected patients in the era of highly active antiretroviral treatment. Am. J. Clin. Nutr. 2000, 72, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Bolarin, D.M.; Azinge, E.C. Biochemical markers, extracellular components in liver fibrosis and cirrhosis. Nig. Q. J. Hosp. Med. 2007, 17, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y. Liver Cirrhosis: Evaluation, Nutritional Status, and Prognosis. Mediators Inflamm. 2015, 2015, 872152. [Google Scholar] [CrossRef] [PubMed]
- Pose, E.; Cardenas, A. Translating Our Current Understanding of Ascites Management into New Therapies for Patients with Cirrhosis and Fluid Retention. Dig. Dis. 2017, 35, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Fukui, H.; Yoshiji, H. Treatment for cirrhotic ascites. Hepatol. Res. 2017, 47, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Solà, E.; Solé, C.; Ginès, P. Management of uninfected and infected ascites in cirrhosis. Liver Int. 2016, 36, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Comparison of Prognostic Impact between the Child-Pugh Score and Skeletal Muscle Mass for Patients with Liver Cirrhosis. Nutrients 2017, 9, E595. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Iwata, Y.; Nishimura, T.; Iijima, H.; Nishiguchi, S. Clinical utility of bioimpedance analysis in liver cirrhosis. J. Hepato-Biliary-Pancreat. Sci. 2017, 24, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Kiriyama, S.; Tanikawa, M.; Hisanaga, Y.; Kanamori, A.; Kitabatake, S.; Yama, T.; Tanaka, J. Long-term prognosis of patients with chronic hepatitis C who did not receive interferon-based therapy: Causes of death and analysis based on the FIB-4 index. J. Gastroenterol. 2016, 51, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Kumada, T.; Toyoda, H.; Tsuji, K.; Hiraoka, A.; Tanaka, J. Impact of FIB-4 index on hepatocellular carcinoma incidence during nucleos(t) ide analogue therapy in patients with chronic hepatitis B: An analysis using time-dependent receiver operating characteristic. J. Gastroenterol. Hepatol. 2017, 32, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Nishijima, N.; Enomoto, H.; Sakamoto, A.; Nasu, A.; Komekado, H.; Nishimura, T.; Kita, R.; Kimura, T.; Iijima, H.; et al. Comparison of FIB-4 index and aspartate aminotransferase to platelet ratio index on carcinogenesis in chronic hepatitis B treated with entecavir. J. Cancer 2017, 8, 152–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malczyk, E.; Dzięgielewska-Gęsiak, S.; Fatyga, E.; Ziółko, E.; Kokot, T.; Muc-Wierzgon, M. Body composition in healthy older persons: Role of the ratio of extracellular/total body water. J. Biol. Regul. Homeost. Agents 2016, 30, 767–772. [Google Scholar] [PubMed]
- Tajiri, K.; Shimizu, Y. Liver physiology and liver diseases in the elderly. World, J. Gastroenterol. 2013, 19, 8459–8467. [Google Scholar] [CrossRef] [PubMed]
- Asahina, Y.; Tsuchiya, K.; Tamaki, N.; Hirayama, I.; Tanaka, T.; Sato, M.; Yasui, Y.; Hosokawa, T.; Ueda, K.; Kuzuya, T.; et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 2010, 52, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhaveri, M.A.; Manne, V.; Kowdley, K.V. Chronic Hepatitis C in Elderly Patients: Current Evidence with Direct-Acting Antivirals. Drugs Aging 2018, 35, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bruguera, M. Liver diseases in the elderly. Gastroenterol. Hepatol. 2014, 37, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Ianni Filho, D.; Boin, I.F.S.F.; Yamanaka, A. Bioimpedance: New approach to non-invasive detection of liver fibrosis-A pilot study. Arq. Gastroenterol. 2018, 55, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Ricci, C.S.; Zaccherini, G. Role of human albumin in the management of complications of liver cirrhosis. J. Clin. Exp. Hepatol. 2014, 4, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Review article: Sarcopenia in cirrhosis-aetiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Panorchan, K.; Nongnuch, A.; El-Kateb, S.; Goodlad, C.; Davenport, A. Changes in muscle and fat mass with haemodialysis detected by multi-frequency bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2015, 69, 1109–1112. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Cases (n = 525) | HCV Group (n = 440) | HBV Group (n = 85) | p Value (HCV vs. HBV) |
|---|---|---|---|---|
| Age (years) | 64.4 (20.8–94.0) | 65.0 (20.8–94.0) | 55.8 (28.8–77.0) | <0.0001 |
| Gender, male/female | 268/257 | 218/222 | 50/35 | 0.1247 |
| Body mass index (kg/m2) | 22.2 (13.1–34.4) | 22.2 (13.1–34.4) | 22.2 (15.9–30.4) | 0.6839 |
| ECW to TBW ratio | 0.387 (0.363–0.433) | 0.388 (0.365–0.433) | 0.381 (0.363–0.425) | <0.0001 |
| Skeletal muscle mass index (cm2/m2) | 6.56 (3.50–10.21) | 6.51 (3.50–10.21) | 6.92 (5.21–9.90) | 0.0099 |
| Liver histology (F0-1, F2, F3, Child-Pugh A/B/C) | 106/53/64/226/72/4 | 79/41/56/198/62/4 | 27/12/8/28/10/0 | 0.0394 |
| Total bilirubin (mg/dL) | 0.9 (0.2–5.1) | 0.9 (0.2–5.1) | 0.9 (0.3–2.8) | 0.5264 |
| Serum albumin (g/dL) | 3.9 (2.3–5.1) | 3.9 (2.3–5.1) | 4.0 (2.7–5.1) | 0.0017 |
| Prothrombin time (%) | 87.0 (39.2–130.3) | 86.4 (39.2–130.3) | 89.6 (43.3–123.0) | 0.2371 |
| Platelet count (× 104/mm3) | 12.8 (2.6–48.1) | 12.4 (3.0–48.1) | 14.9 (2.6–37.9) | 0.0022 |
| Serum creatinine (mg/dL) | 0.68 (0.30–7.69) | 0.67 (0.30–7.69) | 0.75 (0.46–6.0) | 0.0021 |
| Total cholesterol (mg/dL) | 156 (73–293) | 153 (73–293) | 171 (82–293) | 0.0100 |
| Triglyceride (mg/dL) | 85 (25–554) | 85 (25–554) | 85 (30–256) | 0.5929 |
| AST (IU/L) | 38 (10–756) | 40 (10–349) | 30 (13–756) | <0.0001 |
| ALT (IU/L) | 33 (7–1079) | 34 (7–258) | 29 (7–1979) | 0.0304 |
| BTR | 4.95 (1.65–19.68) | 4.77 (1.65–19.68) | 5.85 (1.82–11.28) | 0.0002 |
| FIB-4 index | 3.56 (0.22–20.04) | 3.80 (0.22–20.04) | 1.95 (0.37–19.14) | <0.0001 |
| Fasting blood glucose (mg/dL) | 99 (64–403) | 100 (64–403) | 95 (70–283) | 0.1764 |
| rs | p Value | |
|---|---|---|
| Age | 0.6085 | <0.0001 |
| Body mass index | −0.0742 | 0.0896 |
| Skeletal muscle index | −0.3059 | <0.0001 |
| Total bilirubin | 0.0432 | 0.3228 |
| Serum albumin | −0.4145 | <0.0001 |
| Prothrombin time | −0.4210 | <0.0001 |
| Platelet count | −0.3241 | <0.0001 |
| Serum creatinine | −0.1764 | <0.0001 |
| Total cholesterol | −0.2509 | <0.0001 |
| Triglyceride | −0.1980 | <0.0001 |
| AST | 0.0478 | 0.2740 |
| ALT | −0.2709 | <0.0001 |
| FIB-4 index | 0.4915 | <0.0001 |
| BTR | −0.4076 | <0.0001 |
| Fasting blood glucose | 0.0558 | 0.2025 |
| Variables | ECW to TBW Ratio ≥0.4 (n = 52) | ECW to TBW Ratio <0.4 (n = 473) | p Value |
|---|---|---|---|
| Age (years) | 73.0 (49.5–94.0) | 63.2 (20.8–89.8) | <0.0001 |
| Gender, male/female | 22/30 | 246/227 | 0.1919 |
| Cause of liver disease HBV/HCV | 9/43 | 76/397 | 0.8428 |
| Body mass index (kg/m2) | 23.0 (16.5–31.8) | 22.1 (13.1–34.4) | 0.3275 |
| Skeletal muscle index | 6.46 (4.40–10.21) | 6.58 (3.50–9.57) | 0.5380 |
| Total bilirubin (mg/dL) | 0.9 (0.2–3.1) | 0.9 (0.2–5.1) | 0.3063 |
| Serum albumin (g/dL) | 3.2 (2.3–5.0) | 3.9 (2.6–5.1) | <0.0001 |
| Prothrombin time (%) | 71.75 (39.2–118.8) | 88 (43.3–130.3) | <0.0001 |
| Platelet count (× 104/mm3) | 11.05 (3.0–37.9) | 13.0 (2.6–48.1) | 0.1081 |
| Serum creatinine (mg/dl) | 0.73 (0.35–4.51) | 0.68 (0.3–7.69) | 0.3141 |
| Total cholesterol (mg/dl) | 132 (73–266) | 158 (82–293) | 0.0002 |
| Triglyceride (mg/dl) | 72 (25–149) | 88 (25–554) | 0.0035 |
| AST (IU/L) | 39 (15–105) | 38 (10–756) | 0.7295 |
| ALT (IU/L) | 23.5 (10–82) | 35 (7–1079) | <0.0001 |
| BTR | 3.75 (1.65–9.63) | 5.06 (1.76–19.68) | 0.0002 |
| Fasting blood sugar (mg/dl) | 98.5 (72–215) | 99 (64–403) | 0.3624 |
| FIB-4 index | 6.40 (1.16–19.14) | 3.40 (0.22–20.04) | <0.0001 |
| Variables | Multivariate Analysis | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p Value | |
| Age ≥64.4 years | 3.921 | 1.802–8.526 | 0.0006 |
| Serum albumin <3.9 g/dL | 4.295 | 1.890–9.761 | 0.0005 |
| PT <87.0% | 2.607 | 1.089–6.241 | 0.0314 |
| ALT <33 IU/l | 4.143 | 2.059–8.336 | <0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients 2018, 10, 1072. https://doi.org/10.3390/nu10081072
Nishikawa H, Yoh K, Enomoto H, Ishii N, Iwata Y, Nakano C, Takata R, Nishimura T, Aizawa N, Sakai Y, et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients. 2018; 10(8):1072. https://doi.org/10.3390/nu10081072
Chicago/Turabian StyleNishikawa, Hiroki, Kazunori Yoh, Hirayuki Enomoto, Noriko Ishii, Yoshinori Iwata, Chikage Nakano, Ryo Takata, Takashi Nishimura, Nobuhiro Aizawa, Yoshiyuki Sakai, and et al. 2018. "Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis" Nutrients 10, no. 8: 1072. https://doi.org/10.3390/nu10081072
APA StyleNishikawa, H., Yoh, K., Enomoto, H., Ishii, N., Iwata, Y., Nakano, C., Takata, R., Nishimura, T., Aizawa, N., Sakai, Y., Ikeda, N., Hasegawa, K., Takashima, T., Iijima, H., & Nishiguchi, S. (2018). Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients, 10(8), 1072. https://doi.org/10.3390/nu10081072




