Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes
Abstract
1. Introduction
2. Changes in Clinical Presentation
3. Variable Definitions for Symptoms and Findings
4. Epidemiology and Pathogenesis of Extraintestinal Manifestations
4.1. Poor Growth
4.2. Anemia
4.3. Neurologic Manifestations
4.4. Dental Enamel Defects
4.5. Liver Abnormalities
4.6. Joint Manifestations
4.7. Dermatitis Herpetiformis
4.8. Bone Disease
4.9. Problems in Reproductive and Endocrine Systems
4.10. Other Extraintestinal Manifestations
5. Effects of Dietary Treatment to the Extraintestinal Manifestations
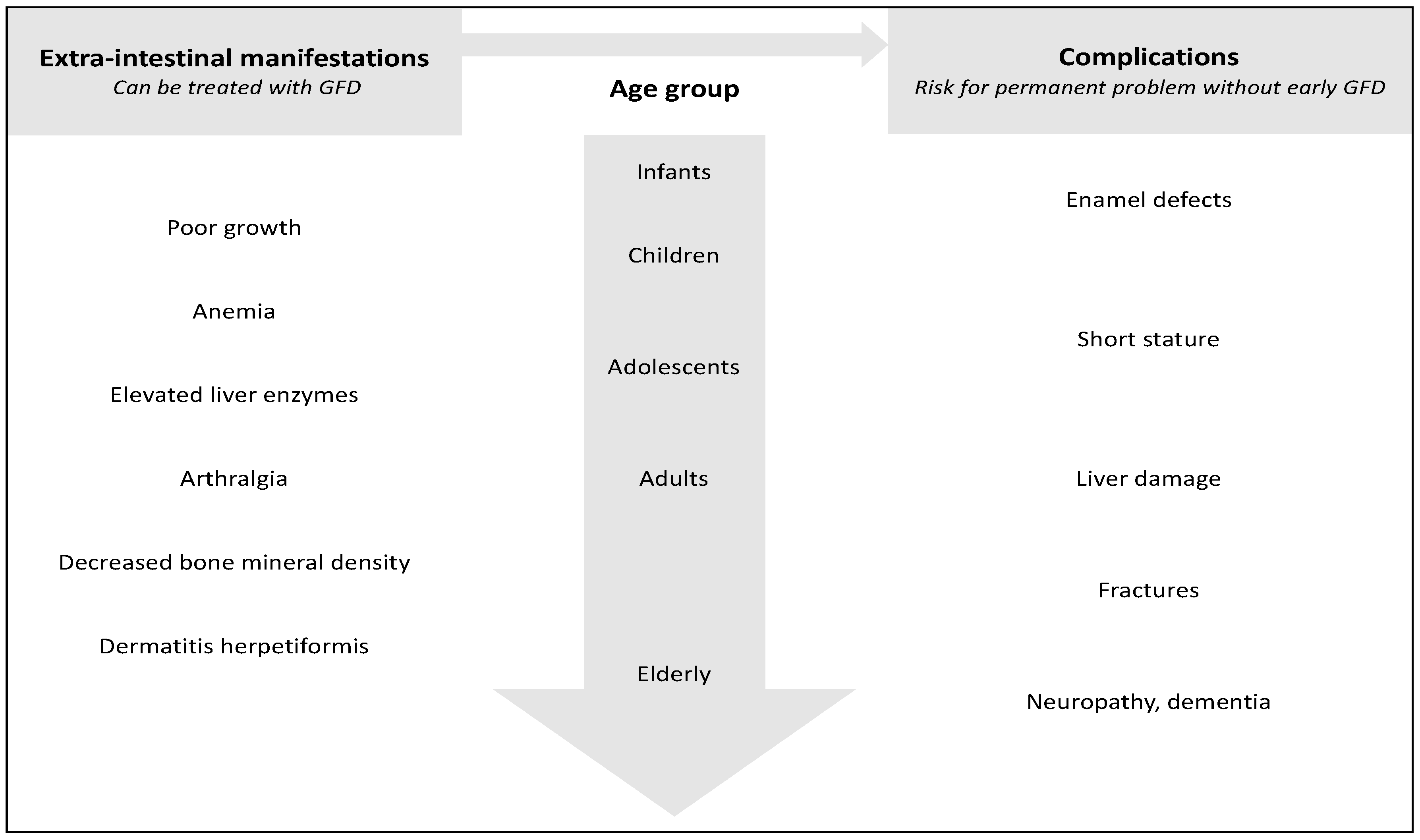
6. Importance of Early Diagnosis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global prevalence of celiac disease: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Virta, L.J.; Kaukinen, K.; Collin, P. Incidence and prevalence of diagnosed coeliac disease in Finland: Results of effective case finding in adults. Scand. J. Gastroenterol. 2009, 44, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Mårild, K.; Kahrs, C.R.; Tapia, G.; Stene, L.C.; Størdal, K. Infections and risk of celiac disease in childhood: A prospective nationwide cohort study. Am. J. Gastroenterol. 2015, 110, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Dong, F.; Barón, A.E.; Taki, I.; Norris, J.M.; Frohnert, B.I.; Hoffenberg, E.J.; Rewers, M. High incidence of celiac disease in a long-term study of adolescents with susceptibility genotypes. Gastroenterology 2017, 152, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Kaukinen, K.; Lähdeaho, M.-L.; Huhtala, H.; Ashorn, M.; Ruuska, T.; Hiltunen, P.; Visakorpi, J.; Mäki, M.; Kurppa, K. Presentation of celiac disease in Finnish children is no longer changing: A 50-year perspective. J. Pediatr. 2015, 167, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Mäki, M.; Sanders, D.S.; Williamson, C.A.; Grünewald, R.A.; Woodroofe, N.M.; Korponay-Szabó, I.R. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 2006, 66, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Hervonen, K.; Kautiainen, H.; Collin, P.; Reunala, T. Prevalence and incidence of dermatitis herpetiformis: A 40-year prospective study from Finland. Br. J. Dermatol. 2011, 165, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Äärelä, L.; Nurminen, S.; Kivelä, L.; Huhtala, H.; Mäki, M.; Viitasalo, A.; Kaukinen, K.; Lakka, T.; Kurppa, K. Prevalence and associated factors of abnormal liver values in children with celiac disease. Dig. Liver Dis. 2016, 48, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Tersigni, C.; Castellani, R.; De Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Update 2014, 20, 582–593. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.E.; Castiglione, D.A.; Butzner, J.D. The changing face of childhood celiac disease in North America: Impact of serological testing. Pediatrics 2009, 124, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, S.; Kivelä, L.; Taavela, J.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Factors associated with growth disturbance at celiac disease diagnosis in children: A retrospective cohort study. BMC Gastroenterol. 2015, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Rajalahti, T.; Repo, M.; Kivelä, L.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Lindfors, K.; Kurppa, K. Anemia in pediatric celiac disease: Association with clinical and histological features and response to gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Björck, S.; Brundin, C.; Karlsson, M.; Agardh, D. Reduced bone mineral density in children with screening-detected celiac disease. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, W.; Brow, J.; Parker, F.; Rubin, C. The small intestinal mucosa in dermatitis herpetiformis. Gastroenterology 1971, 60, 362–369. [Google Scholar] [PubMed]
- Jericho, H.; Sansotta, N.; Guandalini, S. Extraintestinal manifestations of celiac disease: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Bonura, A.; Garavaglia, D.; Rulli, E.; Floriani, I.; Tagliabue, G.; Contiero, P.; Bardella, M.T. Immunological comorbity in coeliac disease: Associations, risk factors and clinical implications. J. Clin. Immunol. 2012, 32, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Sategna Guidetti, C.; Solerio, E.; Scaglione, N.; Aimo, G.; Mengozzi, G. Duration of gluten exposure in adult coeliac disease does not correlate with the risk for autoimmune disorders. Gut 2001, 49, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.; Magazzù, G.; Greco, L. Duration of exposure to gluten and risk for autoimmune disorders in patients with celiac disease. SIGEP Study Group for Autoimmune Disorders in Celiac Disease. Gastroenterology 1999, 117, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nurminen, S.; Kivelä, L.; Huhtala, H.; Kaukinen, K.; Kurppa, K. Extraintestinal manifestations were common in children with celiac disease and were more prevalent in patients with more severe clinical and histological presentation. Acta Paediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabó, I.R.; Halttunen, T.; Szalai, Z.; Laurila, K.; Király, R.; Kovács, J.B.; Fésüs, L.; Mäki, M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Aeschlimann, P.; Sanders, D.S.; Mäki, M.; Kaukinen, K.; Grünewald, R.A.; Bandmann, O.; Woodroofe, N.; Haddock, G.; Aeschlimann, D.P. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology 2013, 80, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- De Leo, L.; Aeschlimann, D.; Hadjivassiliou, M.; Aeschlimann, P.; Salce, N.; Vatta, S.; Ziberna, F.; Cozzi, G.; Martelossi, S.; Ventura, A.; et al. Anti-transglutaminase 6 antibody development in children with celiac disease correlates with duration of gluten exposure. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Sárdy, M.; Kárpáti, S.; Merkl, B.; Paulsson, M.; Smyth, N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 2002, 195, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Riches, P.L.; McRorie, E.; Fraser, W.D.; Determann, C.; van’t Hof, R.; Ralston, S.H. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N. Engl. J. Med. 2009, 361, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Volta, C.; Ziveri, M.A.; Zanacca, C.; Banchini, G.; Viani, I.; Rossi, M.; Virdis, R.; Bernasconi, S. Changes and relationships of IGFS and IGFBPS and cytokines in coeliac disease at diagnosis and on gluten-free diet. Clin. Endocrinol. (Oxf.) 2008, 68, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hervonen, K.; Karell, K.; Holopainen, P.; Collin, P.; Partanen, J.; Reunala, T. Concordance of dermatitis herpetiformis and celiac disease in monozygous twins. J. Investig. Dermatol. 2000, 115, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.; Kolho, K.L.; Westerholm-Ormio, M.; Verkasalo, M. Clinics of coeliac disease in children in the 2000s. Acta Paediatr. Int. J. Paediatr. 2010, 99, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M. Celiac Disease: Evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics 2005, 116, e754–e759. [Google Scholar] [CrossRef] [PubMed]
- Roma, E.; Panayiotou, J.; Karantana, H.; Constantinidou, C.; Siakavellas, S.I.; Krini, M.; Syriopoulou, V.P.; Bamias, G. Changing pattern in the clinical presentation of pediatric celiac disease: A 30-year study. Digestion 2009, 80, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zelnik, N.; Pacht, A.; Obeid, R.; Lerner, A. Range of neurologic disorders in patients with celiac disease. Pediatrics 2004, 113, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, G.; Cataldo, F.; Rotolo, N.; Spina, M.; Corazza, G.R. The clinical pattern of subclinical/silent celiac disease: An analysis on 1026 consecutive cases. Am. J. Gastroenterol. 1999, 94, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Spierings, E.; Wolters, V.M.; Otten, H.G.; ten Kate, F.J.W.; Houwen, R.H.J. Children with celiac disease and high tTGA are genetically and phenotypically different. World J. Gastroenterol. 2013, 19, 7114–7120. [Google Scholar] [CrossRef] [PubMed]
- Abu Daya, H.; Lebwohl, B.; Lewis, S.K.; Green, P.H. Celiac disease patients presenting with anemia have more severe disease than those presenting with diarrhea. Clin. Gastroenterol. Hepatol. 2013, 11, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Malahias, T.; Brar, P.; Minaya, M.T.; Green, P.H.R. The Association between celiac disease, dental enamel defects, and aphthous ulcers in a United States cohort. J. Clin. Gastroenterol. 2010, 44, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Aine, L.; Maki, M.; Collin, P.; Keyrilainen, O. Dental enamel defects in celiac disease. J. Oral Pathol. Med. 1990, 19, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Stavropoulos, S.N.; Panagi, S.G.; Goldstein, S.L.; Mcmahon, D.J.; Absan, H.; Neugut, A.I. Characteristics of adult celiac disease in the USA: Results of a national survey. Am. J. Gastroenterol. 2001, 96, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Jansson, U.H.G.; Kristiansson, B.; Magnusson, P.; Larsson, L.; Albertsson-Wikland, K.; Bjarnason, R. The decrease of IGF-I, IGF-binding protein-3 and bone alkaline phosphatase isoforms during gluten challenge correlates with small intestinal inflammation in children with coeliac disease. Eur. J. Endocrinol. 2001, 144, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, E.; Giavoli, C.; Elli, L.; Redaelli, A.; Novati, E.; De Bellis, A.; Ronchi, C.L.; Bergamaschi, S.; Lania, A.; Bardella, M.T.; et al. Evaluation of GH-IGF-I axis in adult patients with coeliac disease. Horm. Metab. Res. 2010, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Wierdsma, N.; van Bokhorst-de van der Schueren, M.A.; Berkenpas, M.; Mulder, C.; van Bodegraven, A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef] [PubMed]
- Repo, M.; Lindfors, K.; Mäki, M.; Huhtala, H.; Laurila, K.; Lähdeaho, M.-L.; Saavalainen, P.; Kaukinen, K.; Kurppa, K. Anemia and iron deficiency in children with potential celiac disease. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, L.; Pirttilä, T.; Collin, P. Coeliac disease presenting with neurological disorders. Eur. Neurol. 1999, 42, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Grünewald, R.; Sharrack, B.; Sanders, D.; Lobo, A.; Williamson, C.; Woodroofe, N.; Wood, N.; Davies-Jones, A. Gluten ataxia in perspective: Epidemiology, genetic susceptibility and clinical characteristics. Brain 2003, 126, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Majorana, A.; Bardellini, E.; Ravelli, A.; Plebani, A.; Pol, A.; Campus, G. Implications of gluten exposure period, CD clinical forms, and HLA typing in the association between celiac disease and dental enamel defects in children. A case-control study. Int. J. Paediatr. Dent. 2010, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Halme, L.; Collin, P.; Färkkilä, M.; Mäki, M.; Vehmanen, P.; Partanen, J.; Höckerstedt, K. Celiac disease in patients with severe liver disease: Gluten-free diet may reverse hepatic failure. Gastroenterology 2002, 122, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Baschè, R.; Ferraresi, A.; Pigozzi, M.G.; Ricci, C.; Lanzarotto, F.; Villanacci, V.; Lanzini, A. Factors that contribute to hypertransaminasemia in patients with celiac disease or functional gastrointestinal syndromes. Clin. Gastroenterol. Hepatol. 2014, 12, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Novacek, G.; Miehsler, W.; Wrba, F.; Ferenci, P.; Penner, E.; Vogelsang, H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur. J. Gastroenterol. Hepatol. 2000, 11, 283–288. [Google Scholar] [CrossRef]
- Marciano, F.; Savoia, M.; Vajro, P. Celiac disease-related hepatic injury: Insights into associated conditions and underlying pathomechanisms. Dig. Liver Dis. 2016, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Daron, C.; Soubrier, M.; Mathieu, S. Occurrence of rheumatic symptoms in celiac disease: A meta-analysis: Comment on the article “Osteoarticular manifestations of celiac disease and non-celiac gluten hypersensitivity” by Dos Santos and Lioté. Joint Bone Spine 2016. Jt. Bone Spine 2017, 84, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Usai, P.; Francesa, M.; Piga, M.; Cacace, E.; Antonia Lai, M.; Beccaris, A.; Piras, E.; La Nasa, G.; Mulargia, M.; Balestrieri, A. Adult celiac disease is frequently associated with sacroiliitis. Dig. Dis. Sci. 1995, 40, 1906–1908. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, E.; Ciacci, C.; Ames, P.R.; Mazzacca, G.; Oriente, P.; Scarpa, R. The arthritis of coeliac disease: Prevalence and pattern in 200 adult patients. Br. J. Rheumatol. 1996, 35, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Iagnocco, A.; Ceccarelli, F.; Mennini, M.; Rutigliano, I.M.; Perricone, C.; Nenna, R.; Petrarca, L.; Mastrogiorgio, G.; Valesini, G.; Bonamico, M. Subclinical synovitis detected by ultrasound in children affected by coeliac disease: A frequent manifestation improved by a gluten-free diet. Clin. Exp. Rheumatol. 2014, 32, 137–142. [Google Scholar] [PubMed]
- Iltanen, S.; Collin, P.; Korpela, M.; Holm, K. Celiac disease and markers of celiac disease latency in patients with primary Sjogren’s syndrome. Am. J. Gastroenterol. 1999, 94, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Giani, T.; Simonini, G.; Falcini, F. Thyroid function, autoimmune thyroiditis and coeliac disease in juvenile idiopathic arthritis. Rheumatology 2005, 44, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Shor, D.B.A.; Comaneshter, D.; Tekes-Manova, D.; Shovman, O.; Amital, H.; Cohen, A.D. All disease begins in the gut: Celiac disease co-existence with SLE. Autoimmun. Rev. 2016, 15, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Zone, J.J.; Meyer, L.J.; Petersen, M.J. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch. Dermatol. 1996, 132, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Mansikka, E.; Hervonen, K.; Kaukinen, K.; Collin, P.; Huhtala, H.; Reunala, T.; Salmi, T. Prognosis of dermatitis herpetiformis patients with and without villous atrophy at diagnosis. Nutrients 2018, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Salmi, T.T.; Hervonen, K.; Kurppa, K.; Collin, P.; Kaukinen, K.; Reunala, T. Celiac disease evolving into dermatitis herpetiformis in patients adhering to normal or gluten-free diet. Scand. J. Gastroenterol. 2015, 50, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Mautalen, C.; Gonzalez, D.; Mazure, R.; Vazquez, H.; Lorenzetti, M.P.; Maurino, E.; Niveloni, S.; Pedreira, S.; Smecuol, E.; Boerr, L.A.; et al. Effect of treatment on bone mass, mineral metabolism, and body composition in untreated celiac disease patients. Am. J. Gastroenterol. 1997, 92, 313–318. [Google Scholar] [PubMed]
- Fornari, M.C.; Pedreira, S.; Niveloni, S.; González, D.; Diez, R.A.; Vázquez, H.; Mazure, R.; Sugai, E.; Smecuol, E.; Boerr, L.; et al. Pre- and post-treatment serum levels of cytokines IL-1beta, IL-6, and IL-1 receptor antagonist in celiac disease. Are they related to the associated osteopenia? Am. J. Gastroenterol. 1998, 93, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.E.; Pennisi, P.; Ferro, G.; Ximenes, B.; Privitelli, L.; Mangiafico, R.A.; Santoro, F.; Parisi, N.; Lombardo, T. Altered osteoprotegerin/RANKL ratio and low bone mineral density in celiac patients on long-term treatment with gluten-free diet. Horm. Metab. Res. 2006, 38, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Suraci, E.; Nazionale, I.; Leone, I.; Montalcini, T.; Abenavoli, L.; Imeneo, M.; Pujia, A.; Luzza, F. No evidence of circulating autoantibodies against osteoprotegerin in patients with celiac disease. World J. Gastroenterol. 2012, 18, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Mustalahti, K.; Collin, P.; Sievänen, H.; Salmi, J.; Mäki, M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet 1999, 354, 744–745. [Google Scholar] [CrossRef]
- Kurppa, K.; Collin, P.; Sievänen, H.; Huhtala, H.; Mäki, M.; Kaukinen, K. Gastrointestinal symptoms, quality of life and bone mineral density in mild enteropathic coeliac disease: A prospective clinical trial. Scand. J. Gastroenterol. 2010, 45, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Naiyer, A.J.; Shah, J.; Hernandez, L.; Kim, S.-Y.; Ciaccio, E.J.; Cheng, J.; Manavalan, S.; Bhagat, G.; Green, P.H.R. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid 2008, 18, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Larizza, D.; Calcaterra, V.; De Giacomo, C.; De Silvestri, A.; Asti, M.; Badulli, C.; Autelli, M.; Coslovich, E.; Martinetti, M. Celiac disease in children with autoimmune thyroid disease. J. Pediatr. 2001, 139, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Jaruvongvanich, V.; Cheungpasitporn, W.; Ungprasert, P. Association between celiac disease and schizophrenia: A meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Mollazadegan, K.; Kugelberg, M.; Tallstedt, L.; Ludvigsson, J.F. Increased risk of uveitis in coeliac disease: A nationwide cohort study. Br. J. Ophthalmol. 2012, 96, 857–861. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; De Polo, M.; et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Spiers, E.; Reynolds, N.; Walsh, S.; Fahey, T.; MacDonald, T.M. The association between coeliac disease and cardiovascular disease. Aliment. Pharmacol. Ther. 2008, 27, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Pitter, G.; Ludvigsson, J.F.; Romor, P.; Zanier, L.; Zanotti, R.; Simonato, L. Coeliac disease and asthma association in children: The role of antibiotic consumption. Eur. Respir. J. 2015, 46, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Gerdes, L.U. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr. Scand. 2012, 125, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac disease symptom resolution: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Laurikka, P.; Salmi, T.; Collin, P.; Huhtala, H.; Mäki, M.; Kaukinen, K.; Kurppa, K. Gastrointestinal symptoms in celiac disease patients on a long-term gluten-free diet. Nutrients 2016, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Hervonen, K.; Salmi, T.T.; Kurppa, K.; Kaukinen, K.; Collin, P.; Reunala, T. Dermatitis herpetiformis in children: A long-term follow-up study. Br. J. Dermatol. 2014, 171, 1242–1243. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.G.; Kansu, A.; Girgin, N.; Kucuk, O.; Aras, G. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics 2001, 108, E89. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Iovino, P.; Cappello, C.; Capone, P.; Andreozzi, P.; Ciacci, C. From menarche to menopause: The fertile life span of celiac women. Menopause 2011, 18, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Bode, S.H.; Bachmann, E.H.; Gudmand-Hoyer, E.; Jensen, G.B. Stature of adult coeliac patients: No evidence for decreased attained height. Eur. J. Clin. Nutr. 1991, 45, 145–149. [Google Scholar] [PubMed]
- Pärnänen, A.; Kaukinen, K.; Helakorpi, S.; Uutela, A.; Lähdeaho, M.-L.; Huhtala, H.; Collin, P.; Mäki, M.; Kurppa, K. Symptom-detected and screen-detected celiac disease and adult height. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Bardella, M.T.; Fredella, C.; Prampolini, L.; Molteni, N.; Giunta, A.M.; Bianchi, P.A. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Am. J. Clin. Nutr. 2000, 72, 937–939. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Cosnes, C.; Cosnes, A.; Contou, J.-F.; Reijasse, D.; Carbonnel, F.; Beaugerie, L.; Gendre, J.-P. Undiagnosed celiac disease in childhood. Gastroenterol. Clin. Biol. 2002, 26, 616–623. [Google Scholar] [PubMed]
- Weiss, B.; Skourikhin, Y.; Modan-Moses, D.; Broide, E.; Fradkin, A.; Bujanover, Y. Is adult height of patients with celiac disease influenced by delayed diagnosis? Am. J. Gastroenterol. 2008, 103, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Pitter, G.; Zanier, L.; Simonato, L.; Michaelsson, K.; Ludvigsson, J.F. Risk of fractures in youths with celiac disease—A population-based study. J. Pediatr. 2018, 198, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Tau, C.; Mautalen, C.; De Rosa, S.; Roca, A.; Valenzuela, X. Bone mineral density in children with celiac disease. Effect of a Gluten-free diet. Eur. J. Clin. Nutr. 2006, 60, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Moleski, S.M.; Lindenmeyer, C.C.; Jon Veloski, J.; Miller, R.S.; Miller, C.L.; Kastenberg, D.; Dimarino, A.J. Increased rates of pregnancy complications in women with celiac disease. Ann. Gastroenterol. 2015, 28, 236–240. [Google Scholar] [PubMed]
- Rawal, N. Remission of refractory celiac disease with infliximab in a pediatric patient. ACG Case Rep. J. 2015, 2, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Kurppa, K.; Paavola, A.; Collin, P.; Sievänen, H.; Laurila, K.; Huhtala, H.; Saavalainen, P.; Mäki, M.; Kaukinen, K. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology 2014, 147, 610.e1–617.e1. [Google Scholar] [CrossRef] [PubMed]
- Paarlahti, P.; Kurppa, K.; Ukkola, A.; Collin, P.; Huhtala, H.; Mäki, M.; Kaukinen, K. Predictors of persistent symptoms and reduced quality of life in treated coeliac disease patients: A large cross-sectional study. BMC Gastroenterol. 2013, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, A.; Mäki, M.; Kurppa, K.; Collin, P.; Huhtala, H.; Kekkonen, L.; Kaukinen, K. Changes in body mass index on a gluten-free diet in coeliac disease: A nationwide study. Eur. J. Intern. Med. 2012, 23, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Agardh, D.; Lee, H.-S.; Kurppa, K.; Simell, V.; Aronsson, C.A.; Jorneus, O.; Hummel, M.; Liu, E.; Koletzko, S. Clinical features of celiac disease: A prospective birth cohort. Pediatrics 2015, 135, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Kaukinen, K.; Huhtala, H.; Lähdeaho, M.L.; Mäki, M.; Kurppa, K. At-risk screened children with celiac disease are comparable in disease severity and dietary adherence to those found because of clinical suspicion: A large cohort study. J. Pediatr. 2017, 183, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kinos, S.; Kurppa, K.; Ukkola, A.; Collin, P.; Lähdeaho, M.L.; Huhtala, H.; Kekkonen, L.; Mäki, M.; Kaukinen, K. Burden of illness in screen-detected children with celiac disease and their families. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Mattila, E.; Kurppa, K.; Ukkola, A.; Collin, P.; Huhtala, H.; Forma, L.; Lähdeaho, M.L.; Kekkonen, L.; Mäki, M.; Kaukinen, K. Burden of illness and use of health care services before and after celiac disease diagnosis in children. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Popp, A.; Arvola, T.; Huhtala, H.; Kaukinen, K.; Kurppa, K. Long-term health and treatment outcomes in adult coeliac disease patients diagnosed by screening in childhood. United Eur. Gastroenterol. J. 2018, 205064061877838. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Tosco, A.; Salvati, V.M.; Auricchio, R.; Maglio, M.; Borrelli, M.; Coruzzo, A.; Paparo, F.; Boffardi, M.; Esposito, A.; D’Adamo, G.; et al. Natural history of potential celiac disease in children. Clin. Gastroenterol. Hepatol. 2011, 9, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Herzstein, J.; et al. Screening for celiac disease: US Preventive Services Task Force recommendation statement. JAMA 2017, 317, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. American College of Gastroenterology ACG Clinical Guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [PubMed]
| Children | Adults | ||
|---|---|---|---|
| % | % | References | |
| Poor growth 1 | 11–70 | - | [20,28,29] |
| Short stature 1 | 4–33 | 3 | [15,30,31,32,33] |
| Anemia | 12–40 | 23–48 | [12,15,20,28,30,32,33,34] |
| Neurological symptoms | 4–52 | 24 | [15,20,28,29,31] |
| Enamel defects | 0–15 | 1–83 | [20,29,32,33,35,36] |
| Liver abnormalities | 1–57 | 2–5 | [8,15,32] |
| Joint manifestations | 1–10 | 2–9 | [15,20,29,30,32,33] |
| Dermatitis herpetiformis | 2–3 | 10–20 | [20,30,32,37] |
| Osteoporosis | 0 | 4–23 | [15,32] |
| Infertility | - | 5 | [9,15] |
| Manifestation | Response | Comments | References |
|---|---|---|---|
| Anemia | Yes | Sometimes slow or incomplete response | [12,15] |
| Dermatitis herpetiformis | Yes | Dietary response may be slow and require additional Dapsone medication | [15,75] |
| Transaminasemia | Usually | Often mild and reversible; in rare cases may lead to liver failure | [8,45] |
| Poor growth | Variable | May lead to reduced adulthood height if not treated before puberty | [15,82] |
| Neurological symptoms | Variable | In children, usually good response, but in adults, possibly irreversible | [6,43] |
| Decreased bone mineral density | Variable | Initiation of GFD before school age may be needed for optimal bone accrual | [13,76,84] |
| Joint problems | Variable | Coexisting musculoskeletal disease should be excluded if poor response | [15] |
| Enamel defects | Infrequently | Early appearance and irreversible in permanent teeth | [44] |
| Infertility | Unclear | Conflicting results | [9,77,85] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurikka, P.; Nurminen, S.; Kivelä, L.; Kurppa, K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients 2018, 10, 1015. https://doi.org/10.3390/nu10081015
Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018; 10(8):1015. https://doi.org/10.3390/nu10081015
Chicago/Turabian StyleLaurikka, Pilvi, Samuli Nurminen, Laura Kivelä, and Kalle Kurppa. 2018. "Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes" Nutrients 10, no. 8: 1015. https://doi.org/10.3390/nu10081015
APA StyleLaurikka, P., Nurminen, S., Kivelä, L., & Kurppa, K. (2018). Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients, 10(8), 1015. https://doi.org/10.3390/nu10081015




