l-Citrulline Supplementation: Impact on Cardiometabolic Health
Abstract
1. Introduction
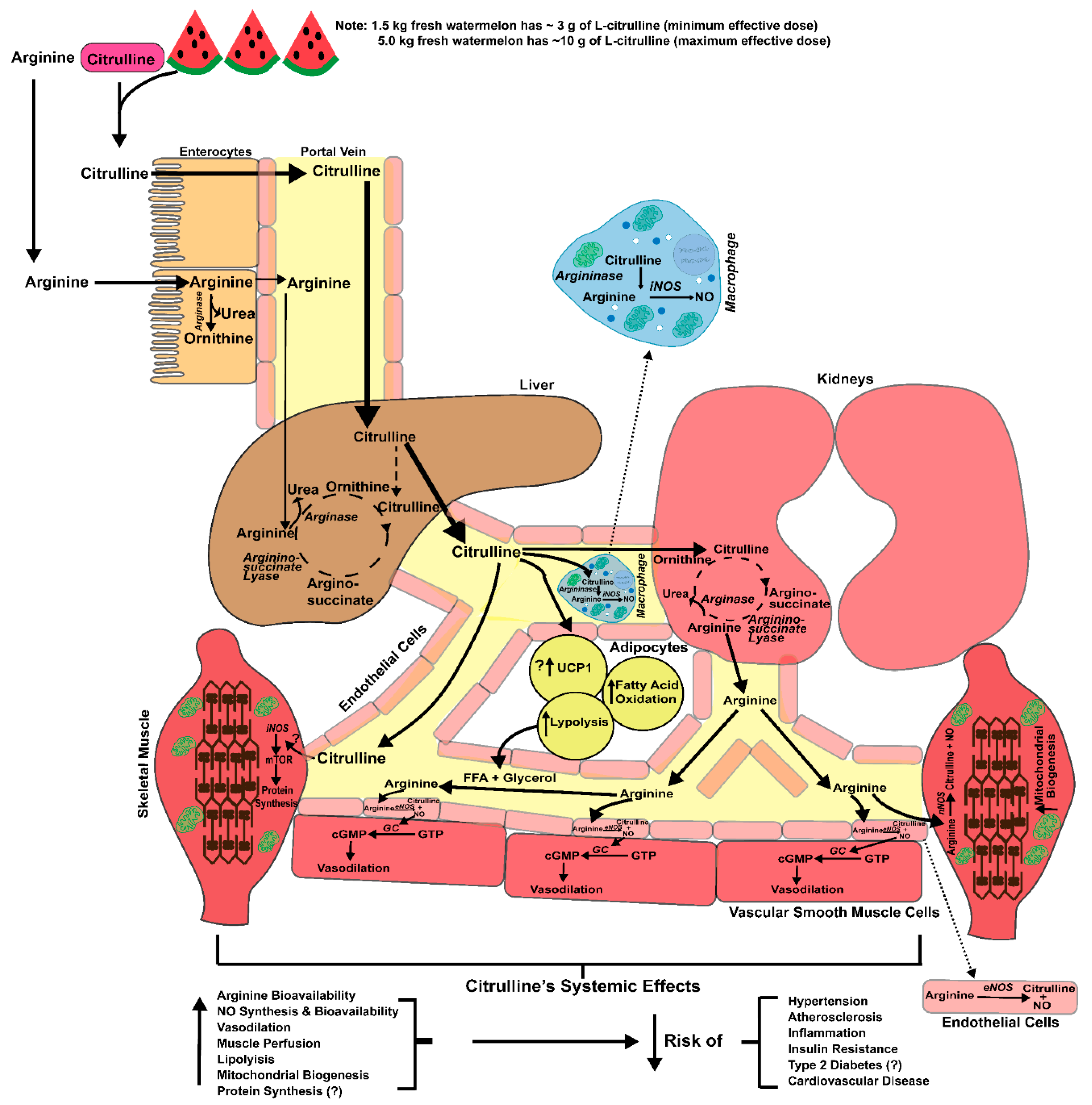
2. Health Applications
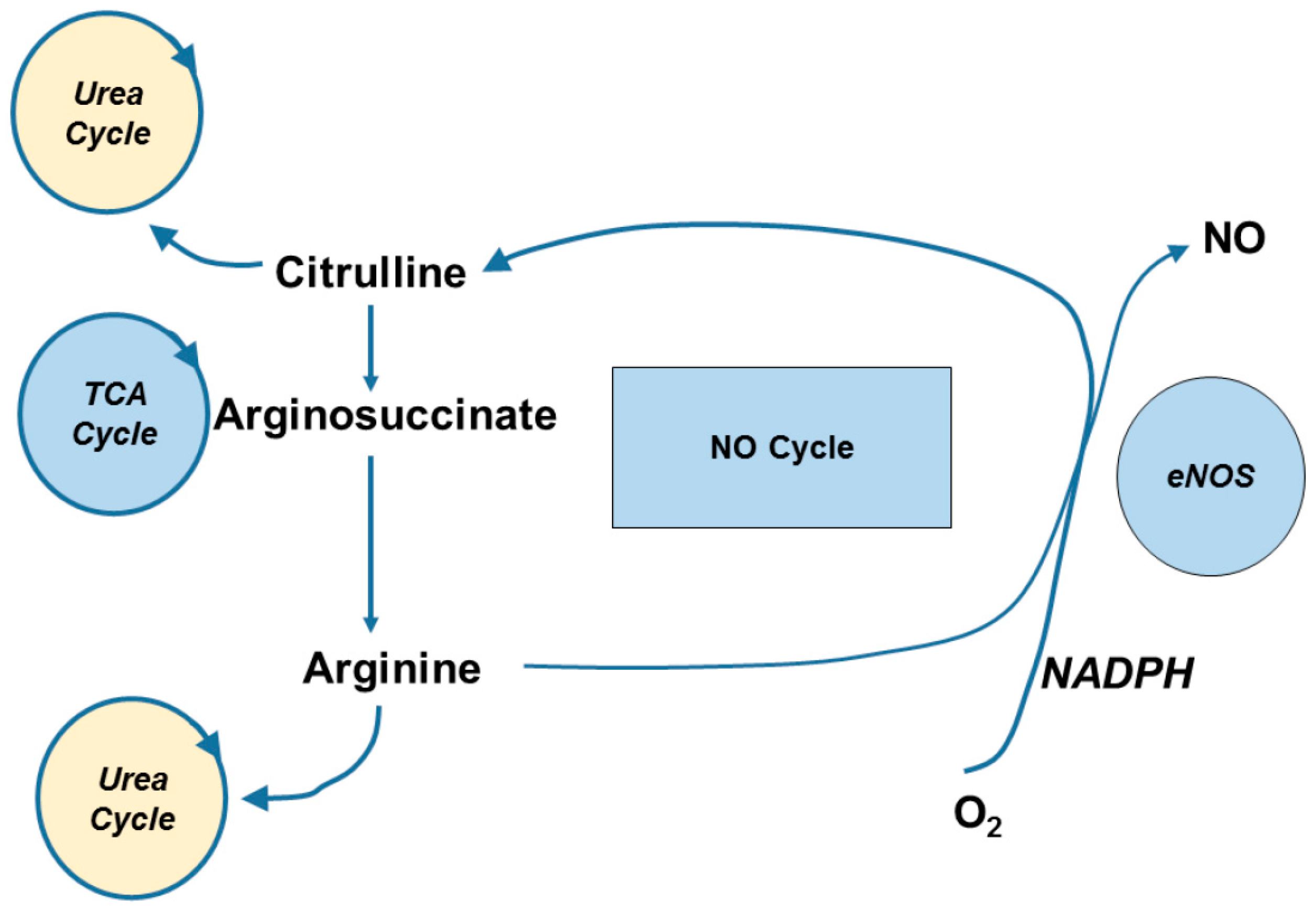
3. Pharmacokinetics, Transport and Metabolism
4. Vasoprotective Effects of l-Citrulline
4.1. Endothelial Vasodilator Function
4.2. Protection against Endothelial Damage
4.3. Antioxidant and Anti-Inflammatory Effects
4.4. Effects on Basal and Hyperemic Limb Blood Flow
5. Anti-Hypertensive Effects of l-Citrulline
5.1. Resting Blood Pressure and Arterial Stiffness
5.2. Blood Pressure Reactivity
5.3. Long-Term Blood Pressure Regulation and Kidney Function
5.4. Cardiac Function
6. Protection against Diabetic Vascular Dysfunction
7. Skeletal Muscle Heath
7.1. Protection against Insulin Resistance
7.2. Protection against Muscle Protein Loss/Wasting
7.3. Enhancement of Mitochondrial Oxidative Capacity
8. Adipose Tissue and Lipolysis Effects of l-citrulline
9. Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Forte, P.; Copland, M.; Smith, L.M.; Milne, E.; Sutherland, J.; Benjamin, N. Basal nitric oxide synthesis in essential hypertension. Lancet 1997, 349, 837–842. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Karim, F.; Straznicky, N.E.; Fernandez, S.; Evans, R.G.; Head, G.A.; Kaye, D.M. Augmented endothelial-specific l-arginine transport prevents obesity-induced hypertension. Acta Physiol. 2014, 212, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Hardy, T.A.; May, J.M. Coordinate regulation of l-arginine uptake and nitric oxide synthase activity in cultured endothelial cells. Free Radic. Biol. Med. 2002, 32, 122–131. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.J.; Rees, D.D.; Ashton, D.S.; Moncada, S. l-arginine is the physiological precursor for the formation of nitric-oxide in endothelium-dependent relaxation. Bioch. Bio. Res. Commun. 1988, 153, 1251–1256. [Google Scholar] [CrossRef]
- Castillo, L.; Chapman, T.E.; Yu, Y.M.; Ajami, A.; Burke, J.F.; Young, V.R. Dietary arginine uptake by the splanchnic region in adult humans. Am. J. Physiol. 1993, 265, E532–E539. [Google Scholar] [CrossRef] [PubMed]
- Grimble, G.K. Adverse gastrointestinal effects of arginine and related amino acids. J. Nutr. 2007, 137, 1693S–1701S. [Google Scholar] [CrossRef] [PubMed]
- Hartman, W.J.; Torre, P.M.; Prior, R.L. Dietary citrulline but not ornithine counteracts dietary arginine deficiency in rats by increasing splanchnic release of citrulline. J. Nutr. 1994, 124, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Benazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Spielmann, G. Does citrulline sit at the nexus of metformin’s pleotropic effects on metabolism and mediate its salutatory effects in individuals with type 2 diabetes? Diabetes 2016, 65, 3537–3540. [Google Scholar] [CrossRef] [PubMed]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin Nutr 2017. [Google Scholar]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Benazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Weber, C.L., III; Fish, W.W. l-citrulline levels in watermelon cultigens tested in two environments. HortScience 2011, 46, 1572–1575. [Google Scholar]
- Tarazona-Diaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From metabolism to therapeutic use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Kaore, S.N.; Amane, H.S.; Kaore, N.M. Citrulline: Pharmacological perspectives and its role as an emerging biomarker in future. Fundam. Clin. Pharmacol. 2013, 27, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L.; Huang, J.M.; Hampl, V.; Nelson, D.P.; Shultz, P.J.; Weir, E.K. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 7583–7587. [Google Scholar] [CrossRef] [PubMed]
- Lyons, D.; Roy, S.; Patel, M.; Benjamin, N.; Swift, C.G. Impaired nitric oxide-mediated vasodilatation and total body nitric oxide production in healthy old age. Clin Sci. 1997, 93, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.; Wolfe, R.R. Acute ingestion of citrulline stimulates nitric oxide synthesis but does not increase blood flow in healthy young and older adults with heart failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, K.L.; Lee, J.L.; Fujita, S.; Dhanani, S.; Dreyer, H.C.; Fry, C.S.; Drummond, M.J.; Sheffield-Moore, M.; Rasmussen, B.B.; Volpi, E. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes 2010, 59, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Ten Have, G.A.; Wolfe, R.R.; Deutz, N.E. Arginine de novo and nitric oxide production in disease states. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1177–E1189. [Google Scholar] [CrossRef] [PubMed]
- Marini, J.C.; Agarwal, U.; Didelija, I.C.; Azamian, M.; Stoll, B.; Nagamani, S.C. Plasma glutamine is a minor precursor for the synthesis of citrulline: A multispecies study. J. Nutr. 2017, 147, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Wood, G.C.; Bennotti, P.N.; Babu, E.; Deshpande, A.; Lent, M.R.; Petrick, A.; Gabrielsen, J.; Strodel, W.; Gerhard, G.S.; et al. Nutrient transporter expression in the jejunum in relation to body mass index in patients undergoing bariatric surgery. Nutrients 2016, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Vadgama, J.V.; Evered, D.F. Characteristics of l-citrulline transport across rat small intestine in vitro. Pediatr. Res. 1992, 32, 472–478. [Google Scholar] [CrossRef] [PubMed]
- van de Poll, M.C.; Ligthart-Melis, G.C.; Boelens, P.G.; Deutz, N.E.; van Leeuwen, P.A.; Dejong, C.H. Intestinal and hepatic metabolism of glutamine and citrulline in humans. J. Physiol. 2007, 581, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.; Didelija, I.C.; Yuan, Y.; Wang, X.Y.; Marini, J.C. Supplemental citrulline is more efficient than arginine in increasing systemic arginine availability in mice. J. Nutr. 2017, 147, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M., Jr. Regulation of enzymes of urea and arginine synthesis. Annu. Rev. Nutr. 1992, 12, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Yu, B.; He, J.; Yu, J.; Mao, X.; Luo, Y.; Luo, J.; Huang, Z.; Tian, G.; Zeng, Q.; et al. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 2017, 117, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Closs, E.I.; Simon, A.; Vekony, N.; Rotmann, A. Plasma membrane transporters for arginine. J. Nutr. 2004, 134, 2752S–2759S. [Google Scholar] [CrossRef] [PubMed]
- Wileman, S.M.; Mann, G.E.; Pearson, J.D.; Baydoun, A.R. Role of l-citrulline transport in nitric oxide synthesis in rat aortic smooth muscle cells activated with LPS and interferon-gamma. Br. J. Pharmacol. 2003, 140, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Intestinal mucosal amino acid catabolism. J. Nutr. 1998, 128, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Levillain, O.; Parvy, P.; Hassler, C. Amino acid handling in uremic rats: Citrulline, a reliable marker of renal insufficiency and proximal tubular dysfunction. Metabolism 1997, 46, 611–618. [Google Scholar] [CrossRef]
- Jourdan, M.; Nair, K.S.; Carter, R.E.; Schimke, J.; Ford, G.C.; Marc, J.; Aussel, C.; Cynober, L. Citrulline stimulates muscle protein synthesis in the post-absorptive state in healthy people fed a low-protein diet—A pilot study. Clin. Nutr. 2015, 34, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Mitchell, J.A.; Swierkosz, T.A.; Sessa, W.C.; Vane, J.R. Inhibition by l-glutamine of the release of endothelium-derived relaxing factor from cultured endothelial cells. Br. J. Pharmacol. 1990, 101, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Sessa, W.C.; Harris, H.J.; Anggard, E.E.; Vane, J.R. The metabolism of l-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: Cultured endothelial cells recycle l-citrulline to l-arginine. Proc. Natl. Acad. Sci. USA 1990, 87, 8612–8616. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Brosnan, J.T. Macrophages can convert citrulline into arginine. Biochem. J. 1992, 281, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Campbell, E.B.; Gross, S.S. Argininosuccinate synthetase mRNA and activity are induced by immunostimulants in vascular smooth muscle. Role in the regeneration or arginine for nitric oxide synthesis. J. Biol. Chem. 1994, 269, 9405–9408. [Google Scholar] [PubMed]
- Churchward-Venne, T.A.; Cotie, L.M.; MacDonald, M.J.; Mitchell, C.J.; Prior, T.; Baker, S.K.; Phillips, S.M. Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E71–E83. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Gori, T.; Keaney, J.F., Jr.; Maack, C.; Daiber, A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur. Heart J. 2015, 36, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, J.; Hildreth, K.L.; Christians, U.; Kohrt, W.M.; Moreau, K.L. A relative l-arginine deficiency contributes to endothelial dysfunction across the stages of the menopausal transition. Physiol. Rep. 2017, 5, e13409. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.; Meesters, D.M.; van Barneveld, K.W.; Visschers, R.G.; Briede, J.J.; Vandendriessche, B.; van Eijk, H.M.; Bessems, B.A.; van den Hoven, N.; von Wintersdorff, C.J.; et al. Citrulline supplementation improves organ perfusion and arginine availability under conditions with enhanced arginase activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.J.; Lin, K.M.; Kuo, H.C.; Huang, C.F.; Lin, Y.J.; Huang, L.T.; Tain, Y.L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal N(G)-nitro-l-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, C.T.; Huang, L.T. Long-term effects of maternal citrulline supplementation on renal transcriptome prevention of nitric oxide depletion-related programmed hypertension: The impact of gene-nutrient interactions. Int. J. Mol. Sci. 2014, 15, 23255–23268. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L. Pharmacokinetics of arginine and related amino acids. J. Nutr. 2007, 137, 1646S–1649S. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Sakurada, M.; Watanabe, F.; Yamasaki, T.; Doi, H.; Ezaki, H.; Morishita, K.; Miyakex, T. Effects of oral l-citrulline supplementation on lipoprotein oxidation and endothelial dysfunction in humans with vasospastic angina. Immunol. Endocr. Metab. Agents Med. Chem. 2013, 13, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, L.; Yang, T.; Sun, K.; Chen, J.; Hui, R. Increase in fasting vascular endothelial function after short-term oral l-arginine is effective when baseline flow-mediated dilation is low: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gates, P.E.; Boucher, M.L.; Silver, A.E.; Monahan, K.D.; Seals, D.R. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J. Appl. Physiol. (1985) 2007, 102, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R., 2nd; Boyle, K.E.; Koves, T.R.; Ilkayeva, O.R.; Muoio, D.M.; Houmard, J.A.; Friedman, J.E. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity 2015, 23, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct evidence of endothelial oxidative stress with aging in humans: Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappab. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Manas, L.; El-Assar, M.; Vallejo, S.; Lopez-Doriga, P.; Solis, J.; Petidier, R.; Montes, M.; Nevado, J.; Castro, M.; Gomez-Guerrero, C.; et al. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- El-Kirsh, A.A.; Abd El-Wahab, H.M.; Abd-Ellah Sayed, H.F. The effect of l-arginine or l-citrulline supplementation on biochemical parameters and the vascular aortic wall in high-fat and high-cholesterol-fed rats. Cell Biochem. Funct. 2011, 29, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Erdely, A.; Kepka-Lenhart, D.; Salmen-Muniz, R.; Chapman, R.; Hulderman, T.; Kashon, M.; Simeonova, P.P.; Morris, S.M., Jr. Arginase activities and global arginine bioavailability in wild-type and apoe-deficient mice: Responses to high fat and high cholesterol diets. PLoS ONE 2010, 5, e15253. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.J.; Nubret, E.; Butel, M.J.; Bergheim, I.; De Bandt, J.P. Preventive effects of citrulline on western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Lun, L.M.; Zhao, J.X.; Wang, H.W.; Wang, J.; Ning, C.P.; Liu, Z.; Zhang, B.B.; He, G.W. l-citrulline for protection of endothelial function from ADMA-induced injury in porcine coronary artery. Sci. Rep. 2015, 5, 10987. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Juliet, P.A.; Matsui-Hirai, H.; Miyazaki, A.; Fukatsu, A.; Funami, J.; Iguchi, A.; Ignarro, L.J. l-citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc. Natl. Acad Sci. USA 2005, 102, 13681–13686. [Google Scholar] [CrossRef] [PubMed]
- Konior, A.; Schramm, A.; Czesnikiewicz-Guzik, M.; Guzik, T.J. NADPH oxidases in vascular pathology. Antioxid. Redox Signal. 2014, 20, 2794–2814. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.M.; Gambetti, S.; Dondi, A.; Cervellati, C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des. 2004, 10, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Nagy, I.; Floyd, R.A. Hydroxyl free radical reactions with amino acids and proteins studied by electron spin resonance spectroscopy and spin-trapping. Biochim. Biophys. Acta 1984, 790, 238–250. [Google Scholar] [CrossRef]
- Coles, K.E. An Investiagation into the Antioxidant Capacity of l-Arginine and l-Citrulline in Relation to Their Vascular Protective Properties. Ph.D. Disseration, Cardiff University, Ann Arobor, MI, USA, 2007. [Google Scholar]
- Joffin, N.; Jaubert, A.M.; Durant, S.; Barouki, R.; Forest, C.; Noirez, P. Citrulline counteracts overweight- and aging-related effects on adiponectin and leptin gene expression in rat white adipose tissue. Biochim. Open 2015, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barkhidarian, B.; Seyedhamzeh, S.; Hashemy, S.I.; Nematy, M.; Rahbari, A.; Ranjbar, R.; Safarian, M. Effects of arginine and citrulline supplementation on inflammatory markers in critically ill patients. J. Nutr. Sci. Diet. 2016, 2. [Google Scholar] [CrossRef]
- Breuillard, C.; Bonhomme, S.; Couderc, R.; Cynober, L.; De Bandt, J.P. In vitro anti-inflammatory effects of citrulline on peritoneal macrophages in zucker diabetic fatty rats. Br. J. Nutr. 2015, 113, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, T.; Zhang, S.; Nunoo, R.; Mascarenas, C.; Dujovny, N.; Luchtefeld, M.; Cavey, G.S.; Senagore, A. Citrulline: A potential immunomodulator in sepsis. Surgery 2011, 150, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, K.A.; Vink, H.; Briede, J.J.; van Faassen, E.E.; Lamers, W.H.; Buurman, W.A.; Poeze, M. Citrulline a more suitable substrate than arginine to restore no production and the microcirculation during endotoxemia. PLoS ONE 2012, 7, e37439. [Google Scholar] [CrossRef] [PubMed]
- MacIver, N.J.; Michalek, R.D.; Rathmell, J.C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 2013, 31, 259–283. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.M.; McKell, M.C.; Schmidt, S.M.; Hossfeld, A.P.; Chaturvedi, V.; Kinder, J.M.; McAlees, J.W.; Lewkowich, I.P.; Way, S.S.; Turner, J.; et al. l-citrulline metabolism in mice augments CD4(+) T cell proliferation and cytokine production in vitro, and accumulation in the mycobacteria-infected lung. Front. Immunol. 2017, 8, 1561. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. l-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Candales, A.; Hernandez Burgos, P.M.; Hernandez-Suarez, D.F.; Harris, D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017, 3, e341. [Google Scholar] [PubMed]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two weeks of watermelon juice supplementation improves nitric oxide bioavailability but not endurance exercise performance in humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Le Roux-Mallouf, T.; Vibert, F.; Doutreleau, S.; Verges, S. Effect of acute nitrate and citrulline supplementation on muscle microvascular response to ischemia-reperfusion in healthy humans. Appl. Physiol. Nutr. Metab. 2017, 42, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. (1985) 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Alacid, F.; Rubio-Arias, J.A.; Fernandez-Lobato, B.; Ramos-Campo, D.J.; Aguayo, E. Consumption of watermelon juice enriched in l-citrulline and pomegranate ellagitannins enhanced metabolism during physical exercise. J. Agric. Food Chem. 2017, 65, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Shabeeh, H.; Seddon, M.; Brett, S.; Melikian, N.; Casadei, B.; Shah, A.M.; Chowienczyk, P. Sympathetic activation increases NO release from enos but neither enos nor nnos play an essential role in exercise hyperemia in the human forearm. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1225–H1230. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon extract supplementation reduces ankle blood pressure and carotid augmentation index in obese adults with prehypertension or hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-term effects of l-citrulline supplementation on arterial stiffness in middle-aged men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, M.A.; Koutnik, A.P.; Ramirez, K.; Wong, A.; Figueroa, A. The effects of short term l-citrulline supplementation on wave reflection responses to cold exposure with concurrent isometric exercise. Am. J. Hypertens. 2013, 26, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects of watermelon supplementation on arterial stiffness and wave reflection amplitude in postmenopausal women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Trivino, J.A.; Sanchez-Gonzalez, M.A.; Vicil, F. Oral l-citrulline supplementation attenuates blood pressure response to cold pressor test in young men. Am. J. Hypertens. 2010, 23, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Gutierrez, J.J.; Castillo-Martinez, L.; Orea-Tejeda, A.; Vazquez-Diaz, O.; Valdespino-Trejo, A.; Narvaez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sanchez-Santillan, R. Effect of l-arginine or l-citrulline oral supplementation on blood pressure and right ventricular function in heart failure patients with preserved ejection fraction. Cardiol. J. 2010, 17, 612–618. [Google Scholar] [PubMed]
- Alsop, P.; Hauton, D. Oral nitrate and citrulline decrease blood pressure and increase vascular conductance in young adults: A potential therapy for heart failure. Eur. J. Appl. Physiol. (1985) 2016, 116, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kalfon, R. l-citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br. J. Nutr. 2016, 116, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Massa, N.M.; Silva, A.S.; Toscano, L.T.; Silva, J.D.; Persuhn, D.C.; Goncalves Mda, C. Watermelon extract reduces blood pressure but does not change sympathovagal balance in prehypertensive and hypertensive subjects. Blood Press. 2016, 25, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Chernykh, O.; Figueroa, A. Chronic l-citrulline supplementation improves cardiac sympathovagal balance in obese postmenopausal women: A preliminary report. Auton. Neurosci. 2016, 198, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Does l-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.G.; Gauthier, A.L.; Hayman, M.A.; Lang, J.T.; Kenefick, R.W. Acute effects of cold exposure on central aortic wave reflection. J. Appl. Physiol. (1985) 2006, 100, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Menkes, M.S.; Matthews, K.A.; Krantz, D.S.; Lundberg, U.; Mead, L.A.; Qaqish, B.; Liang, K.Y.; Thomas, C.B.; Pearson, T.A. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension 1989, 14, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Lifton, R.P.; Gharavi, A.G.; Geller, D.S. Molecular mechanisms of human hypertension. Cell 2001, 104, 545–556. [Google Scholar] [CrossRef]
- Koeners, M.P.; Braam, B.; Joles, J.A. Blood pressure follows the kidney: Perinatal influences on hereditary hypertension. Organogenesis 2008, 4, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Alexandre-Gouabau, M.C.; Pagniez, A.; Ouguerram, K.; Boquien, C.Y.; Winer, N.; Darmaun, D. Neonatal citrulline supplementation and later exposure to a high fructose diet in rats born with a low birth weight: A preliminary report. Nutrients 2017, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Blacher, J.; Safar, M.E. Large-artery stiffness, hypertension and cardiovascular risk in older patients. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Dinenno, F.A.; Jones, P.P.; Seals, D.R.; Tanaka, H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 1999, 100, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Paulus, W.J. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur. Heart J. 2011, 32, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Balderas-Munoz, K.; Castillo-Martinez, L.; Orea-Tejeda, A.; Infante-Vazquez, O.; Utrera-Lagunas, M.; Martinez-Memije, R.; Keirns-Davis, C.; Becerra-Luna, B.; Sanchez-Vidal, G. Improvement of ventricular function in systolic heart failure patients with oral l-citrulline supplementation. Cardiol. J. 2012, 19, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.I.; Stratton, I.M.; Neil, H.A.; Yudkin, J.S.; Matthews, D.R.; Cull, C.A.; Wright, A.D.; Turner, R.C.; Holman, R.R. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): Prospective observational study. BMJ 2000, 321, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, C.; Chobanian, A.V.; Jones, D.W.; Roccella, E.J. Seventh report of the joint national committee on the prevention, detection, evaluation, and treatment of high blood pressure (JNC 7): Resetting the hypertension sails. Hypertension 2003, 41, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Lazaridis, A.A.; Ruiz-Hurtado, G.; Ruilope, L.M. Blood pressure reduction in diabetes: Lessons from accord, sprint and empa-reg outcome. Nat. Rev. Endocrinol. 2017, 13, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.; Zanchetti, A.; Carruthers, S.G.; Dahlof, B.; Elmfeldt, D.; Julius, S.; Menard, J.; Rahn, K.H.; Wedel, H.; Westerling, S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: Principal results of the hypertension optimal treatment (hot) randomised trial. Hot study group. Lancet 1998, 351, 1755–1762. [Google Scholar] [CrossRef]
- Calver, A.; Collier, J.; Vallance, P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J. Clin. Invest. 1992, 90, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, M.T.; Creager, S.J.; Scales, K.M.; Cusco, J.A.; Lee, B.K.; Creager, M.A. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation 1993, 88, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- McVeigh, G.E.; Brennan, G.M.; Johnston, G.D.; McDermott, B.J.; McGrath, L.T.; Henry, W.R.; Andrews, J.W.; Hayes, J.R. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1992, 35, 771–776. [Google Scholar] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Hishikawa, K.; Katusic, Z.S.; Luscher, T.F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997, 96, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Thallas, V.; Thomas, M.C.; Founds, H.W.; Burns, W.C.; Jerums, G.; Cooper, M.E. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. Faseb. J. 2003, 17, 1762–1764. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Yee, L.T.; Thallas, V.; Lassila, M.; Candido, R.; Jandeleit-Dahm, K.A.; Thomas, M.C.; Burns, W.C.; Deemer, E.K.; Thorpe, S.R.; et al. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes 2004, 53, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ji, Y.; Yao, K.; Cao, Y.X.; Ferro, A. Inhibition of human endothelial cell nitric oxide synthesis by advanced glycation end-products but not glucose: Relevance to diabetes. Clin. Sci. 2005, 109, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sobrevia, L.; Cesare, P.; Yudilevich, D.L.; Mann, G.E. Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: Association with membrane hyperpolarization. J. Physiol. 1995, 489, 183–192. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef] [PubMed]
- Galgani, J.E.; Moro, C.; Ravussin, E. Metabolic flexibility and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1009–E1017. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, D.; Jacot, E.; DeFronzo, R.A.; Maeder, E.; Jequier, E.; Felber, J.P. The effect of graded doses of insulin on total glucose uptake, glucose oxidation, and glucose storage in man. Diabetes 1982, 31, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Chai, W.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.D.; Levisetti, M.; Reichart, D.; Khoursheed, M.; Moossa, A.R.; Olefsky, J.M. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 1995, 44, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Stewart Coats, A.J.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Robinson, M.M.; Nair, K.S. Age effect on myocellular remodeling: Response to exercise and nutrition in humans. Aging Res. Rev. 2012, 11, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Roubenoff, R. Sarcopenia: A major modifiable cause of frailty in the elderly. J. Nutr. Health Aging 2000, 4, 140–142. [Google Scholar] [PubMed]
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.K.; Fielding, R.A.; Evans, W.J.; Leigh, J.S., Jr.; Posner, J.D. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J. Appl. Physiol. (1985) 1993, 75, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Tatpati, L.L.; Irving, B.A.; Tom, A.; Bigelow, M.L.; Klaus, K.; Short, K.R.; Nair, K.S. The effect of branched chain amino acids on skeletal muscle mitochondrial function in young and elderly adults. J. Clin. Endocrinol. Metab. 2010, 95, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-i expression in older women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Nilsson, P.A.; Nilsson, J.; Chibalin, A.V.; Zierath, J.R.; Blomstrand, E. Branched-chain amino acids increase p70s6k phosphorylation in human skeletal muscle after resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C.; Irving, B.A.; Nair, K.S. Potential application of essential amino acid supplementation to treat sarcopenia in elderly people. J. Clin. Endocrinol. Metab. 2009, 94, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Drummond, M.J.; Pennings, B.; Fujita, S.; Glynn, E.L.; Chinkes, D.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mtor signaling and protein synthesis in human muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E392–E400. [Google Scholar] [CrossRef] [PubMed]
- Gran, P.; Cameron-Smith, D. The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol. 2011, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIf4E and phosphorylation of ribosomal protein S6. J. Bio. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef]
- Barazzoni, R.; Short, K.R.; Asmann, Y.; Coenen-Schimke, J.M.; Robinson, M.M.; Nair, K.S. Insulin fails to enhance mtor phosphorylation, mitochondrial protein synthesis, and ATP production in human skeletal muscle without amino acid replacement. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1117–E1125. [Google Scholar] [CrossRef] [PubMed]
- Fryburg, D.A.; Jahn, L.A.; Hill, S.A.; Oliveras, D.M.; Barrett, E.J. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J. Clin. Invest. 1995, 96, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Keske, M.A.; Clerk, L.H.; Price, W.J.; Jahn, L.A.; Barrett, E.J. Obesity blunts microvascular recruitment in human forearm muscle after a mixed meal. Diabetes Care 2009, 32, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Ham, D.J.; Gleeson, B.G.; Chee, A.; Baum, D.M.; Caldow, M.K.; Lynch, G.S.; Koopman, R. l-citrulline protects skeletal muscle cells from cachectic stimuli through an inos-dependent mechanism. PLoS ONE 2015, 10, e0141572. [Google Scholar] [CrossRef] [PubMed]
- Osowska, S.; Duchemann, T.; Walrand, S.; Paillard, A.; Boirie, Y.; Cynober, L.; Moinard, C. Citrulline modulates muscle protein metabolism in old malnourished rats. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E582–E586. [Google Scholar] [CrossRef] [PubMed]
- Bouillanne, O.; Melchior, J.C.; Faure, C.; Paul, M.; Canoui-Poitrine, F.; Boirie, Y.; Chevenne, D.; Forasassi, C.; Guery, E.; Herbaud, S.; et al. Impact of 3-week citrulline supplementation on postprandial protein metabolism in malnourished older patients: The ciproage randomized controlled trial. Clin. Nutr. 2018, S0261–S5614, 30080–30083. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.N.; Koch, D.W.; Newcomer, S.C.; Le, K.U.; Leuenberger, U.A. Impaired leg vasodilation during dynamic exercise in healthy older women. J. Appl. Physiol. (1985) 2003, 95, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Ridout, S.J.; Parker, B.A.; Smithmyer, S.L.; Gonzales, J.U.; Beck, K.C.; Proctor, D.N. Age and sex influence the balance between maximal cardiac output and peripheral vascular reserve. J. Appl. Physiol. (1985) 2010, 108, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Clementi, E.; Paolucci, C.; Cozzi, V.; Tonello, C.; Sciorati, C.; Bracale, R.; Valerio, A.; Francolini, M.; Moncada, S.; et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science 2003, 299, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; D’Antona, G.; Nisoli, E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging 2011, 3, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Barbato, D.L.; Tatulli, G.; Aquilano, K.; Ciriolo, M.R. The role of nNOS and PGC-1 alpha in skeletal muscle cells. J. Cell. Sci. 2014, 127, 4813–4820. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Brown, D.L.; Lira, A.K.; Kavazis, A.N.; Soltow, Q.A.; Zeanah, E.H.; Criswell, D.S. Nitric oxide and AMPK cooperatively regulate pgc-1 in skeletal muscle cells. J. Physiol. 2010, 588, 3551–3566. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Nuclear recruitment of neuronal nitric-oxide synthase by alpha-syntrophin is crucial for the induction of mitochondrial biogenesis. J. Biol. Chem. 2014, 289, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Matsukawa, T.; Isoda, H. l-citrulline supplementation-increased skeletal muscle pgc-1alpha expression is associated with exercise performance and increased skeletal muscle weight. Mol. Nutr. Food Res. 2018, e1701043. [Google Scholar] [CrossRef] [PubMed]
- Jobgen, W.S.; Fried, S.K.; Fu, W.J.; Meininger, C.J.; Wu, G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J. Nutr. Biochem. 2006, 17, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Janke, J.; Gorzelniak, K.; Bohnke, J.; Ghose, N.; Lindschau, C.; Luft, F.C.; Sharma, A.M. Regulation of the nitric oxide system in human adipose tissue. J. Lipid Res. 2004, 45, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Penfornis, P.; Marette, A. Inducible nitric oxide synthase modulates lipolysis in adipocytes. J. Lipid Res. 2005, 46, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gaudiot, N.; Jaubert, A.M.; Charbonnier, E.; Sabourault, D.; Lacasa, D.; Giudicelli, Y.; Ribiere, C. Modulation of white adipose tissue lipolysis by nitric oxide. J. Biol. Chem. 1998, 273, 13475–13481. [Google Scholar] [CrossRef] [PubMed]
- Lincova, D.; Misekova, D.; Kmonickova, E.; Canova, N.; Farghali, H. Effect of nitric oxide donors on isoprenaline-induced lipolysis in rat epididymal adipose tissue: Studies in isolated adipose tissues and immobilized perfused adipocytes. Physiol. Res. 2002, 51, 387–394. [Google Scholar] [PubMed]
- Klatt, P.; Cacho, J.; Crespo, M.D.; Herrera, E.; Ramos, P. Nitric oxide inhibits isoproterenol-stimulated adipocyte lipolysis through oxidative inactivation of the beta-agonist. Biochem. J. 2000, 351, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.J.; Haynes, T.E.; Kohli, R.; Hu, J.; Shi, W.; Spencer, T.E.; Carroll, R.J.; Meininger, C.J.; Wu, G. Dietary l-arginine supplementation reduces fat mass in zucker diabetic fatty rats. J. Nutr. 2005, 135, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Joffin, N.; Jaubert, A.M.; Bamba, J.; Barouki, R.; Noirez, P.; Forest, C. Acute induction of uncoupling protein 1 by citrulline in cultured explants of white adipose tissue from lean and high-fat-diet-fed rats. Adipocyte 2015, 4, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Joffin, N.; Jaubert, A.M.; Durant, S.; Bastin, J.; De Bandt, J.P.; Cynober, L.; Moinard, C.; Coumoul, X.; Forest, C.; Noirez, P. Citrulline reduces glyceroneogenesis and induces fatty acid release in visceral adipose tissue from overweight rats. Mol. Nutr. Food Res. 2014, 58, 2320–2330. [Google Scholar] [CrossRef] [PubMed]
- Joffin, N.; Jaubert, A.M.; Durant, S.; Bastin, J.; De Bandt, J.P.; Cynober, L.; Moinard, C.; Forest, C.; Noirez, P. Citrulline induces fatty acid release selectively in visceral adipose tissue from old rats. Mol. Nutr. Food Res. 2014, 58, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Harada, R.; Nair, N.; Balasubramanian, N.; Cooke, J.P. l-arginine supplementation in peripheral arterial disease: No benefit and possible harm. Circulation 2007, 116, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.P.; Becker, L.C.; Kass, D.A.; Champion, H.C.; Terrin, M.L.; Forman, S.; Ernst, K.V.; Kelemen, M.D.; Townsend, S.N.; Capriotti, A.; et al. l-arginine therapy in acute myocardial infarction: The vascular interaction with age in myocardial infarction (vintage mi) randomized clinical trial. JAMA 2006, 295, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Wu, C.C.; Shin, S.; Fung, H.L. Continuous exposure to l-arginine induces oxidative stress and physiological tolerance in cultured human endothelial cells. Amino Acids 2012, 43, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Irving, B.A.; Carter, R.E.; Soop, M.; Weymiller, A.; Syed, H.; Karakelides, H.; Bhagra, S.; Short, K.R.; Tatpati, L.; Barazzoni, R.; et al. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 2015, 64, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Brandmaier, S.; Leonhardt, J.; Scheerer, M.F.; Mohney, R.P.; Xu, T.; Bi, J.; Rotter, M.; Troll, M.; Chi, S.; et al. Metformin effect on nontargeted metabolite profiles in patients with type 2 diabetes and in multiple murine tissues. Diabetes 2016, 65, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
| Reference | Population | BP Status | Formulation | Dose | Duration | Resting Function | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular Reactivity | |||||||||
| Figueroa et al. (2010) [91] | 17 M | Normotensive | l-Citrulline | 6 g/day | 4 weeks | ↓ bSBP, aSBP, aPP | |||
| Orozco-Gutierrez et al. (2010) [92] | 9 M 6 F | Heart failure w/ preserved EF | l-Citrulline- Malate | 3 g/day | 8 weeks | ↓bSPB, bDBP, | ↑ RVEF during exercise | ||
| Figueroa et al. (2011) [90] | 4 M 5 W | Pre-hypertensive | Watermelon Extract | 2.7 g/day | 6 weeks | ↓bPP, aSBP, aPP, AIx | |||
| Figueroa et al. (2012) [86] | 3 M 11 W | Pre-hypertensive | Watermelon Extract | 2.7 g/day | 6 weeks | ↓ ankle SBP, DBP, MAP, ↓ bSBP, bDBP, bMAP ↓ carotid AIx | |||
| Figueroa et al. (2013) [89] | 12 W | Hypertensive Post-menopausal | Watermelon Extract | 6 g/day | 6 weeks | ↓b-aPWV, aSBP, aDBP, aSBP2 ↔ AIx | |||
| Sanchez-Gonzalez et al. (2013) [88] | 16 M | Normotensive | l-Citrulline | 100 mg/kg | 2 weeks | ↓ CI and IHG increases in bSBP, aSBP and AIx | |||
| Alsop et al. (2016) [93] | 4 M 8 F | Normotensive | l-Citrulline | 3 d/day | 1 week | ↓ bSBP, bDBP, MAP, pulse interval | ↓ Pulse interval, Pulse Amplitude Ratio, ↑ HRV post 30% MVC exercise | ||
| Figueroa et al. (2016) [94] | 16 M | Normotensive Overweight/obese | l-Citrulline | 6 d/day | 2 weeks | Attenuated the increase in aSBP and AIx during IHG and reduced MAP aDBP | ↓ aSBP, aPP, AIx during IHG ↓ aDBP, MAP, AIx during PEMI ↓ aSBP, DBP, aPP, an baPWV during PEMI + CPT | ||
| Bailey et al. (2016) [81] | 8 M | Normotensive | Watermelon Juice | ~3.4 g/day | 2 weeks | ↑ aSBP and MAP | |||
| Massa et al. (2016) [95] | 10 M 10 W | Pre-hypertensive | Watermelon Extract | 6 g/day | 6 weeks | ↓ bSBP and bDBP ↔ cardiac autonomic function | |||
| Wong et al. (2016) [96] | 25 F * | Normotensive/ Pre-hypertensive | l-Citrulline | 6 g/day | 8 weeks | ↓bSBP, bDBP, and nLF (SNS activity), LnLF/LnHF (sympathovagal balance) | |||
| Gonzales et al. (2017) [97] | 12M 13W | Normotensive/ Pre-hypertensive | l-Citrulline | 6 g/day | 2 weeks | ↓ seated bSBP | ↑muscle blood flow during submaximal exercise in men | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. https://doi.org/10.3390/nu10070921
Allerton TD, Proctor DN, Stephens JM, Dugas TR, Spielmann G, Irving BA. l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients. 2018; 10(7):921. https://doi.org/10.3390/nu10070921
Chicago/Turabian StyleAllerton, Timothy D., David N. Proctor, Jacqueline M. Stephens, Tammy R. Dugas, Guillaume Spielmann, and Brian A. Irving. 2018. "l-Citrulline Supplementation: Impact on Cardiometabolic Health" Nutrients 10, no. 7: 921. https://doi.org/10.3390/nu10070921
APA StyleAllerton, T. D., Proctor, D. N., Stephens, J. M., Dugas, T. R., Spielmann, G., & Irving, B. A. (2018). l-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients, 10(7), 921. https://doi.org/10.3390/nu10070921






