Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Assessment
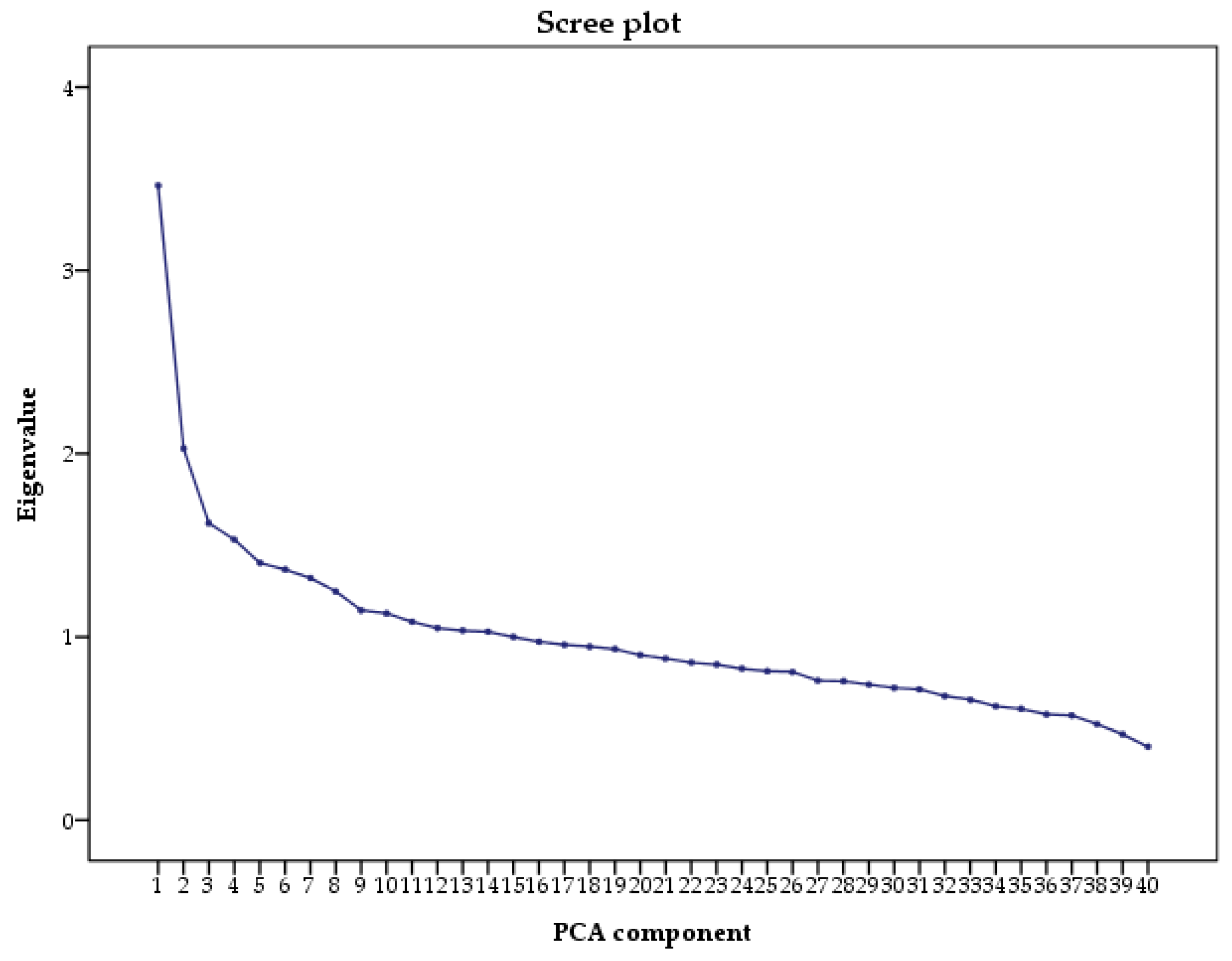
2.3. Principal Component Analysis
2.4. Anthropometric Measurements
2.5. Biochemical Analyses and Physical Examination
2.6. Definition of Metabolic Syndrome
2.7. Lifestyle Factors
2.8. Other Covariates
2.9. Statistical Analyses
3. Results
3.1. Study Population and Prevalence of MetS
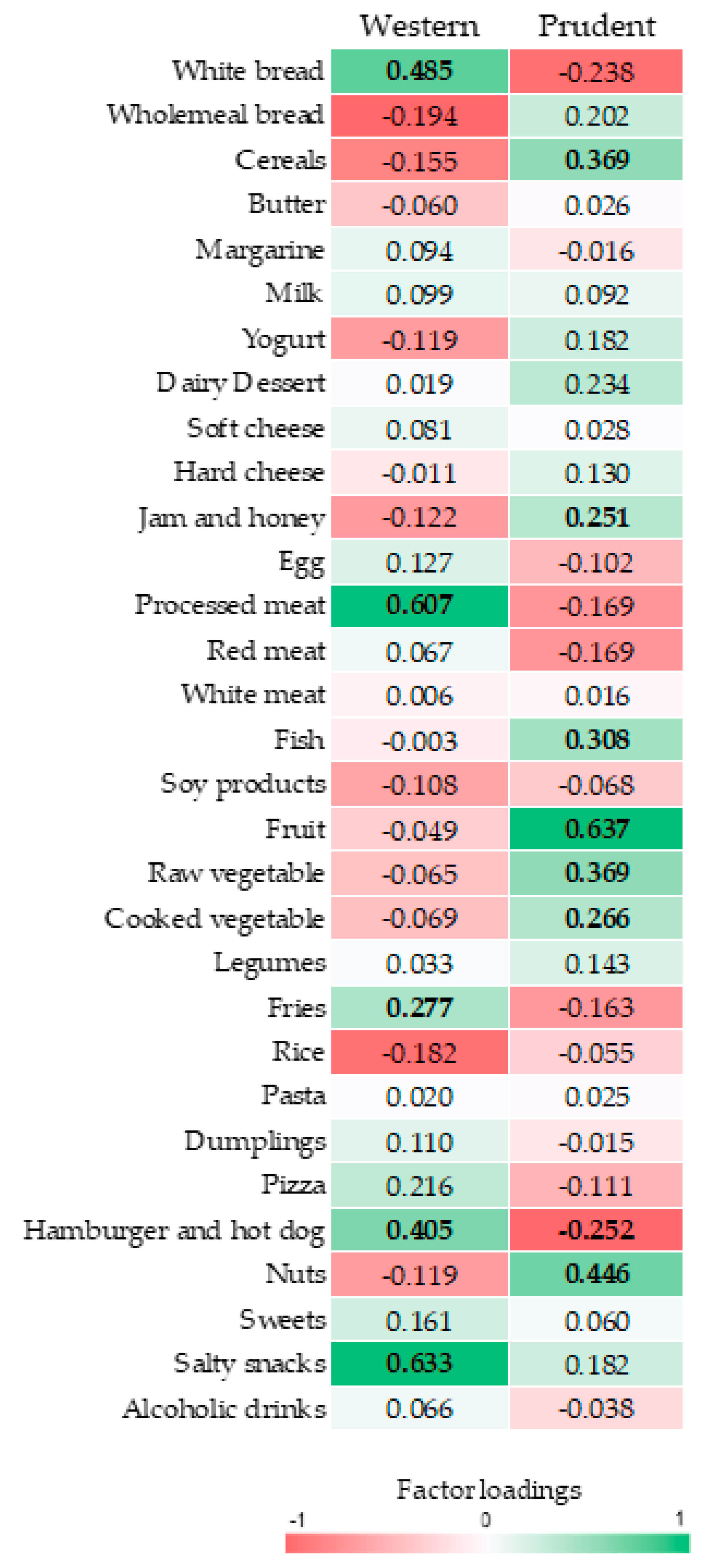
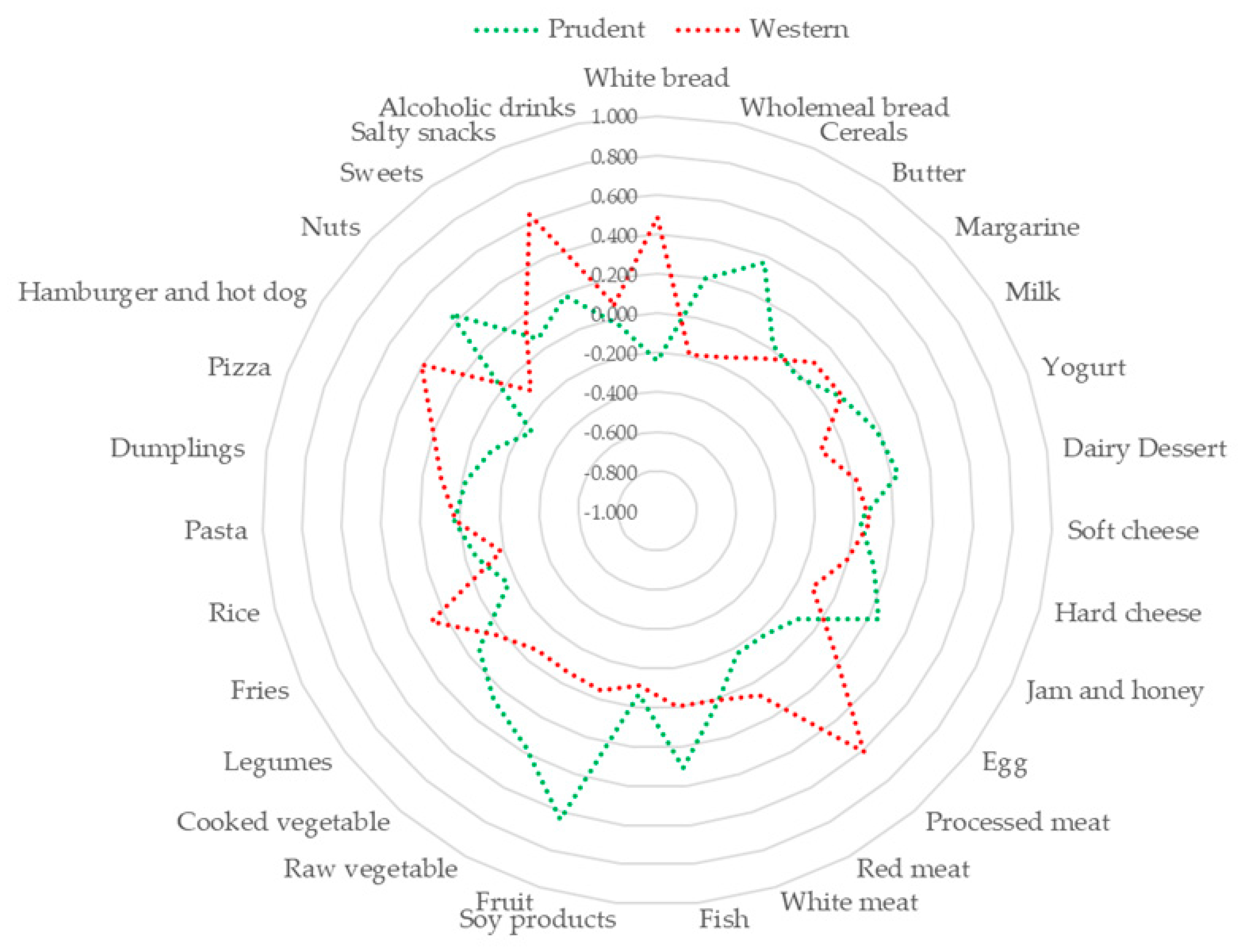
3.2. Dietary Patterns
3.3. The Association of Dietary Patterns with Cardio-Metabolic Parameters
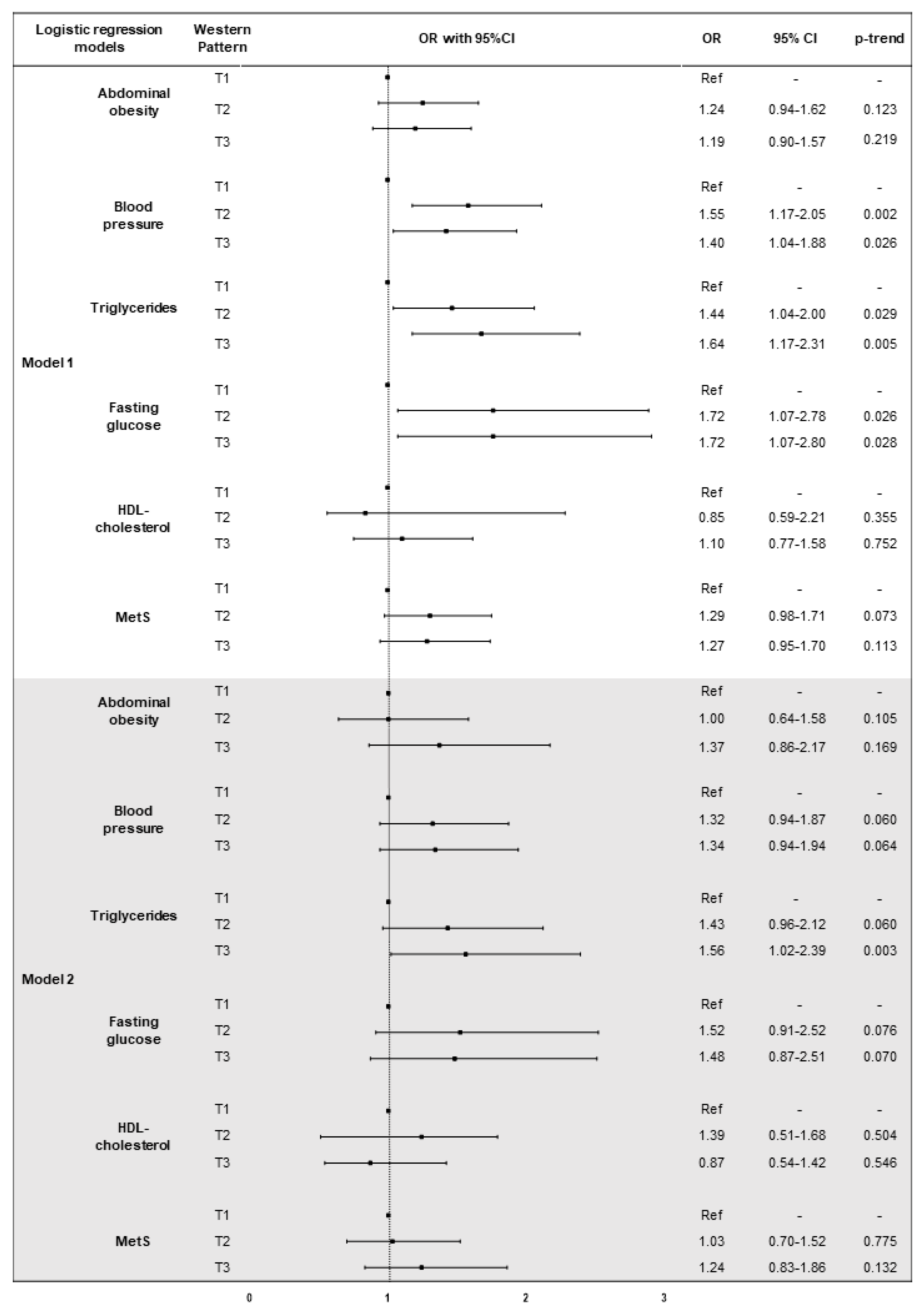
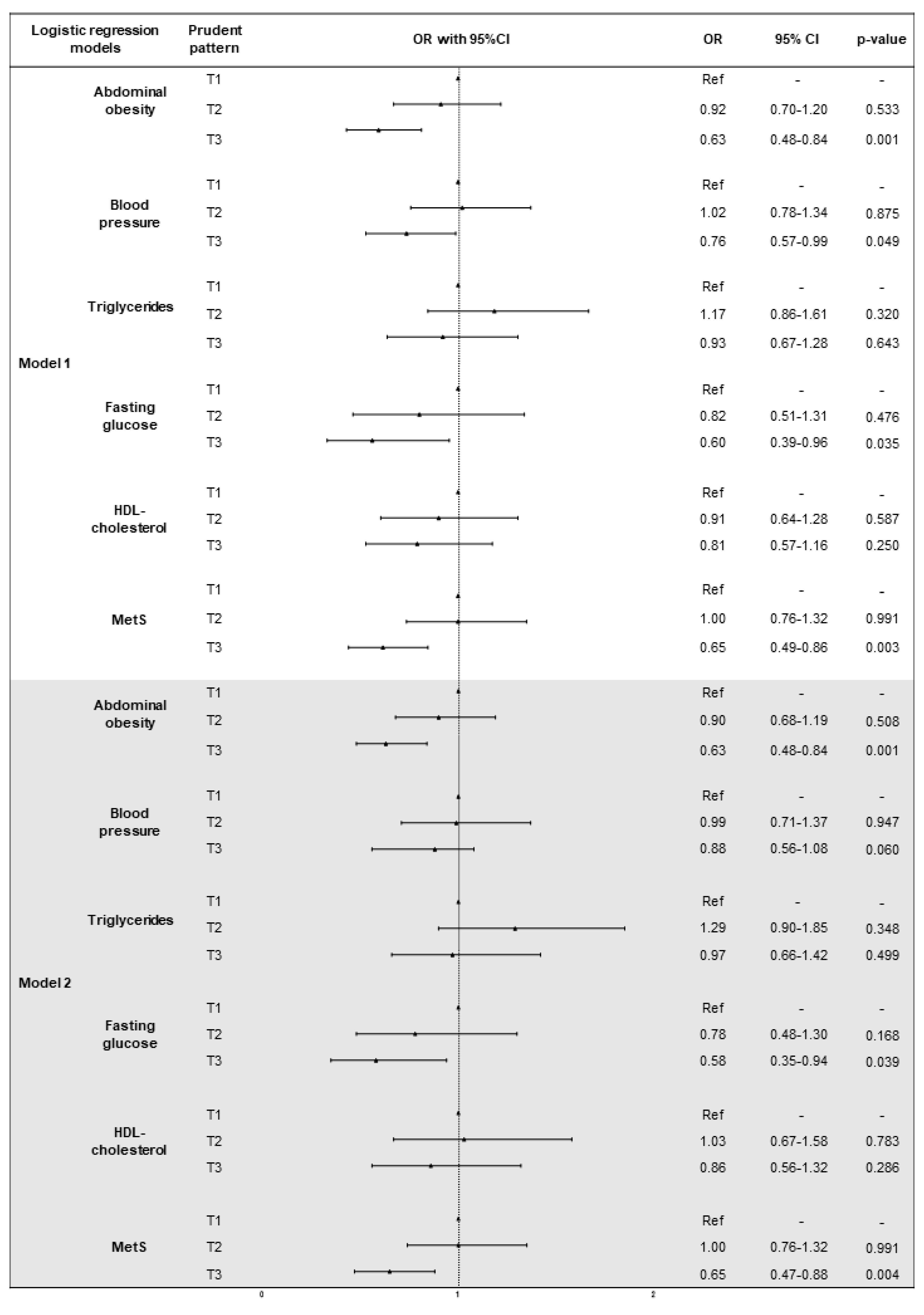
3.4. The Association of Dietary Patterns with MetS and Its Components
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ruotsalainen, S.; Moilanen, L.; Lepistö, P.; Laakso, M.; Kuusisto, J. The metabolic syndrome predicts cardiovascular mortality: A 13-year follow-up study in elderly non-diabetic Finns. Eur. Heart J. 2007, 28, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Aggarwal, S.; Sharma, V. Cardiovascular risk factors in young patients of coronary artery disease: Differences over a decade. J. Cardiovasc. Thorac. Res. 2014, 6, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Jahangiry, L.; Asghari-Jafarabadi, M.; Najafi, M. Association between dietary patterns and metabolic syndrome in a sample of Tehranian adults. Obes. Res. Clin. Pract. 2016, 10 (Suppl. 1), S64–S73. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome. An American heart association/national heart, lung, and blood institute scientific statement. Executive summary. Cardiol. Rev. 2005, 13, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Shab-Bidar, S.; Golzarand, M.; Hajimohammadi, M.; Mansouri, S. A posteriori dietary patterns and metabolic syndrome in adults: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2018, 21, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, C.; Scheidt-Nave, C.; Richter, A.; Mensink, G.B. Dietary patterns are associated with cardiometabolic risk factors in a representative study population of German adults. Br. J. Nutr. 2011, 106, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, Y.; Lee, K.H.; Lee, H.S.; Lee, M.; Jee, S.H.; Joung, H. A fruit and dairy dietary pattern is associated with a reduced risk of metabolic syndrome. Metabolism 2012, 61, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.A.; Kim, J.; Cho, E.R.; Shin, A. Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. N. Am 2004, 33, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Day, C. Metabolic syndrome, or what you will: Definitions and epidemiology. Diabetes Vasc. Dis. Res. 2007, 4, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Mertens, E.; Kuijsten, A.; Dofková, M.; Mistura, L.; D’Addezio, L.; Turrini, A.; Dubuisson, C.; Favret, S.; Havard, S.; Trolle, E.; et al. Geographic and socioeconomic diversity of food and nutrient intakes: A comparison of four European countries. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, N.K.; Vinciguerra, M.; Lopez-Jimenez, F.; Kunzová, Š.; Homolka, M.; Jaresova, J.; Cífková, R.; Sochor, O. Kardiovize brno 2030, a prospective cardiovascular health study in central Europe: Methods, baseline findings and future directions. Eur. J. Prev. Cardiol. 2017, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Hruskova, J.; Maugeri, A.; Podroužková, H.; Štípalová, T.; Jakubík, J.; Barchitta, M.; Medina-Inojosa, J.R.; Homolka, M.; Agodi, A.; Kunzova, S.; et al. Association of cardiovascular health with epicardial adipose tissue and intima media thickness: The kardiovize study. J. Clin. Med. 2018, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Kunzova, S.; Medina-Inojosa, J.R.; Agodi, A.; Barchitta, M.; Homolka, M.; Kiacova, N.; Bauerova, H.; Sochor, O.; Lopez-Jimenez, F.; et al. Association between eating time interval and frequency with ideal cardiovascular health: Results from a random sample Czech urban population. Nutr. Metab. Cardiovasc. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (redcap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, J.H.; Löwik, M.R.; Steingrímsdóttir, L.; Møller, A.; Kearney, J.; De Henauw, S.; Becker, W.; Group, E. A European food consumption survey method—Conclusions and recommendations. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 2), S89–S94. [Google Scholar] [CrossRef] [PubMed]
- Ruprich, J.; Dofkova, M.; Rehurkova, I.; Slamenikova, E.; Resova, D. Individual Food Consumption—The National Study SISP04. CHFCH NIPH in Prague. Available online: http://www.chpr.szu.cz/spotrebapotravin.htm (accessed on 1 April 2018).
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Kozieł, D.; Cieśla, E.; Rębak, D.; Głuszek, S. Dietary patterns in relation to metabolic syndrome among adults in Poland: A cross-sectional study. Nutrients 2017, 9, 1366. [Google Scholar] [CrossRef] [PubMed]
- Kimokoti, R.W.; Gona, P.; Zhu, L.; Newby, P.K.; Millen, B.E.; Brown, L.S.; D’Agostino, R.B.; Fung, T.T. Dietary patterns of women are associated with incident abdominal obesity but not metabolic syndrome. J. Nutr. 2012, 142, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Ängquist, L.; Du, H.; Jakobsen, M.U.; Forouhi, N.G.; Halkjær, J.; Feskens, E.J.; van der A, D.L.; Masala, G.; Steffen, A.; et al. Food composition of the diet in relation to changes in waist circumference adjusted for body mass index. PLoS ONE 2011, 6, e23384. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Siegert, S.; Koch, M.; Aits, I.; Lieb, W.; Nikolaus, S.; Laudes, M.; Jacobs, G.; Nöthlings, U. Comparison of two exploratory dietary patterns in association with the metabolic syndrome in a northern german population. Br. J. Nutr. 2014, 112, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, X.; Liu, Z.; Varma, D.S.; Wan, R.; Wan, Q.; Zhao, S. Dietary patterns in relation to general and central obesity among adults in southwest china. Int. J. Environ. Res. Public Health 2016, 13, 1080. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, A.; Tsutsumi, A.; Gotoh, T.; Ishikawa, S.; Ojima, T.; Kario, K.; Nakamura, Y.; Kayaba, K. Dietary patterns and levels of blood pressure and serum lipids in a Japanese population. J. Epidemiol. 2008, 18, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Kim, J.M.; Kim, Y. Associations between dietary patterns and hypertension among Korean adults: The Korean national health and nutrition examination survey (2008–2010). Nutr. Res. Pract. 2013, 7, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Buys, N.J.; Hills, A.P. Dietary pattern and its association with the prevalence of obesity, hypertension and other cardiovascular risk factors among Chinese older adults. Int. J. Environ. Res. Public Health 2014, 11, 3956–3971. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.E.; Kim, J.E.; Campbell, W.W. Total red meat intake of ≥0.5 servings/d does not negatively influence cardiovascular disease risk factors: A systemically searched meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Xia, Y.; Zhang, Q.; Wu, H.; Du, H.; Liu, L.; Wang, C.; Shi, H.; Guo, X.; Liu, X.; et al. Dietary patterns are associated with prevalence of fatty liver disease in adults. Eur. J. Clin. Nutr. 2015, 69, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Shu, L.; Wang, S.; Wang, J.J.; Zhou, Y.; Xuan, Y.J.; Wang, S.F. Dietary patterns modulate the risk of non-alcoholic fatty liver disease in Chinese adults. Nutrients 2015, 7, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; Jones, B.; Ullah, I.; McNaughton, S.A.; Haslett, S.J.; Stonehouse, W. Associations between dietary patterns, socio-demographic factors and anthropometric measurements in adult New Zealanders: An analysis of data from the 2008/09 New Zealand adult nutrition survey. Eur. J. Nutr. 2018, 57, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Kengne, A.P. Nutrient patterns and their relationship with general and central obesity in US adults. Eur. J. Clin. Investig. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizadeh, F.; Djafarian, K.; Khosravi, S.; Shab-Bidar, S. A posteriori healthy dietary patterns may decrease the risk of central obesity: Findings from a systematic review and meta-analysis. Nutr. Res. 2017, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, Y.; Li, P.; Chang, H.; Wang, X.; Liu, W.; Zhang, Y.; Huang, G. Associations between dietary patterns and impaired fasting glucose in Chinese men: A cross-sectional study. Nutrients 2015, 7, 8072–8089. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Salim, A.; Flynn, A.; Perry, I.J. Prudent diet and the risk of insulin resistance. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 334–343. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Härkänen, T.; Järvinen, R.; Heliövaara, M.; Aromaa, A.; Reunanen, A. Dietary patterns and the incidence of type 2 diabetes. Am. J. Epidemiol. 2005, 161, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Adriano, L.S.; Sampaio, H.A.; Arruda, S.P.; Portela, C.L.; de Melo, M.L.P.; Carioca, A.A.; Soares, N.T. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br. J. Nutr. 2016, 115, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The association of dietary patterns with high-risk human papillomavirus infection and cervical cancer: A cross-sectional study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Dallongeville, J.; Haas, B.; Ruidavets, J.B.; Amouyel, P.; Ferrières, J.; Simon, C.; Arveiler, D. Sedentary behaviour, physical activity and dietary patterns are independently associated with the metabolic syndrome. Diabetes Metab. 2012, 38, 428–435. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (N = 1934) | MetS (N = 739) | Control (N = 1195) | p-Value a |
|---|---|---|---|---|
| Age, years b | 47.0 (19.0) | 54.0 (15.0) | 41.5 (17.0) | <0.001 |
| Sex (% male) | 44.6% | 48.6% | 42.2% | 0.017 |
| Marital status (% married) | 61.7% | 69.1% | 58.3% | <0001 |
| Employment (% unemployed) | 18.1% | 25.7% | 13.3% | <0.001 |
| Smoking (% current smokers) | 23.7% | 26.7% | 22.1% | 0.041 |
| Total energy intake, kcal b | 2006 (916) | 1963 (912) | 2045 (910) | 0.013 |
| Physical activity, MET min/week b | 3175 (4782) | 2994 (4388) | 3252 (4340) | 0.009 |
| Hypertension | 34.6% | 66.0% | 18.2% | <0.001 |
| Use of antihypertensives | 17.0% | 36.5% | 5.2% | <0.001 |
| Hyperlipidemia | 65.4% | 84.3% | 54.0 | <0.001 |
| Use of hypolipidemics | 5.4% | 11.2% | 1.9% | <0.001 |
| Weight, kg b | 75.7 (23.0) | 88.3 (20.2) | 69.8 (17.9) | |
| BMI, kg/m2 b | 24.8 (5.8) | 28.6 (5.3) | 23.1 (3.9) | <0.001 |
| BMI categories (%) | ||||
| Underweight | 2.9% | 0% | 4.6% | <0.001 |
| Normal weight | 48.5% | 14.7% | 69.3% | |
| Overweight | 33.6% | 50.5% | 23.1% | |
| Obese | 15.1% | 34.8% | 3.0% | |
| Waist circumference b | 87.0 (20.0) | 100.0 (13.0) | 81.0 (14.0) | <0.001 |
| Hip circumference b | 102.0 (10.0) | 107.0 (10.0) | 99.0 (8.0) | <0.001 |
| WHR b | 0.85 (0.14) | 0.92 (0.12) | 0.81 (0.12) | <0.001 |
| Abdominal Obesity (%) | 52.2% | 100% | 22.6% | <0.001 |
| Body Fat Mass b | 18.4 (12.1) | 25.5 (11.1) | 14.6 (8.0) | <0.001 |
| Systolic Blood Pressure, mmHg b | 117.0 (19.5) | 127.5 (19.8) | 112.5 (14.0) | <0.001 |
| Diastolic Blood Pressure, mmHg b | 79.0 (13.0) | 85.5 (11.3) | 76.3 (10.5) | <0.001 |
| Glycated Haemoglobin, nmol/mol b | 39.0 (6.0) | 41.0 (5.0) | 38.0 (5.0) | <0.001 |
| Fasting Glucose, nmol/L b | 4.8 (0.7) | 5.1 (0.7) | 4.7 (0.6) | <0.001 |
| Creatinine, nmol/L b | 75.0 (18.0) | 76.0 (18.5) | 75.0 (18.0) | 0.312 |
| Triglycerides, nmol/L b | 1.0 (0.8) | 1.4 (0.9) | 0.8 (0.5) | <0.001 |
| Total Cholesterol, nmol/L b | 5.1 (1.3) | 5.4 (1.4) | 4.9 (1.3) | <0.001 |
| HDL Cholesterol, nmol/L b | 1.5 (0.5) | 1.3 (0.5) | 1.6 (0.5) | <0.001 |
| LDL Cholesterol, nmol/L b | 3.0 (1.2) | 3.3 (1.3) | 2.9 (1.2) | <0.001 |
| Total Cholesterol/HDL-ratio b | 3.3 (1.4) | 4.0 (1.5) | 3.0 (1.1) | <0.001 |
| Characteristics | Western Dietary Pattern | Prudent Dietary Pattern | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 (N = 639) | T2 (N = 651) | T3 (N = 644) | p-Value a | T1 (N = 646) | T2 (N = 633) | T3 (N = 655) | p-Value a | |
| Age, years b | 50.0 (19.0) | 48.0 (18.0) | 43.0 (18.0) | <0.001 | 47.0 (19.0) | 48.0 (19.0) | 46.0 (20.0) | 0.215 |
| Sex, (% male) | 32.9% | 43.3% | 56.5% | <0.001 | 44.3% | 45.2% | 43.4% | 0.805 |
| Marital status, (% married) | 36.2% | 38.3% | 40.4% | 0.298 | 38.2% | 37.5% | 39.3% | 0.807 |
| Employment, (% unemployed) | 21.7% | 17.4% | 14.8% | 0.006 | 16.3% | 17.6% | 20.0% | 0.224 |
| Smoking, (% current smokers) | 20.7% | 23.4% | 27.2% | 0.058 | 26.2% | 22.2% | 22.8% | 0.275 |
| Total energy intake, kcal b | 1795.2 (873.4) | 2000.2 (909.0) | 2214.6 (969.8) | <0.001 | 1992.9 (925.7) | 1993.5 (913.4) | 2031.3 (943.7) | 0.334 |
| Physical activity, MET-min/week b | 3644.5 (4310.2) | 3120.0 (4909.1) | 3338.0 (5235.0) | 0.325 | 3338.0 (5408.3) | 3456.5 (5281.1) | 3300.0 (4292.6) | 0.796 |
| Hypertension | 34.2% | 39.2% | 35.7% | 0.244 | 38.6% | 37.6% | 32.9% | 0.129 |
| Use of antihypertensives | 17.0% | 18.7% | 15.1% | 0.323 | 17.2% | 19.2% | 14.5% | 0.145 |
| Hyperlipidemia | 33.9% | 32.4% | 37.4% | 0.253 | 35.1% | 37.9% | 30.6% | 0.059 |
| Use of hypolipidemics | 6.1% | 4.5% | 5.5% | 0.533 | 5.9% | 5.3% | 4.9% | 0.791 |
| Characteristics | Western Dietary Pattern | Prudent Dietary Pattern | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 (N = 639) | T2 (N = 651) | T3 (N = 644) | p-Value a | T1 (N = 646) | T2 (N = 633) | T3 (N = 655) | p-Value a | |
| Weight, kg b | 72.4 (19.8) | 78.2 (24.0) | 77.5 (23.7) | 0.002 | 77.2 (22.5) | 76.6 (24.7) | 74.1 (22.1) | 0.006 |
| BMI, kg/m2 b | 24.5 (5.2) | 25.2 (6.2) | 24.8 (5.9) | 0.128 | 25.1 (6.0) | 25.2 (5.7) | 24.3 (5.4) | <0.001 |
| BMI categories (%) | ||||||||
| Underweight | 3.9% | 2.5% | 2.3% | 0.425 | 2.5% | 1.6% | 4.5% | 0.005 |
| Normal weight | 49.7% | 45.8% | 48.9% | 45.9% | 45.9% | 52.5% | ||
| Overweight | 32.8% | 34.7% | 34.0% | 34.6% | 35.6% | 31.4% | ||
| Obese | 13.6% | 17.0% | 14.9% | 17.1% | 16.9% | 11.6% | ||
| Waist circumference b | 85.0 (18.0) | 87.5 (21.0) | 88.0 (21.0) | 0.001 | 88.0 (19.8) | 87.0 (20.0) | 85.0 (18.0) | 0.001 |
| Hip circumference b | 102.0 (11.0) | 102.0 (10.8) | 101.0 (10.0) | 0.236 | 102.0 (10.0) | 103.0 (11.0) | 101.0 (9.0) | 0.001 |
| WHR b | 0.83 (0.13) | 0.85 (0.14) | 0.87 (0.14) | <0.001 | 0.86 (0.14) | 0.85 (0.13) | 0.84 (0.15) | 0.075 |
| Abdominal Obesity (%) | 52.8% | 54.6% | 50.3% | 0.401 | 56.3% | 55.9% | 45.8% | 0.001 |
| Body Fat Mass b | 18.4 (12.4) | 18.6 (13.0) | 17.5 (11.4) | 0.121 | 19.3 (12.7) | 18.7 (12.2) | 16.9 (10.8) | <0.001 |
| Systolic Blood Pressure, mmHg b | 116.5 (19.5) | 117.5 (18.0) | 116.5 (19.5) | 0.341 | 117.5 (19.5) | 117.5 (20.3) | 115.5 (18.5) | 0.022 |
| Diastolic Blood Pressure, mmHg b | 78.3 (12.5) | 80.0 (13.0) | 79.0 (14.0) | 0.087 | 79.5 (13.5) | 80.0 (13.3) | 78.0 (12.0) | 0.021 |
| Glycated Haemoglobin, nmol/mol b | 40.0 (5.0) | 39.0 (5.0) | 39.0 (5.0) | 0.002 | 39.0 (6.0) | 39.0 (5.0) | 39.0 (6.0) | 0.279 |
| Fasting Glucose, nmol/L b | 4.8 (0.6) | 4.9 (0.7) | 4.8 (0.6) | 0.187 | 4.8 (0.7) | 4.9 (0.7) | 4.8 (0.7) | 0.157 |
| Creatinine, nmol/L b | 73.0 (17.3) | 75.0 (18.8) | 78.0 (18.0) | <0.001 | 75.0 (19.0) | 76.0 (17.5) | 74.0 (18.0) | 0.281 |
| Triglycerides, nmol/L b | 0.9 (0.7) | 1.0 (0.9) | 1.1 (0.8) | 0.001 | 1.0 (0.7) | 1.0 (0.8) | 1.0 (0.9) | 0.278 |
| Total Cholesterol, nmol/L b | 5.1 (1.4) | 5.2 (1.4) | 5.1 (1.3) | 0.061 | 5.1 (1.3) | 5.2 (1.3) | 5.1 (1.3) | 0.687 |
| HDL Cholesterol, nmol/L b | 1.6 (0.5) | 1.5 (0.5) | 1.4 (0.5) | <0.001 | 1.5 (0.5) | 1.5 (0.5) | 1.5 (0.5) | 0.206 |
| LDL Cholesterol, nmol/L b | 3.1 (1.3) | 3.0 (1.2) | 3.0 (1.2) | 0.388 | 3.0 (1.2) | 3.1 (1.2) | 3.0 (1.3) | 0.761 |
| Total Cholesterol/HDL-ratio b | 3.2 (1.3) | 3.3 (1.4) | 3.4 (1.6) | 0.001 | 3.3 (1.4) | 3.3 (1.4) | 3.3 (1.4) | 0.578 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agodi, A.; Maugeri, A.; Kunzova, S.; Sochor, O.; Bauerova, H.; Kiacova, N.; Barchitta, M.; Vinciguerra, M. Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients 2018, 10, 898. https://doi.org/10.3390/nu10070898
Agodi A, Maugeri A, Kunzova S, Sochor O, Bauerova H, Kiacova N, Barchitta M, Vinciguerra M. Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients. 2018; 10(7):898. https://doi.org/10.3390/nu10070898
Chicago/Turabian StyleAgodi, Antonella, Andrea Maugeri, Sarka Kunzova, Ondrej Sochor, Hana Bauerova, Nikola Kiacova, Martina Barchitta, and Manlio Vinciguerra. 2018. "Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study" Nutrients 10, no. 7: 898. https://doi.org/10.3390/nu10070898
APA StyleAgodi, A., Maugeri, A., Kunzova, S., Sochor, O., Bauerova, H., Kiacova, N., Barchitta, M., & Vinciguerra, M. (2018). Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients, 10(7), 898. https://doi.org/10.3390/nu10070898








