Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. KF Preparation
2.2. Animals and Experiment Design
2.3. Exhaustive Swimming Test
2.4. Forelimb Grip Strength
2.5. Fatigue-Associated Biochemical Indices
2.6. Tissue Glycogen Determination
2.7. Histological Staining of Tissues
2.8. Blood Biochemical Assessments
2.9. Bacterial DNA Extraction and 16S rRNA Sequencing
2.10. Statistical Analysis
3. Results
3.1. Effect of Four-Week KF Supplementation on Tissue Weights, Body Weight, Food Intake, and Water Intake
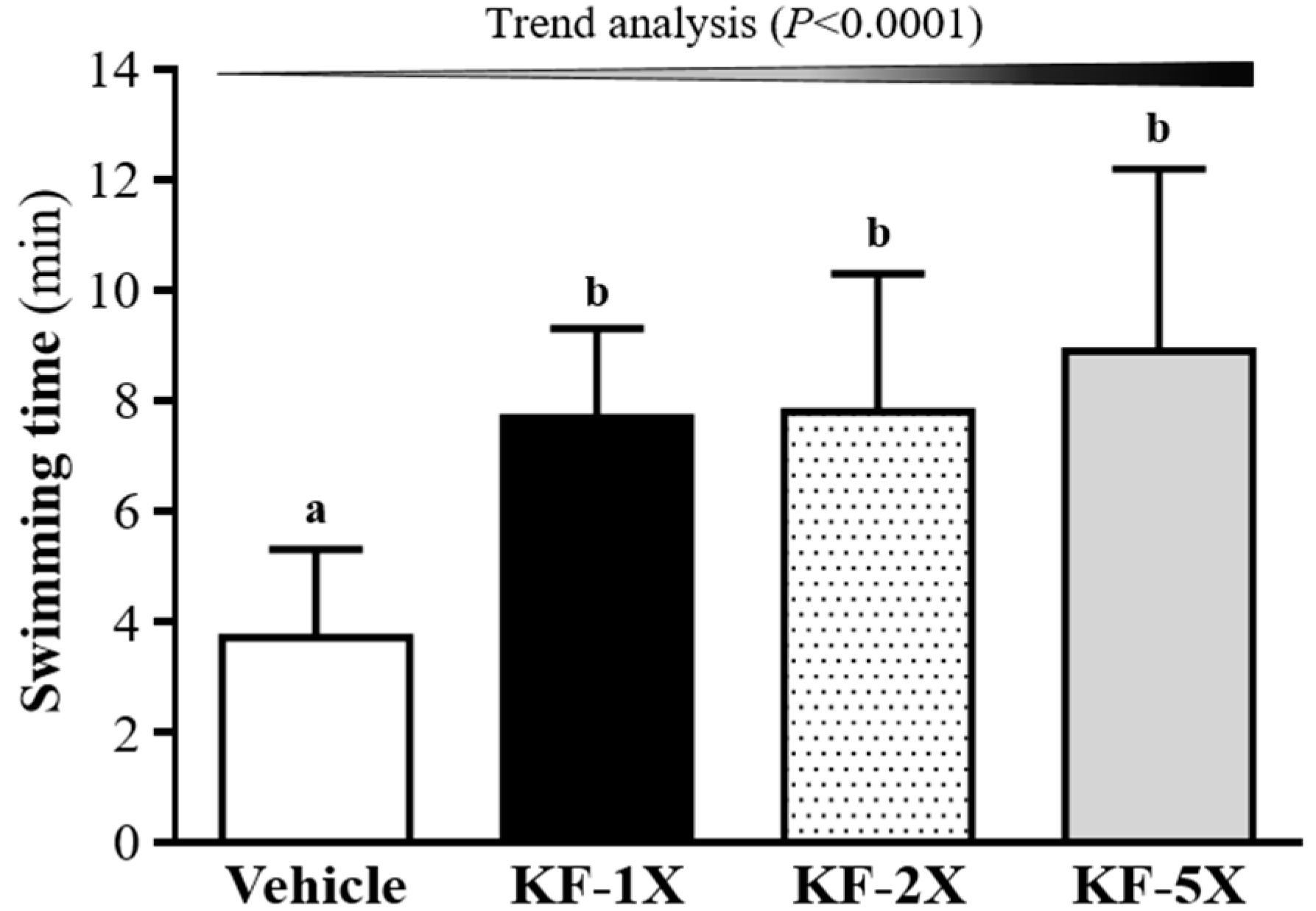
3.2. Effect of Four-Week KF Supplementation on the Exhaustive Swimming Test
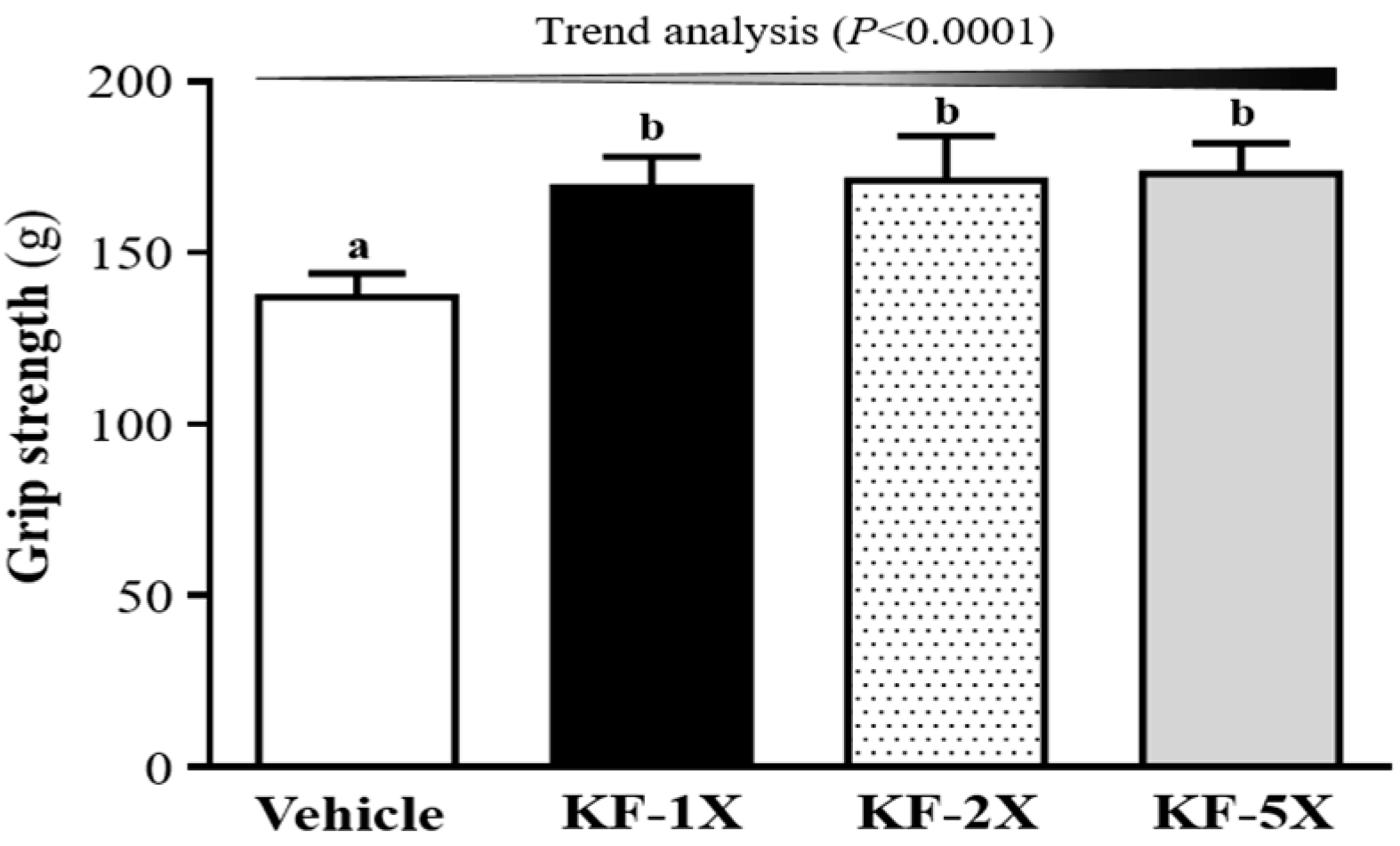
3.3. Effect of Four-Week KF Supplementation on the Forelimb Grip Strength
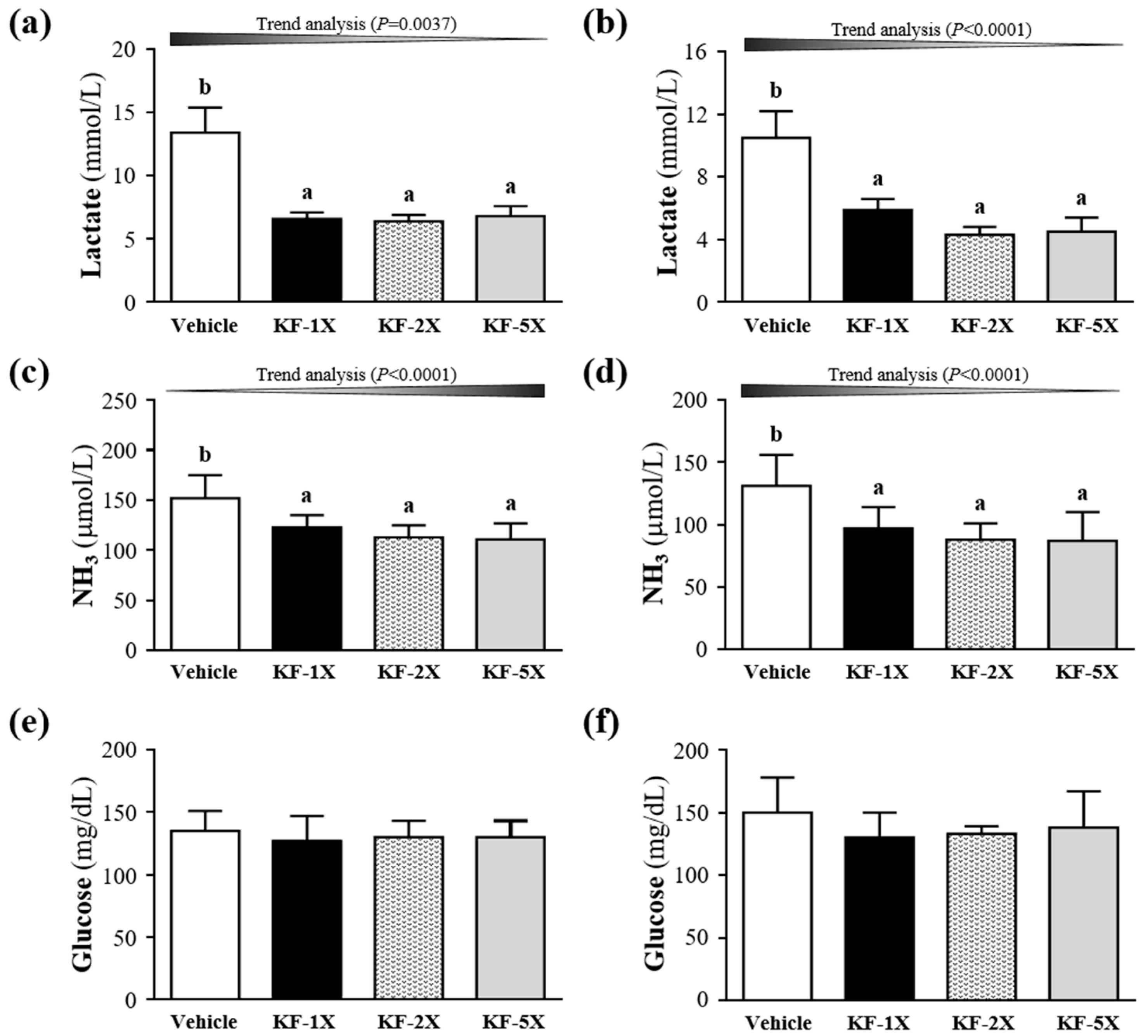
3.4. Effect of Four-Week KF Supplementation on Lactate after the 10-Min Swimming Test
3.5. Effect of Four-Week KF Supplementation on Ammonia and Glucose after the 10-Min Swimming Test
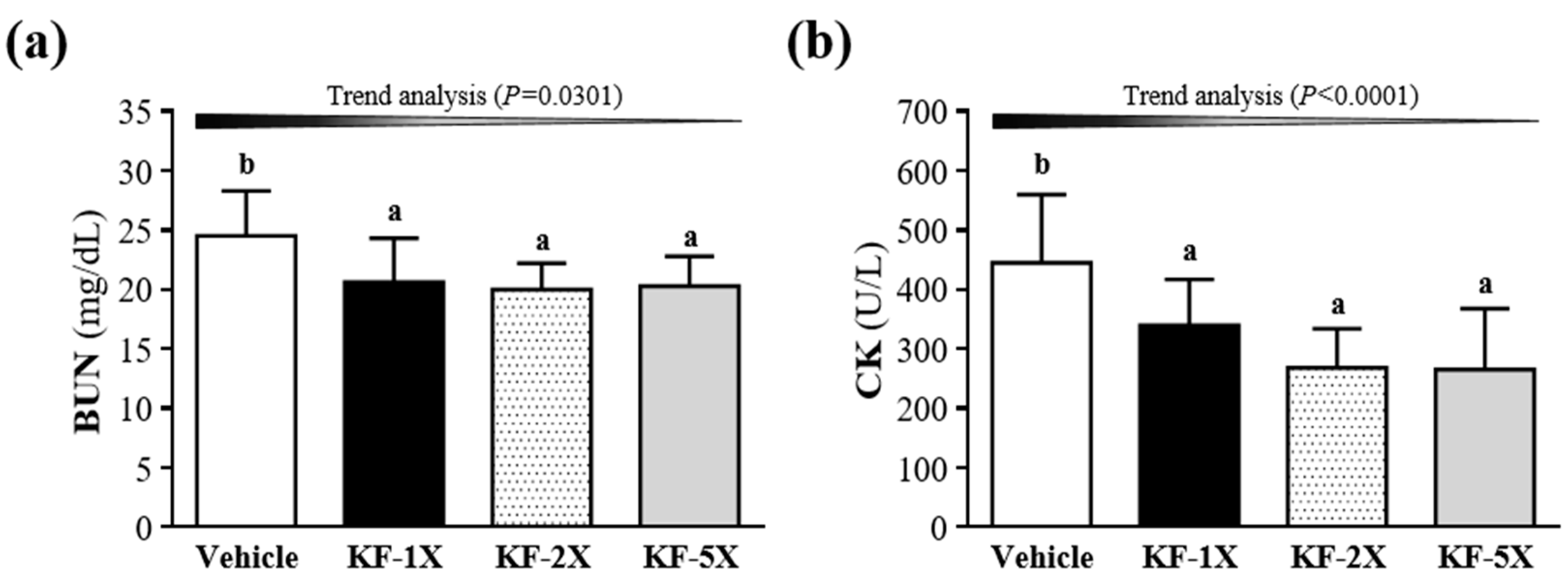
3.6. Effect of Four-Week KF Supplementation on BUN and CK after the 90-Min Swimming Test and 60-Min Rest Period
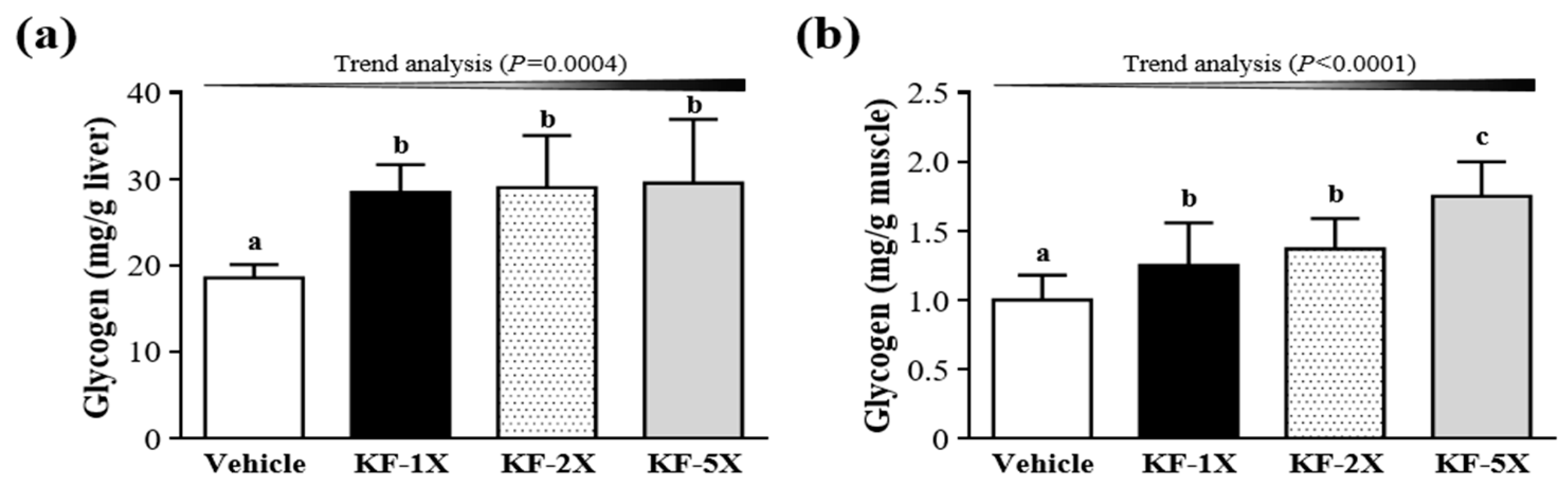
3.7. Effect of Four-Week KF Supplementation on Liver and Muscular Glycogen
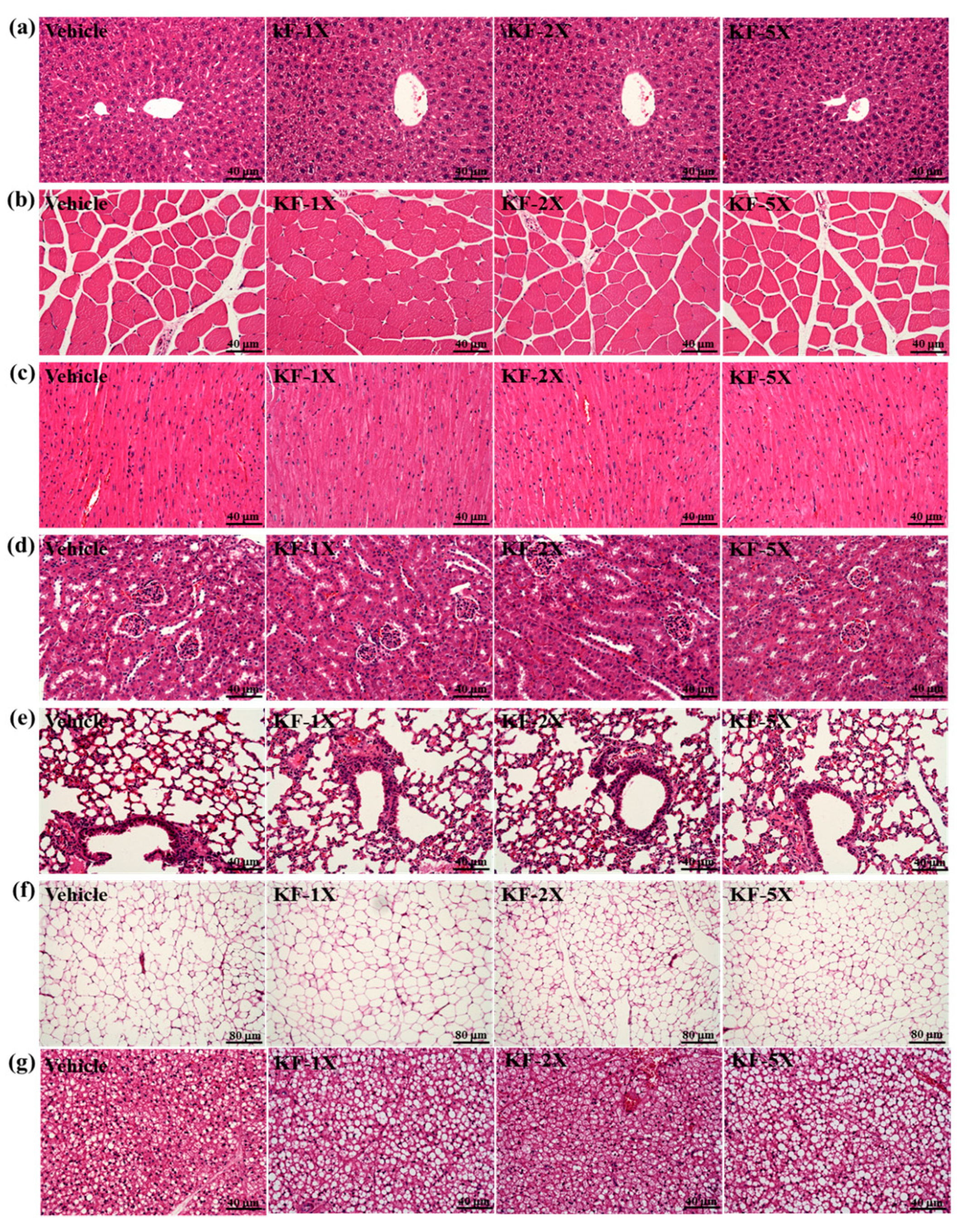
3.8. Effect of Four-Week KF Supplementation on Histopathology of Tissues and Biochemical Variables at the End of the Experiment
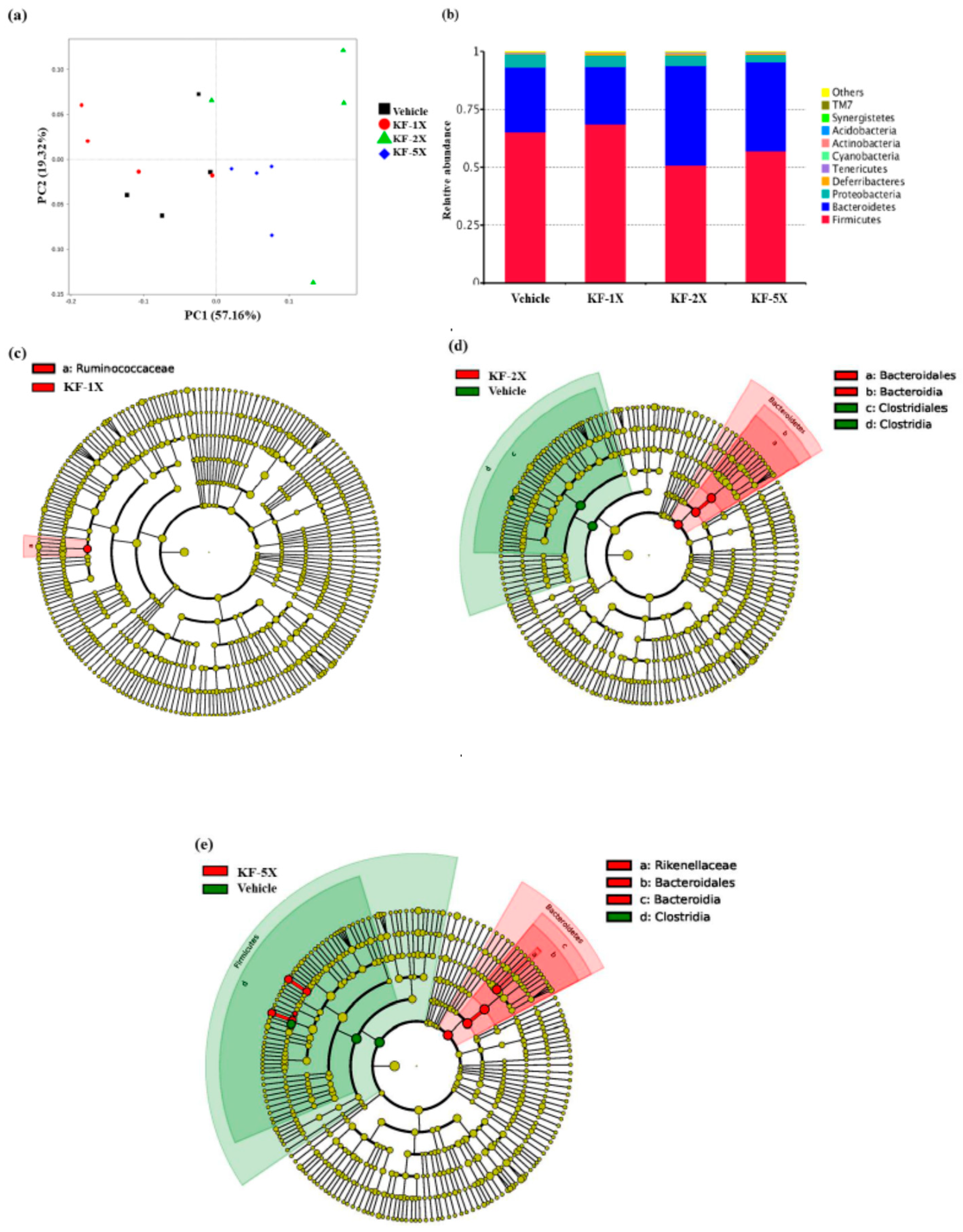
3.9. Effect of Four-Week KF Supplementation on the Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebringer, L.; Ferencík, M.; Krajcovic, J. Beneficial health effects of milk and fermented dairy products—Review. Folia Microbiol. 2008, 53, 378–394. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Farnworth, E.R.; Savard, T.; Chabot, D.; Mafu, A.; Jones, P.J. Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: A randomized controlled trial. BMC Complement. Altern. Med. 2002, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guzel-Seydim, Z.B.; Kok-Tas, T.; Greene, A.K.; Seydim, A.C. Review: Functional properties of kefir. Crit. Rev. Food Sci. Nutr. 2011, 51, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Mainville, I. Kefir: A fermented milk product. In Handbook of Fermented Functional Foods; Farnworth, E.R., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 77–112. [Google Scholar]
- Chen, H.L.; Tung, Y.T.; Tsai, C.L.; Lai, C.W.; Lai, Z.L.; Tsai, H.C.; Lin, Y.L.; Wang, C.H.; Chen, C.M. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int. J. Obes. (Lond.) 2014, 38, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Cagindi, O. Kefir: A probiotic dairy-composition, nutritional and therapeutic aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Wang, L.; Zhang, H.L.; Lu, R.; Zhou, Y.J.; Ma, R.; Lv, J.Q.; Li, X.L.; Chen, L.J.; Yao, Z. The decapeptide CMS001 enhances swimming endurance in mice. Peptides 2008, 29, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Baba, Y.; Kataoka, Y.; Kinbara, N.; Sagesaka, Y.M.; Kakuda, T.; Watanabe, Y. Effects of (-)-epigallocatechin gallate in liver of an animal model of combined (physical and mental) fatigue. Nutrition 2008, 24, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.R. Exercise, oxidative stress, and antioxidants: A review. Int. J. Sport Nutr. 1993, 3, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. 2005, 98, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Cao, S.; Wang, J.; Zheng, H.; Putheti, R. Glabridin from Chinese herb licorice inhibits fatigue in mice. Afr. J. Tradit. Complement. Altern. Med. 2009, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tharakan, B.; Dhanasekaran, M.; Brown-Borg, H.M.; Manyam, B.V. Trichopus zeylanicus combats fatigue without amphetamine-mimetic activity. Phytother. Res. 2006, 20, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef] [PubMed]
- Su, W.L.; Huang, W.C.; Chen, I.N.; Chen, W.C.; Huang, C.C.; Huang, C.H. Investigation of whole-body vibration training on physiological and biochemical characteristics in mice. Phys. Educ. J. 2015, 48, 33–44. [Google Scholar]
- O’Brien, K.V.; Stewart, L.K.; Forney, L.A.; Aryana, K.J.; Prinyawiwatkul, W.; Boeneke, C.A. The effects of postexercise consumption of a kefir beverage on performance and recovery during intensive endurance training. J. Dairy Sci. 2015, 98, 7446–7449. [Google Scholar] [CrossRef] [PubMed]
- Lollo, P.C.B.; Morato, P.N.; Moura, C.S.; Oliveira, M.M.; Cruz, A.G.; Faria, J.A.F.; Amaya-Farfan, J.; Cristianini, M. Ultra-high temperature plus dynamic high pressure processing: An effective combination for potential probiotic fermented milk processing which attenuate exercise-induced immune suppression in Wistar rats. J. Funct. Food 2015, 14, 541–548. [Google Scholar] [CrossRef]
- Frémont, M.; Coomans, D.; Massart, S.; De Meirleir, K. Anaerobe. High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 2013, 22, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Venket Rao, A.; Irani, D. Chronic fatigue syndrome: Lactic acid bacteria may be of therapeutic value. Med. Hypothesis 2003, 60, 915–923. [Google Scholar] [CrossRef]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; McGregor, N.; Stapleton, D.I.; Butt, H.L.; DE Meirleir, K.L. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 2009, 23, 621–628. [Google Scholar] [PubMed]
- Wu, R.E.; Huang, W.C.; Liao, C.C.; Chang, Y.K.; Kan, N.W.; Huang, C.C. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 2013, 18, 4689–4702. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chiu, W.C.; Chuang, H.L.; Tang, D.W.; Lee, Z.M.; Wei, L.; Chen, F.A.; Huang, C.C. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Kan, N.W.; Ho, C.S.; Chiu, Y.S.; Huang, W.C.; Chen, P.Y.; Tung, Y.T.; Huang, C.C. Effects of resveratrol supplementation and exercise training on the exercise performance in middle-aged mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.W. Probiotics and athletic performance: A systematic review. Curr. Sports Med. Rep. 2007, 6, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, A.; Keller, D.; Farmer, S. Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef. Microbes 2009, 1, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Tseng, T.L.; Huang, W.C.; Chung, Y.H.; Chuang, H.L.; Wu, J.H. Whole-body vibration training effect on physical performance and obesity in mice. Int. J. Med. Sci. 2014, 11, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Esgalhado, M.; Kemp, J.A.; Damasceno, N.R.; Fouque, D.; Mafra, D. Short-chain fatty acids: A link between prebiotics and microbiota in chronic kidney disease. Future Microbiol. 2017, 12, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Lambertz, J.; Weiskirchen, S.; Landert, S.; Weiskirchen, R. Fructose: A dietary sugar in crosstalk with microbiota contributing to the development and progression of non-alcoholic liver disease. Front. Immunol. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Simova, E.; Beshkova, D.; Angelov, A.; Hristozova, Ts.; Frengova, G.; Spasov, Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Storey, K.B. A quantitative evaluation of the effect of enzyme complexes on the glycolytic rate in vivo: Mathematical modeling of the glycolytic complex. J. Theor. Biol. 1991, 149, 361–375. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Tsai, Y.H.; Tsai, T.Y.; Chiu, Y.S.; Wei, L.; Chen, W.C.; Huang, C.C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hsu, Y.J.; Li, H.S.; Kan, N.W.; Chen, Y.M.; Lin, J.S.; Hsu, T.K.; Tsai, T.Y.; Chiu, Y.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on improving endurance performance in humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Chen, K.; Liu, P.; Chang, H.; Zhu, J.; Mi, M. Dihydromyricetin improves physical performance under simulated high altitude. Med. Sci. Sports Exerc. 2014, 46, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Huang, W.C.; Chiu, C.C.; Liu, Y.L.; Chiu, W.C.; Chiu, C.H.; Chiu, Y.S.; Huang, C.C. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Nutrients 2016, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Bomhof, M.R.; Saha, D.C.; Reid, D.T.; Paul, H.A.; Reimer, R.A. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity (Silver Spring) 2014, 22, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Hooper, L.; Stappenbeck, T.; Hong, C.; Gordon, J. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.; Liu, C.; Tzianabos, A.; Kasper, D. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Stappenbeck, T.; Hooper, L.; Gordon, J. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl. Acad. Sci. USA 2002, 99, 15451–15455. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Vehicle | KF-1X | KF-2X | KF-5X | Trend Analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 33.4 ± 0.7 | 33.7 ± 0.7 | 33.4 ± 0.9 | 33.6 ± 0.3 | 0.4631 |
| Final BW (g) | 36.2 ± 2.2 | 36.7 ± 1.1 | 36.5 ± 1.4 | 36.0 ± 1.3 | 0.6215 |
| Food intake (g/mouse/day) | 6.2 ± 0.6 | 6.2 ± 0.3 | 6.1 ± 0.3 | 6.0 ± 0.3 | 0.1216 |
| Water intake (mL/mouse/day) | 9.5 ± 2.0 | 9.4 ± 2.0 | 9.5 ± 0.8 | 9.5 ± 2.5 | 0.8155 |
| Liver (g) | 2.03 ± 0.10 | 2.00 ± 0.26 | 2.06 ± 0.13 | 2.03 ± 0.13 | 0.7996 |
| Kidney (g) | 0.52 ± 0.03 | 0.53 ± 0.03 | 0.52 ± 0.05 | 0.51 ± 0.04 | 0.4781 |
| EFP (g) | 0.37 ± 0.03 | 0.35 ± 0.06 | 0.32 ± 0.03 | 0.32 ± 0.11 | 0.1673 |
| Heart (g) | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.21 ± 0.02 | 0.22 ± 0.02 | 0.8400 |
| Lung (g) | 0.23 ± 0.03 | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.2464 |
| Muscle (g) | 0.36 ± 0.03 a | 0.39 ± 0.02 b | 0.39 ± 0.02 b | 0.39 ± 0.02 b | 0.0871 |
| BAT (g) | 0.09 ± 0.02 a | 0.12 ± 0.02 b | 0.11 ± 0.02 b | 0.11 ± 0.01 b | 0.1560 |
| Relative liver weight (%) | 5.60 ± 0.30 | 5.45 ± 0.72 | 5.65 ± 0.45 | 5.65 ± 0.35 | 0.3092 |
| Relative kidney weight (%) | 1.44 ± 0.09 | 1.45 ± 0.11 | 1.42 ± 0.14 | 1.42 ± 0.11 | 0.8171 |
| Relative EFP weight (%) | 1.01 ± 0.11 | 0.95 ± 0.16 | 0.87 ± 0.12 | 0.89 ± 0.32 | 0.1797 |
| Relative heart weight (%) | 0.59 ± 0.08 | 0.62 ± 0.07 | 0.58 ± 0.06 | 0.62 ± 0.07 | 0.6835 |
| Relative lung weight (%) | 0.62 ± 0.07 | 0.59 ± 0.03 | 0.61 ± 0.04 | 0.61 ± 0.05 | 0.2464 |
| Relative muscle weight (%) | 0.99 ± 0.15 a | 1.07 ± 0.04 ab | 1.07 ± 0.06 ab | 1.09 ± 0.05 b | 0.0899 |
| Relative BAT weight (%) | 0.24 ± 0.06 a | 0.33 ± 0.05 b | 0.31 ± 0.05 b | 0.30 ± 0.04 b | 0.1607 |
| Parameter | Vehicle | KF-1X | KF-2X | KF-5X | Trend Analysis |
|---|---|---|---|---|---|
| AST (U/L) | 62 ± 13 | 53 ± 13 | 52 ± 10 | 52 ± 13 | 0.1562 |
| ALT (U/L) | 45 ± 19 b | 37 ± 12 ab | 30 ± 6 a | 30 ± 8 a | 0.0290 |
| Albumin (g/dL) | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.3 ± 0.1 | 3.4 ± 0.1 | 0.0437 |
| Creatinine (mg/dL) | 0.36 ± 0.07 | 0.35 ± 0.03 | 0.33 ± 0.02 | 0.34 ± 0.03 | 0.9029 |
| LDH (mg/dL) | 268 ± 95 | 250 ± 78 | 246 ± 20 | 244 ± 75 | 0.6979 |
| CK (U/L) | 392 ± 106 b | 298 ± 73 a | 271 ± 54 a | 276 ± 88 a | 0.0063 |
| TP (g/dL) | 4.41 ± 0.17 | 4.45 ± 0.05 | 4.51 ± 0.06 | 4.50 ± 0.09 | 0.0914 |
| Glucose (mg/dL) | 79 ± 14 | 80 ± 4 | 75 ± 7 | 80 ± 8 | 0.5622 |
| TC (mg/dL) | 134 ± 16 | 134 ± 10 | 121 ± 8 | 122 ± 13 | 0.0228 |
| TG (mg/dL) | 168 ± 23 | 163 ± 20 | 160 ± 23 | 162 ± 34 | 0.8584 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. https://doi.org/10.3390/nu10070862
Hsu Y-J, Huang W-C, Lin J-S, Chen Y-M, Ho S-T, Huang C-C, Tung Y-T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients. 2018; 10(7):862. https://doi.org/10.3390/nu10070862
Chicago/Turabian StyleHsu, Yi-Ju, Wen-Ching Huang, Jin-Seng Lin, Yi-Ming Chen, Shang-Tse Ho, Chi-Chang Huang, and Yu-Tang Tung. 2018. "Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice" Nutrients 10, no. 7: 862. https://doi.org/10.3390/nu10070862
APA StyleHsu, Y.-J., Huang, W.-C., Lin, J.-S., Chen, Y.-M., Ho, S.-T., Huang, C.-C., & Tung, Y.-T. (2018). Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients, 10(7), 862. https://doi.org/10.3390/nu10070862







