The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement
Abstract
1. Introduction
2. Materials and Methods
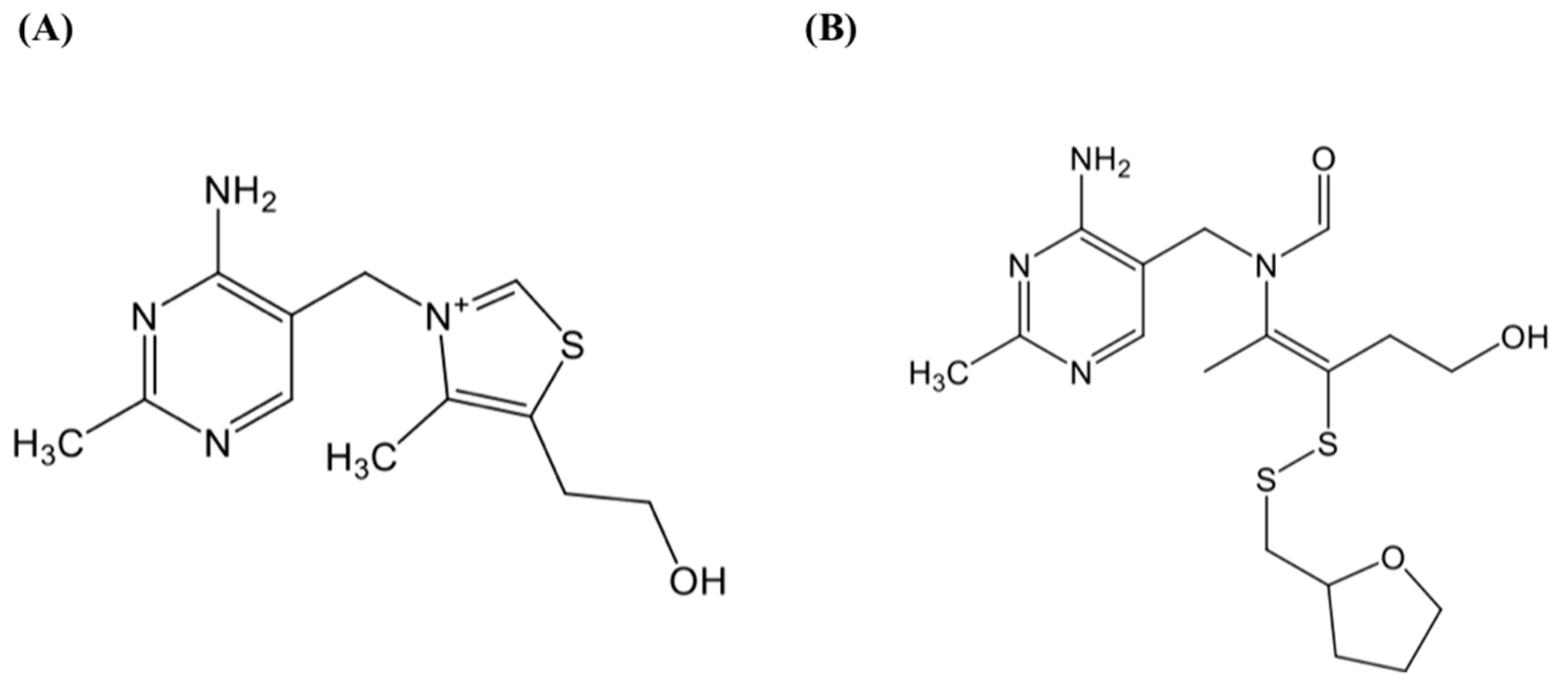
2.1. Materials
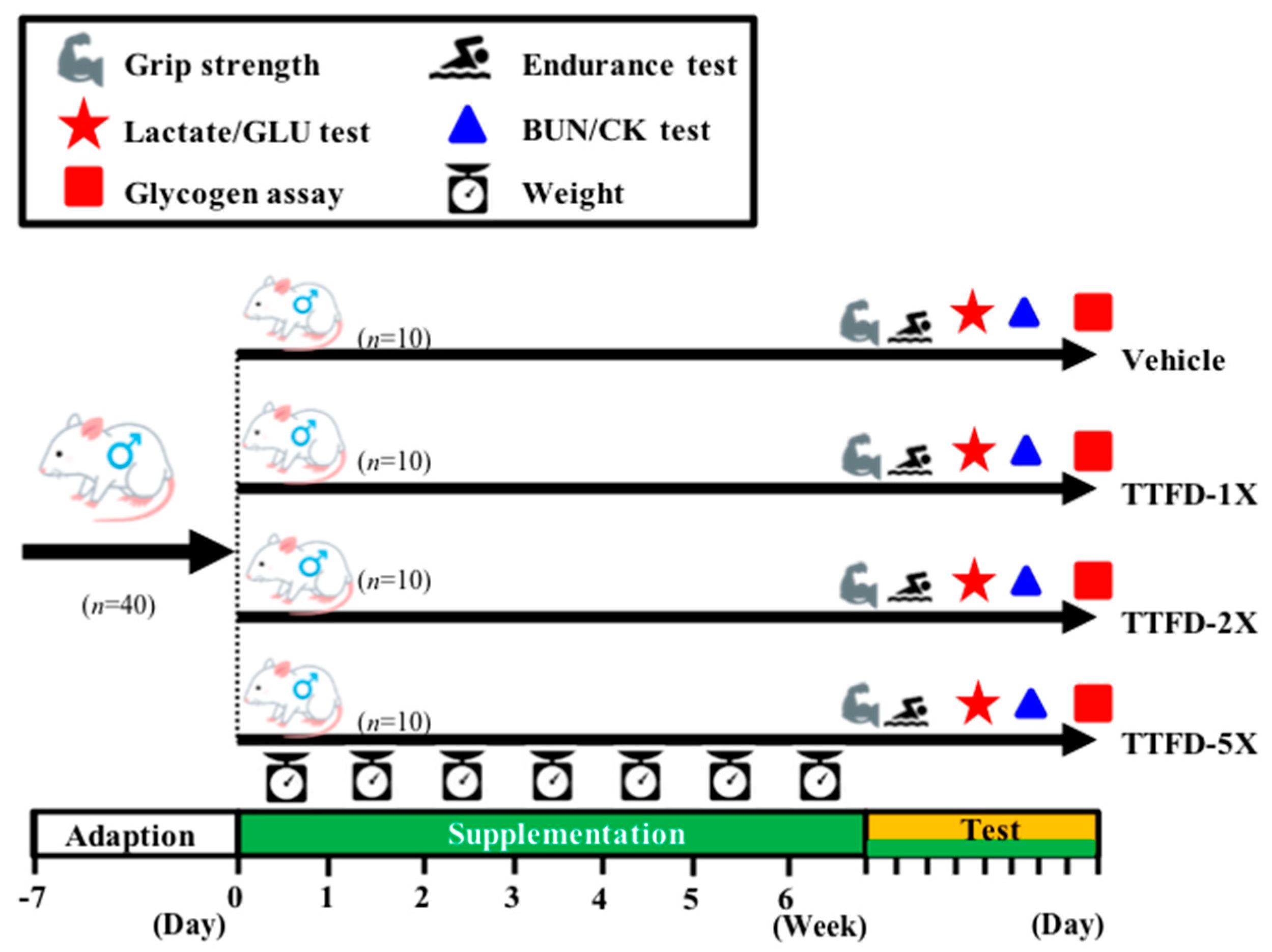
2.2. Animals and Experiment Design
2.3. Exercise Endurance Performance Test
2.4. Forelimb Grip Strength Test
2.5. Determination of Fatigue-Associated Biochemical Variable
2.6. Clinical Biochemical Profiles
2.7. Body Composition and Glycogen Content Analysis
2.8. Histopathology
2.9. Statistical Analysis
3. Results
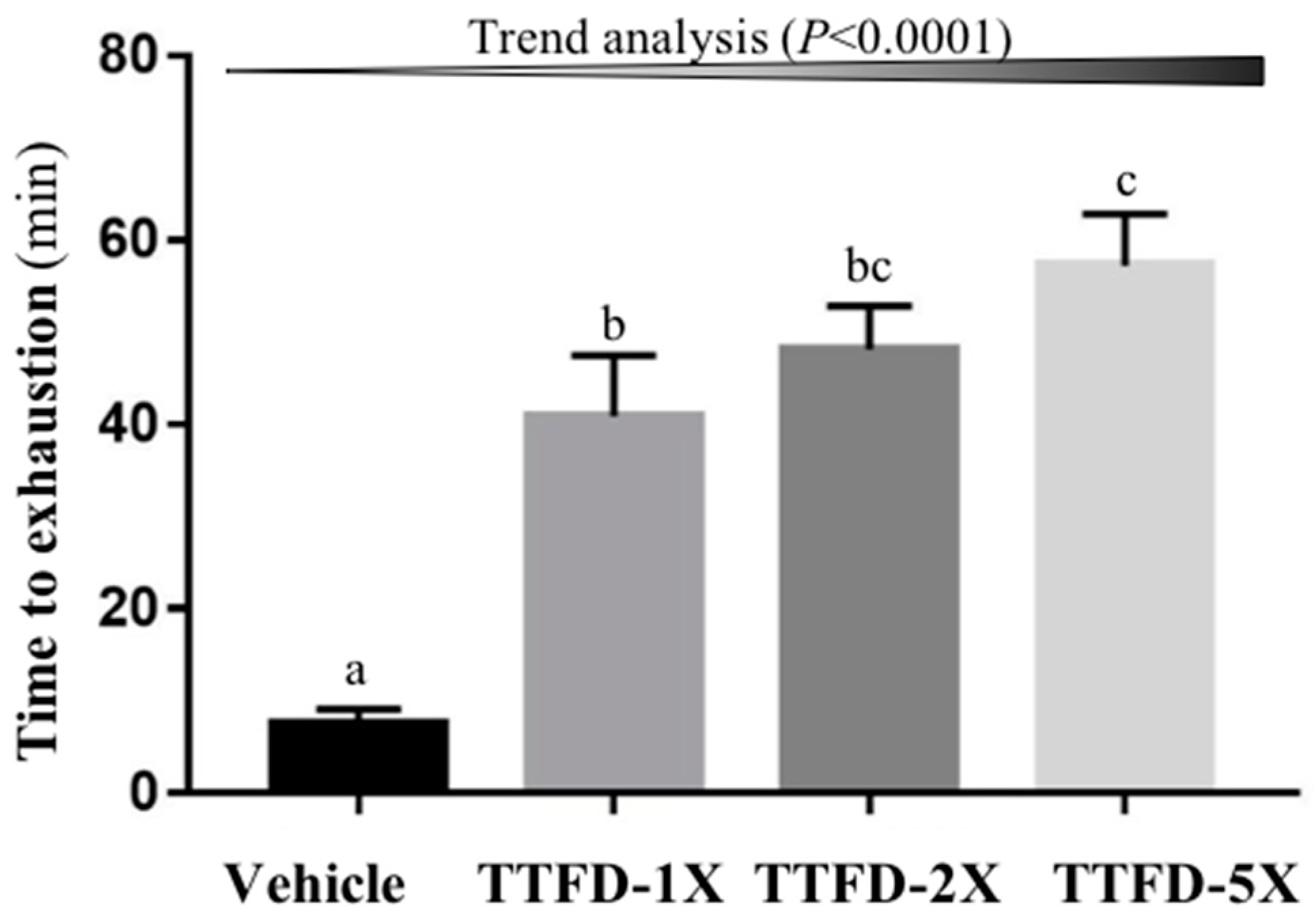
3.1. The Effects of TTFD Supplementation on Endurance Capacity
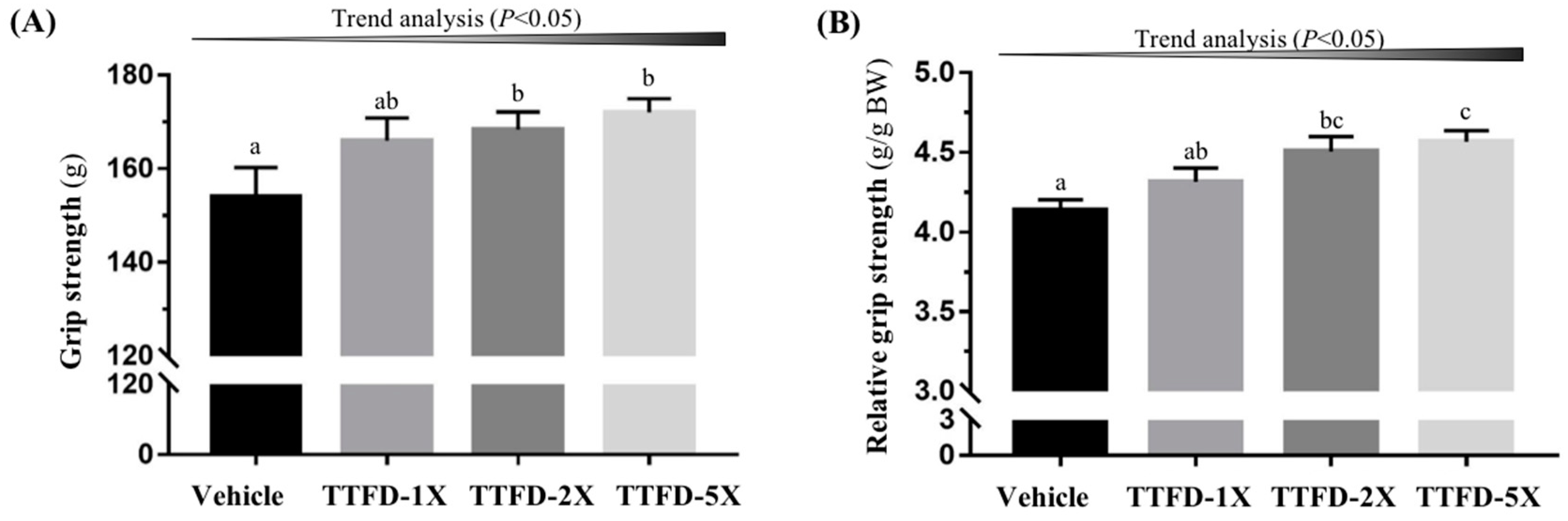
3.2. The Effects of TTFD Supplementation on Grip Strength
3.3. The Effects of TTFD Supplementation on Exercise-Related Biochemical Indexes after Exercise Challenge
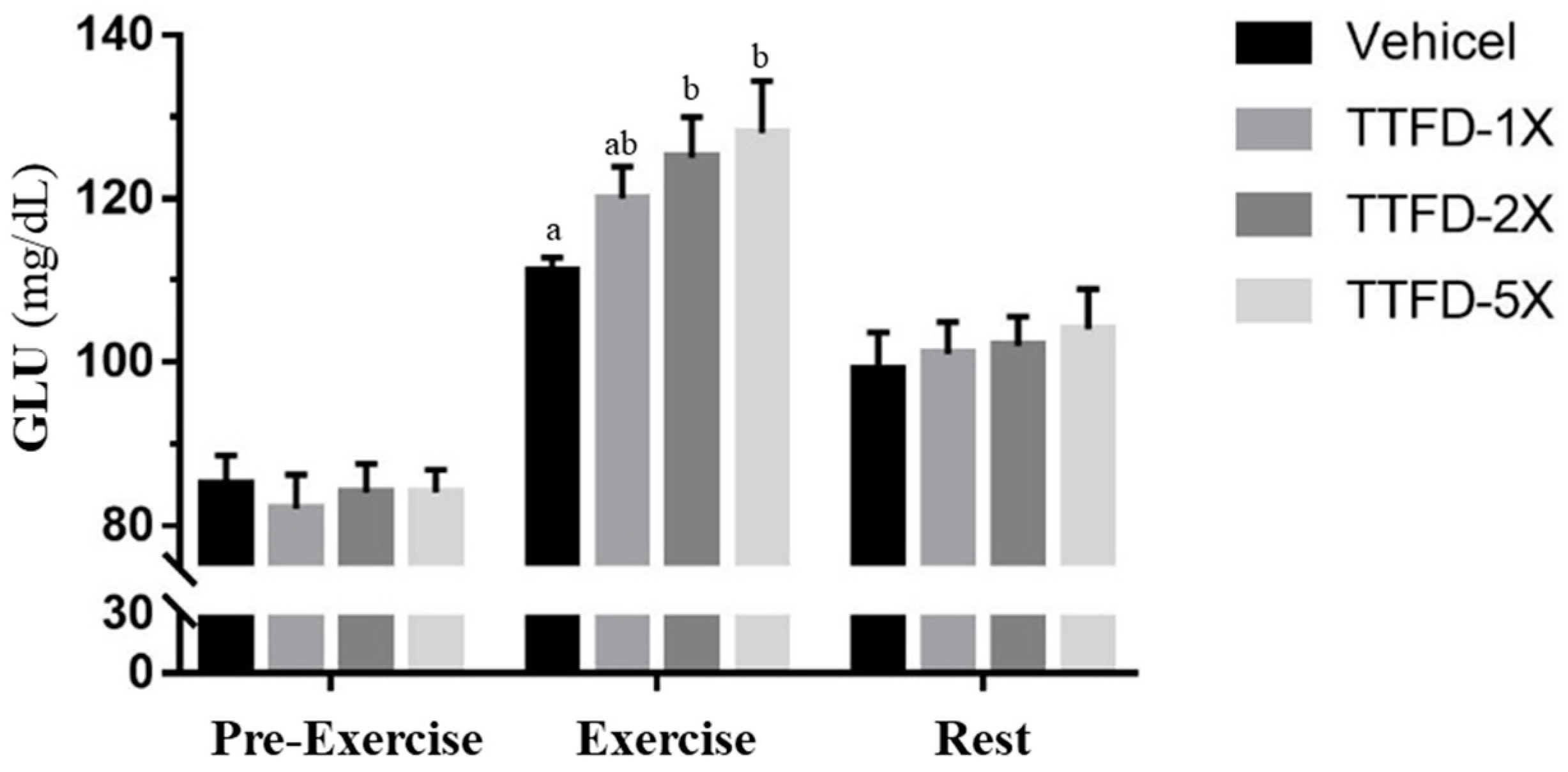
3.4. The Effects of TTFD Supplementation on Energy Metabolism
3.5. The Effects of TTFD Supplementation on Glycogen Content
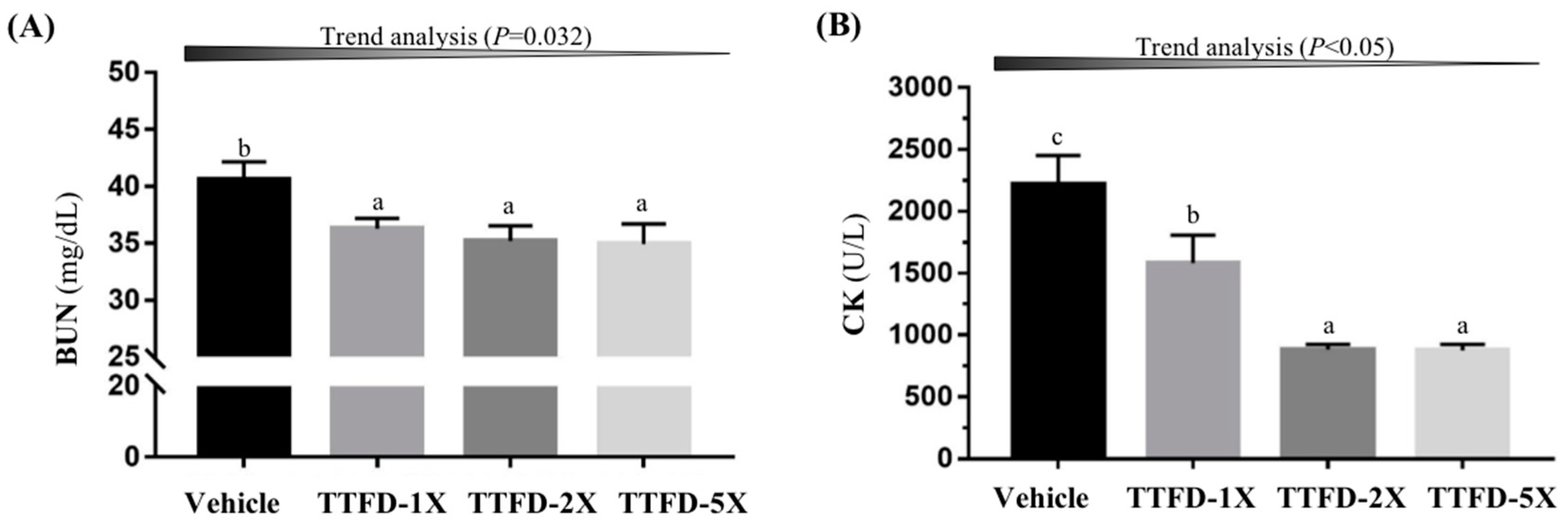
3.6. Subacute Oral Toxicity Evaluation of TTFD Supplementation
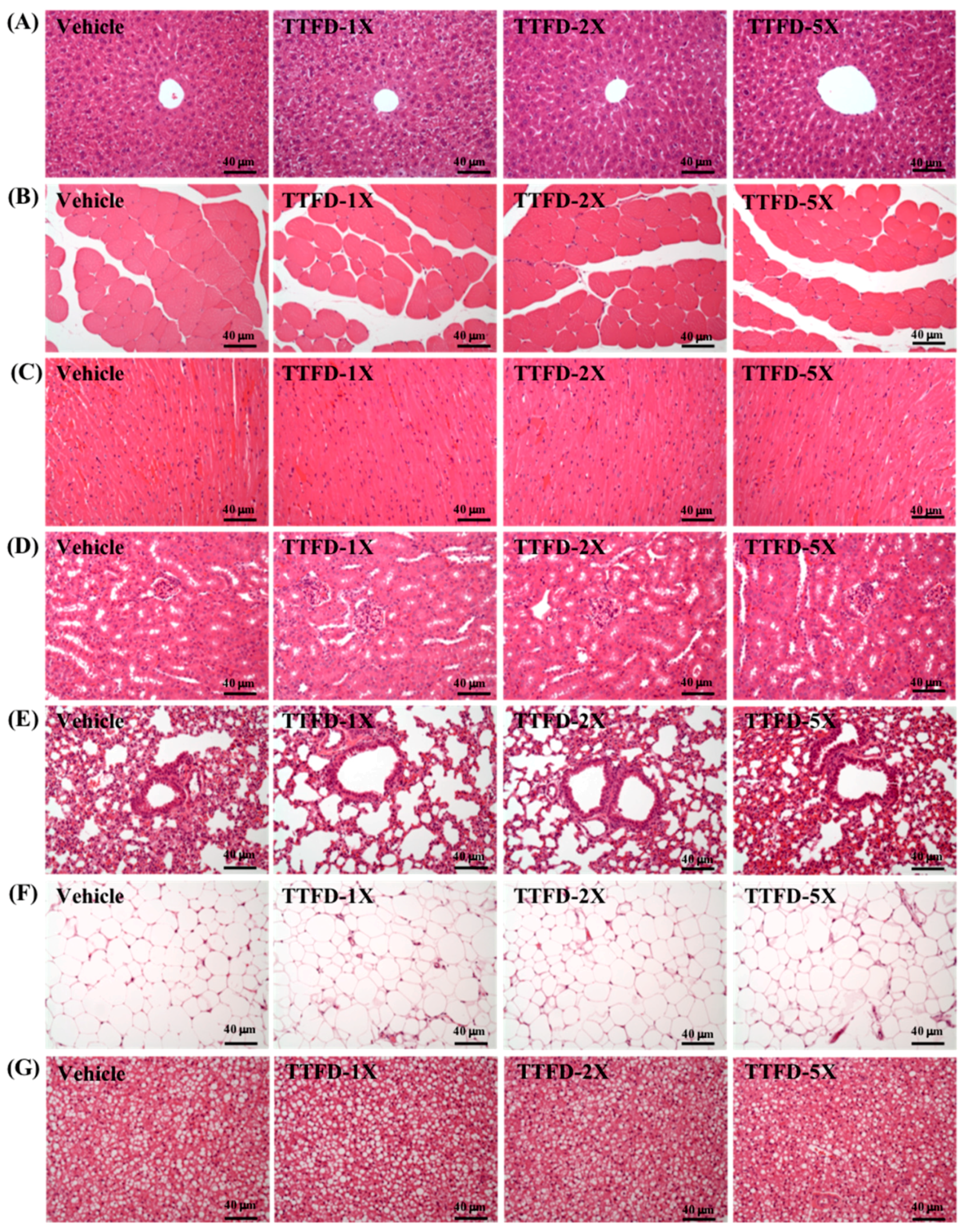
3.7. Histopathological Observation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Butterworth, R.F. Thiamin deficiency and brain disorders. Nutr. Res. Rev. 2003, 16, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fei, G.; Pan, X.; Sang, S.; Wang, L.; Zhong, C.; Jin, L. High thiamine diphosphate level as a protective factor for Alzheimer’s disease. Neurol. Res. 2018, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mehta, R.; Al-Ani, M.; Hill, J.A.; Winchester, D.E. Determining the Role of Thiamine Deficiency in Systolic Heart Failure: A Meta-Analysis and Systematic Review. J. Card. Fail. 2015, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Cuellar, D.; Velazquez-Arellano, A. Thiamine Deprivation Produces a Liver ATP Deficit and Metabolic and Genomic Effects in Mice: Findings Are Parallel to Those of Biotin Deficiency and Have Implications for Energy Disorders. J. Nutrigenet. Nutrigenom. 2016, 9, 287–299. [Google Scholar] [CrossRef]
- Mishra, G.D.; McNaughton, S.A.; O’Connell, M.A.; Prynne, C.J.; Kuh, D. Intake of B vitamins in childhood and adult life in relation to psychological distress among women in a British birth cohort. Public Health Nutr. 2009, 12, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.M.; Suter, P.M. Vitamin requirements of elderly people: An update. Am. J. Clin. Nutr. 1993, 58, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Reports of the Scientific Committee for Food. Nutrient and Energy Intakes for the European Community. 1993. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out89.pdf (accessed on 22 May 2018).
- Smithline, H.A.; Donnino, M.; Greenblatt, D.J. Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects. BMC Clin. Pharmacol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Qin, Z.; Wang, P.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hsu, Y.J.; Li, H.S.; Kan, N.W.; Chen, Y.M.; Lin, J.S.; Hsu, T.K.; Tsai, T.Y.; Chiu, Y.S.; Huang, C.C. Effect of Lactobacillus plantarum TWK10 on improving endurance performance in humans. Chin. J. Physiol. 2018. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wei, L.; Chiu, Y.S.; Hsu, Y.J.; Tsai, T.Y.; Wang, M.F.; Huang, C.C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.D.; Wolden-Hanson, T.; Quinn, L.S. Assessment of murine exercise endurance without the use of a shock grid: An Alternative to Forced Exercise. J. Vis. Exp. 2014, 90, 51846. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.G.; Britton, S.L. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol. Genom. 2001, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Hsu, Y.J.; Wei, L.; Chen, Y.J.; Huang, C.C. Association of physical performance and biochemical profile of mice with intrinsic endurance swimming. Int. J. Med. Sci. 2016, 13, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Bubber, P.; Ke, Z.J.; Gibson, G.E. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem. Int. 2004, 45, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D. A Review of the Biochemistry, Metabolism and Clinical Benefits of Thiamin(e) and Its Derivatives. Evid. Based Complement. Altern. Med. 2006, 3, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Inouye, K.; Katsura, E. Clinical Signs and Metabolism of Beriberi Patients, Review of Japanese Literature on Beriberi and Thiamine, 2nd ed.; Igaku Shoin Ltd.: Tokyo, Japan, 1965; pp. 29–63. [Google Scholar]
- Nozaki, S.; Mizuma, H.; Tanaka, M.; Jin, G.; Tahara, T.; Mizuno, K.; Yamato, M.; Okuyama, K.; Eguchi, A.; Akimoto, K.; et al. Thiamine tetrahydrofurfuryl disulfide improves energy metabolism and physical performance during physical-fatigue loading in rats. Nutr Res. 2009, 29, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Hills, J.I.; Golub, M.S.; Bettendorff, L.; Keen, C.L. The effect of thiamin tetrahydrofurfuryl disulfide on behavior of juvenile DBA/2J mice. Neurotoxicol. Teratol. 2012, 34, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Matsumae, H.; Masuda, T.; Hatta, H. A thiamin derivative inhibits oxidation of exogenous glucose at rest, but not during exercise. J. Nutr. Sci. Vitaminol. 2010, 56, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Tung, Y.T.; Kung, W.M.; Huang, W.C.; Leung, W.K.; Huang, C.C.; Wu, J.H. Effect of Coriolus versicolor Mycelia Extract on Exercise Performance and Physical Fatigue in Mice. Int. J. Med. Sci. 2017, 14, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Huang, C.C.; Chuang, H.L.; Chiu, C.C.; Chen, W.C.; Hsu, M.C. Cornu cervi pantotrichum supplementation improves physiological adaptions during intensive endurance training. J. Vet. Med. Sci. 2017, 79, 674–682. [Google Scholar] [CrossRef]
- Doyle, M.R.; Webster, M.J.; Erdmann, L.D. Allithiamine ingestion does not enhance isokinetic parameters of muscle performance. Int. J. Sport Nutr. 1997, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.J.; Scheett, T.P.; Doyle, M.R.; Branz, M. The effect of a thiamin derivative on exercise performance. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 520–524. [Google Scholar] [PubMed]
- Al-Obaidi, S.; Al-Sayegh, N.; Nadar, M. Smoking impact on grip strength and fatigue resistance: Implications for exercise and hand therapy practice. J. Phys. Act. Health 2014, 11, 1025–1031. [Google Scholar] [PubMed]
- Kristensen, M.; Albertsen, J.; Rentsch, M.; Juel, C. Lactate and force production in skeletal muscle. J. Physiol. 2005, 562, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Baek, S.H.; Choi, S.W. The effects of endurance training and thiamine supplementation on anti-fatigue during exercise. J. Exerc. Nutr. Biochem. 2013, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewski, B. AMP deamination delays muscle acidification during heavy exercise and hypoxia. J. Biol. Chem. 2006, 281, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Lukienko, P.I.; Mel’nichenko, N.G.; Zverinskii, I.V.; Zabrodskaya, S.V. Antioxidant properties of thiamine. Bull Exp. Biol. Med. 2000, 130, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, R. Vitamin B1 (Thiamine). In Vitamin, Physiology, Pathophysiology, and Therapy; Georg Thieme Verlag: Stuttgart, Germany, 1997; pp. 65–74. [Google Scholar]
- Costantini, A.; Pala, M.I.; Tundo, S.; Matteucci, P. High-dose thiamine improves the symptoms of fibromyalgia. BMJ Case Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Pala, M.I.; Catalano, M.L.; Notarangelo, C.; Careddu, P. High-dose thiamine improves fatigue after stroke: A report of three cases. J. Altern. Complement. Med. 2014, 20, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef] [PubMed]

| Time Point | Vehicle | TTFD-1X | TTFD-2X | TTFD-5X |
|---|---|---|---|---|
| Lactate (mmol/L) | ||||
| Before swimming (A) | 2.4 ± 0.2 a | 2.4 ± 0.3 a | 2.3 ± 0.2 a | 2.4 ± 0.1 a |
| After swimming (B) | 6.1 ± 0.3 b | 4.9 ± 0.4 a | 4.5 ± 0.3 a | 4.2 ± 0.2 a |
| After a 20 min rest(C) | 4.1 ± 0.3 b | 3.6 ± 0.2 ab | 3.2 ± 0.2 a | 3.0 ± 0.1 a |
| Rate of lactate production and clearance | ||||
| Production rate = B/A | 2.96 ± 0.2 b | 2.49 ± 0.5 ab | 2.06 ± 0.3 a | 1.82 ± 0.1 a |
| Clearance rate = (B-C)/B | 0.31 ± 0.1 | 0.26 ± 0.1 | 0.26 ± 0.1 | 0.26 ± 0.01 |
| Time Point | Vehicle | TTFD-1X | TTFD-2X | TTFD-5X |
|---|---|---|---|---|
| Initial BW (g) | 31.8 ± 0.2 | 32.2 ± 0.3 | 31.7 ± 0.3 | 32.0 ± 0.3 |
| 1st wk BW (g) | 32.3 ± 0.3 | 32.4 ± 0.3 | 32.2 ± 0.2 | 32.1 ± 0.2 |
| 2nd wk BW (g) | 34.5 ± 0.3 | 34.5 ± 0.5 | 34.0 ± 0.3 | 34.0 ± 0.4 |
| 3rd wk BW (g) | 36.5 ± 0.4 | 36.2 ± 0.7 | 36.2 ± 0.5 | 36.0 ± 0.5 |
| 4th wk BW (g) | 37.7 ± 0.4 | 37.0 ± 0.8 | 37.4 ± 0.6 | 36.8 ± 0.6 |
| 5th wk BW (g) | 38.1 ± 0.7 | 37.5 ± 0.7 | 37.1 ± 0.5 | 37.1 ± 0.6 |
| 6th wk BW (g) | 38.5 ± 0.7 | 37.5 ± 0.7 | 38.2 ± 0.5 | 37.5 ± 0.6 |
| Final BW (g) | 38.4 ± 0.8 | 37.4 ± 0.8 | 38.1 ± 0.5 | 37.6 ± 0.5 |
| Water intake (mL/mouse/day) | 9.1 ± 0.2 | 9.1 ± 0.2 | 9.0 ± 0.2 | 8.9 ± 0.2 |
| Chow 5001 (g/mouse/day) | 7.0 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.1 |
| Characteristic | Vehicle | TTFD-1X | TTFD-2X | TTFD-5X |
|---|---|---|---|---|
| Liver (g) | 2.17 ± 0.15 | 2.18 ± 0.10 | 2.19 ± 0.06 | 2.19 ± 0.04 |
| Muscle (g) | 0.38 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 |
| Kidney (g) | 0.62 ± 0.01 | 0.60 ± 0.02 | 0.60 ± 0.02 | 0.61 ± 0.01 |
| Heart (g) | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| Lung (g) | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 |
| EFP (g) | 0.31 ± 0.03 | 0.30 ± 0.02 | 0.26 ± 0.03 | 0.27 ± 0.03 |
| BAT (g) | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| Relative liver weight (%) | 5.63 ± 0.34 | 5.80 ± 0.19 | 5.75 ± 0.15 | 5.83 ± 0.09 |
| Relative muscle weight (%) | 1.00 ± 0.04 | 1.05 ± 0.04 | 1.01 ± 0.02 | 1.04 ± 0.02 |
| Relative kidney weight (%) | 1.62 ± 0.03 | 1.60 ± 0.04 | 1.58 ± 0.03 | 1.63 ± 0.05 |
| Relative heart weight (%) | 0.51 ± 0.01 | 0.52 ± 0.02 | 0.50 ± 0.02 | 0.52 ± 0.03 |
| Relative lung weight (%) | 0.68 ± 0.02 | 0.68 ± 0.01 | 0.68 ± 0.01 | 0.68 ± 0.02 |
| Relative EFP weight (%) | 0.82 ± 0.08 | 0.81 ± 0.06 | 0.69 ± 0.08 | 0.70 ± 0.06 |
| Relative BAT weight (%) | 0.31 ± 0.02 | 0.31 ± 0.01 | 0.30 ± 0.01 | 0.33 ± 0.02 |
| Parameter | Vehicle | TTFD-1X | TTFD-2X | TTFD-5X |
|---|---|---|---|---|
| AST(U/L) | 77 ± 3 | 77 ± 3 | 77 ± 4 | 77 ± 3 |
| ALT(U/L) | 43 ± 1 | 41± 1 | 42 ± 1 | 42 ± 2 |
| NH3 (µmol/L) | 219 ± 7 | 216 ± 10 | 215 ± 11 | 212 ± 10 |
| CK(U/L) | 194 ± 22 | 189 ± 19 | 171 ± 14 | 179 ± 18 |
| GLU(mg/dL) | 152 ± 8 | 147 ± 11 | 146 ± 10 | 149 ± 8 |
| CREA(mg/dL) | 0.4 ± 0.001 | 0.4 ± 0.001 | 0.4 ± 0.001 | 0.4 ± 0.001 |
| BUN (mg/dL) | 23.3 ± 0.6 | 23.2 ± 0.4 | 22.4 ± 0.5 | 22.3 ± 0.7 |
| UA (mg/dL) | 1.5 ± 0.1 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.6 ± 0.2 |
| TC (mg/dL) | 142 ± 1 b | 137 ± 3 ab | 134 ± 1 a | 135 ± 2 a |
| TG (mg/dL) | 180 ± 7 | 170 ± 7 | 163 ± 8 | 165 ± 9 |
| ALB(g/dL) | 2.6 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 |
| TP(g/dL) | 5.2 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 | 5.3 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Huang, H.-Y.; Hsu, Y.-J.; Su, W.-H.; Shen, S.-Y.; Lee, M.-C.; Lin, C.-L.; Huang, C.-C. The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients 2018, 10, 851. https://doi.org/10.3390/nu10070851
Huang W-C, Huang H-Y, Hsu Y-J, Su W-H, Shen S-Y, Lee M-C, Lin C-L, Huang C-C. The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients. 2018; 10(7):851. https://doi.org/10.3390/nu10070851
Chicago/Turabian StyleHuang, Wen-Ching, Hui-Yu Huang, Yi-Ju Hsu, Wan-Hsiung Su, Sih-Yu Shen, Mon-Chien Lee, Che-Li Lin, and Chi-Chang Huang. 2018. "The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement" Nutrients 10, no. 7: 851. https://doi.org/10.3390/nu10070851
APA StyleHuang, W.-C., Huang, H.-Y., Hsu, Y.-J., Su, W.-H., Shen, S.-Y., Lee, M.-C., Lin, C.-L., & Huang, C.-C. (2018). The Effects of Thiamine Tetrahydrofurfuryl Disulfide on Physiological Adaption and Exercise Performance Improvement. Nutrients, 10(7), 851. https://doi.org/10.3390/nu10070851






