Fat Mass Is Associated with Serum 25-Hydroxyvitamin D Concentration Regardless of Body Size in Men
Abstract
1. Introduction
2. Subjects and Methods
2.1. Data Sources
2.2. Biochemistry and DXA Measurements
2.3. Statistical Analyses
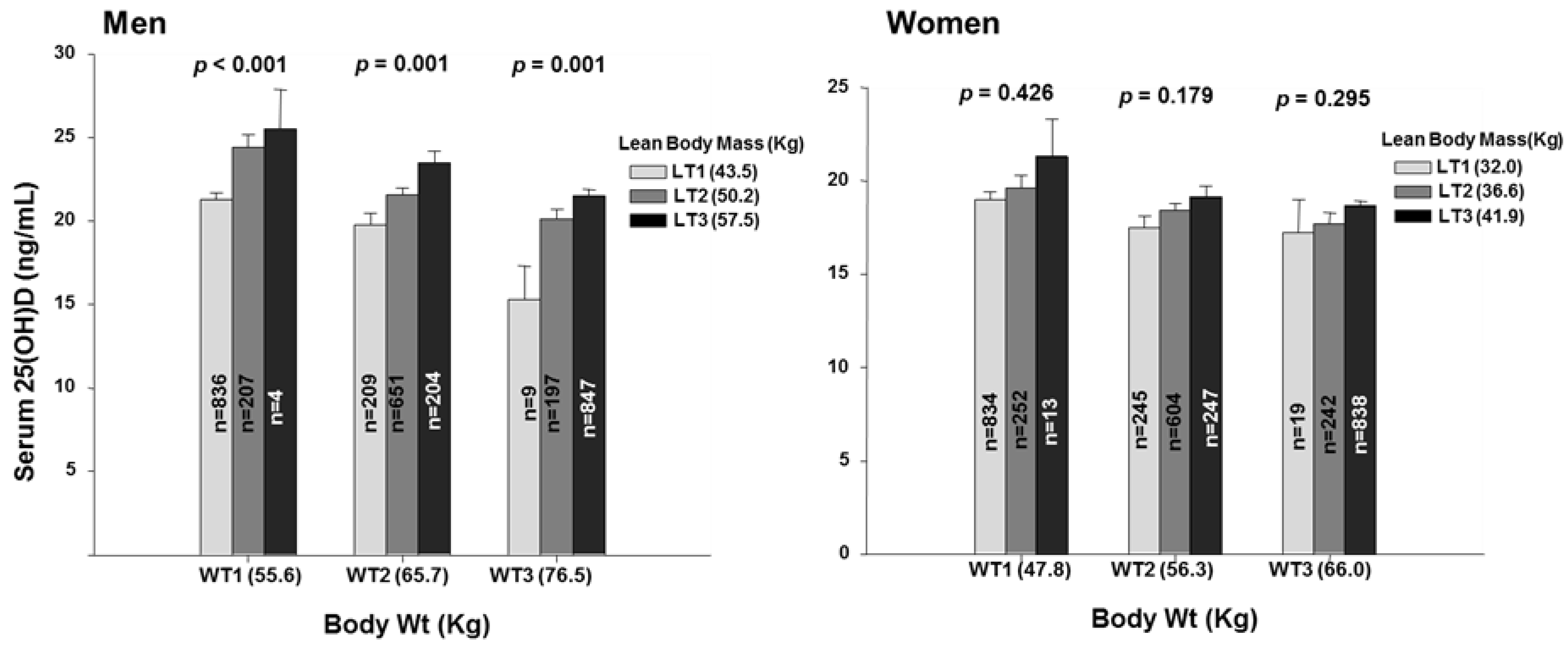
3. Results
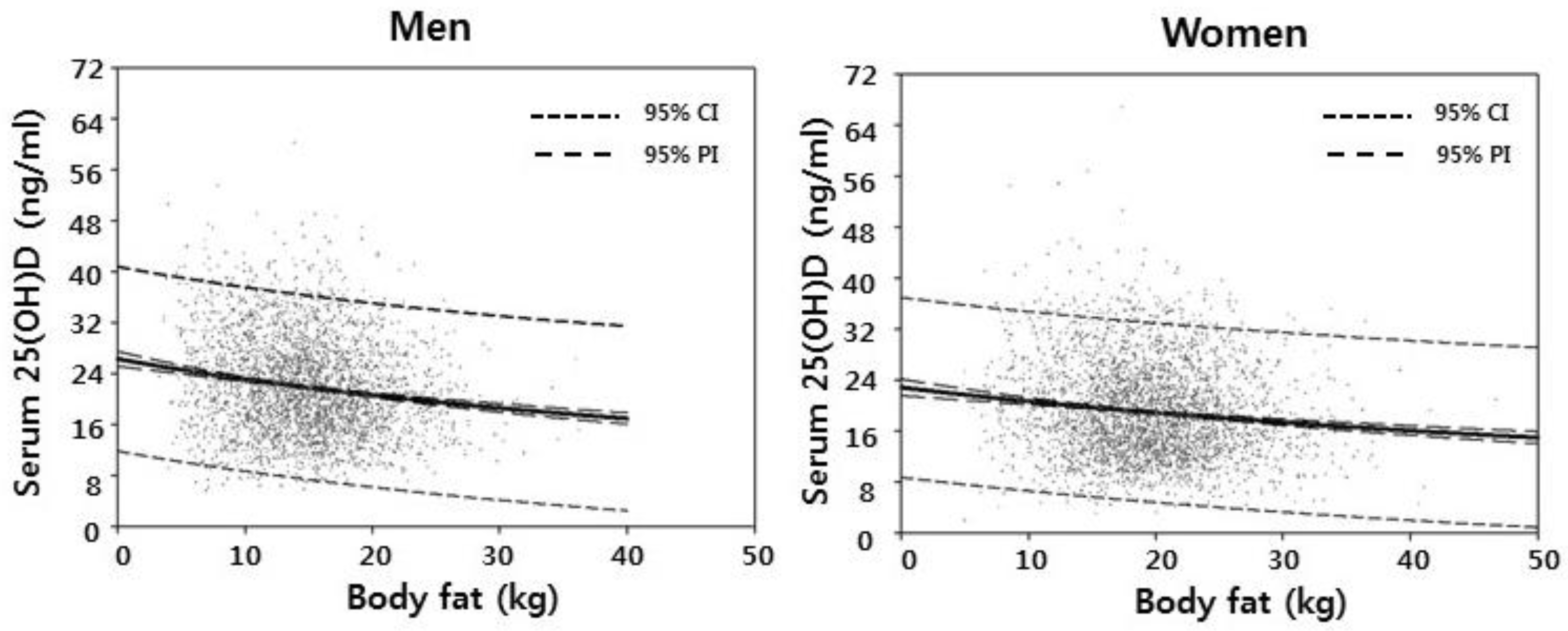
3.1. Weight, Fat and Lean Tissue Associations
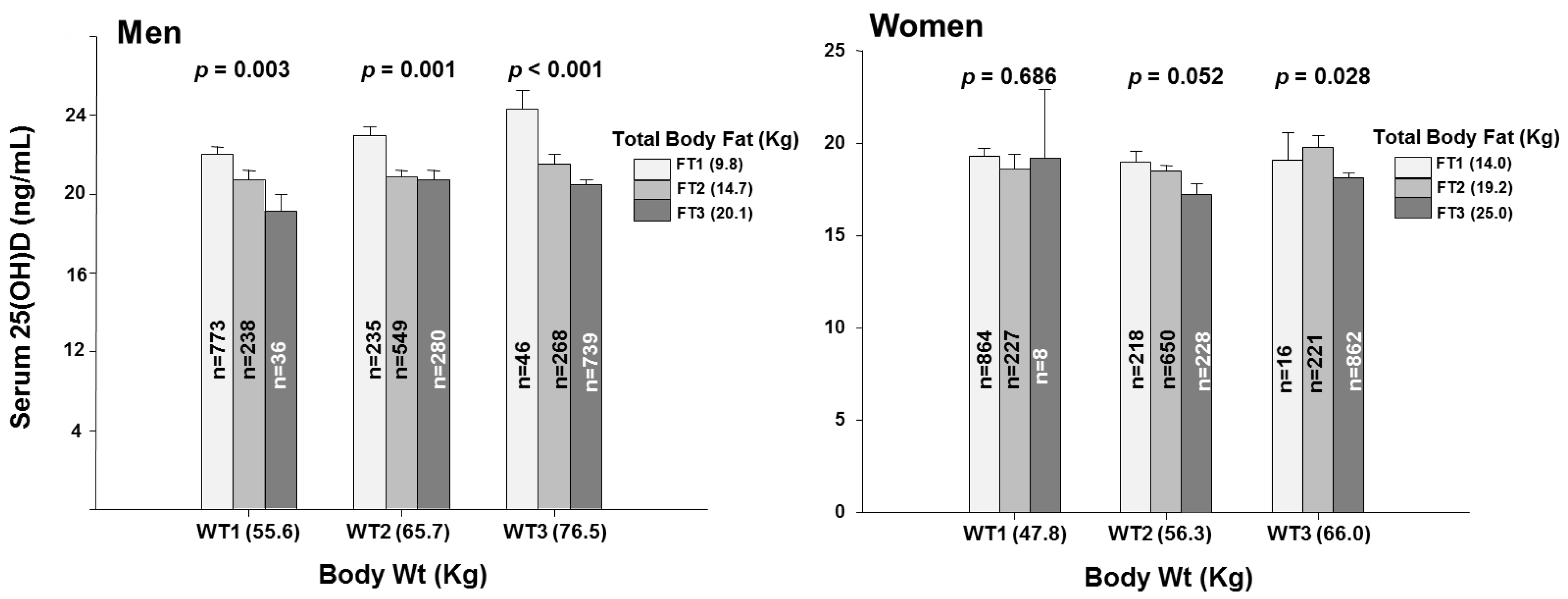
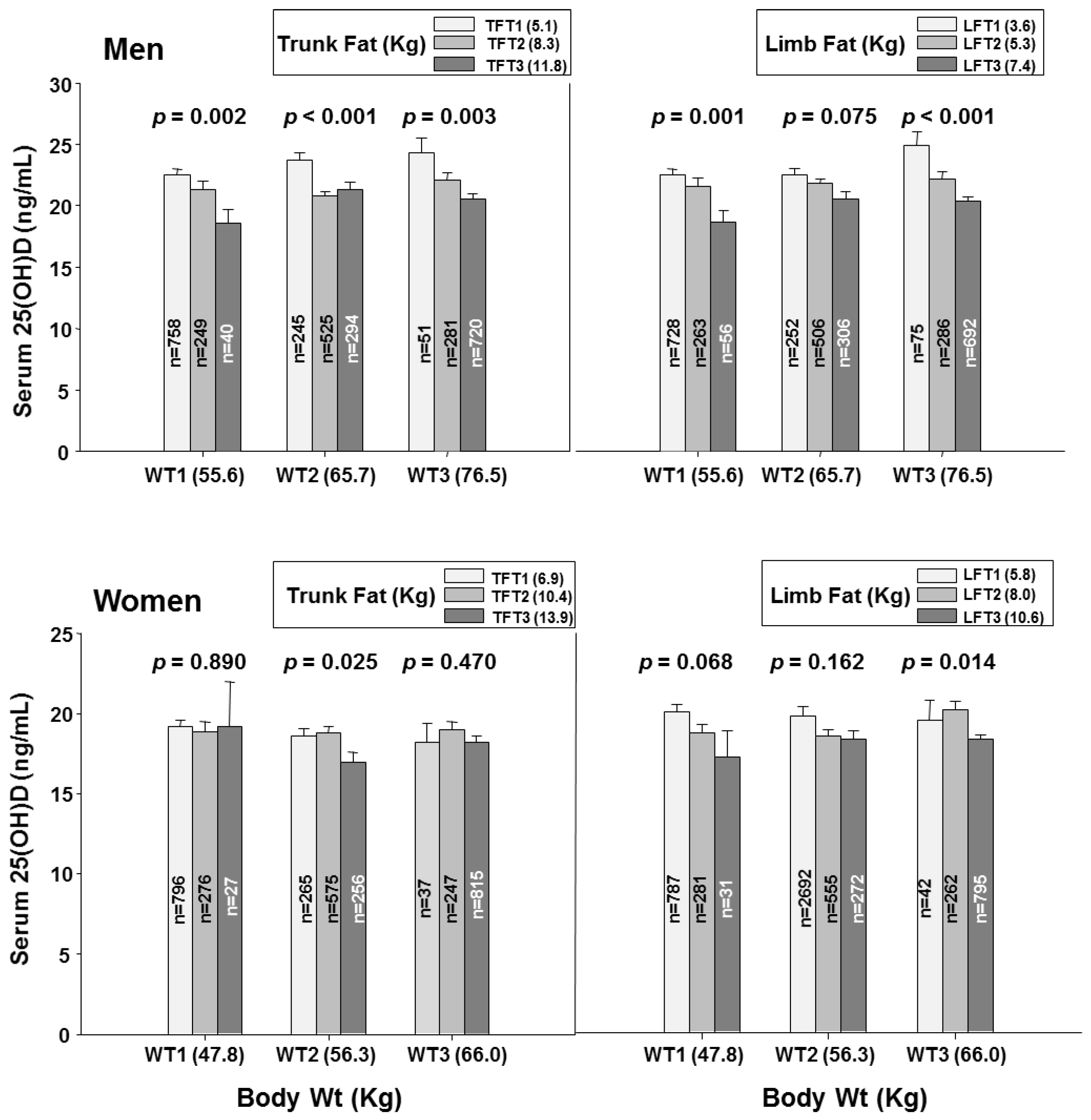
3.2. Fat Mass within Tertiles of Body Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baz-Hecht, M.; Goldfine, A.B. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Pisinger, C.; Jorgensen, T.; Thuesen, B.H.; Fenger, M.; Linneberg, A. Vitamin D status and changes in cardiovascular risk factors: A prospective study of a general population. Cardiology 2012, 123, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Churilla, T.M.; Brereton, H.D.; Klem, M.; Peters, C.A. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: A community oncology experience. Nutr. Cancer 2012, 64, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Heaney, R.P.; Holick, M.F.; Lips, P.; Meunier, P.J.; Vieth, R. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alkharfy, K.M.; Al-Othman, A.; Yakout, S.M.; Al-Saleh, Y.; Fouda, M.A.; Sulimani, R.; Sabico, S. Effect of gender, season, and vitamin D status on bone biochemical markers in Saudi diabetes patients. Molecules 2012, 17, 8408–8418. [Google Scholar] [CrossRef] [PubMed]
- Coney, P.; Demers, L.M.; Dodson, W.C.; Kunselman, A.R.; Ladson, G.; Legro, R.S. Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int. J. Gynaecol. Obstet. 2012, 119, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Valtuena, J.; Gracia-Marco, L.; Vicente-Rodriguez, G.; Gonzalez-Gross, M.; Huybrechts, I.; Rey-Lopez, J.P.; Mouratidou, T.; Sioen, I.; Mesana, M.I.; Martinez, A.E.; et al. Vitamin D status and physical activity interact to improve bone mass in adolescents. The HELENA Study. Osteoporos. Int. 2012, 23, 2227–2237. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Sai, A.; Templin, T., 2nd; Smith, L. Dose response to vitamin D supplementation in postmenopausal women: A randomized trial. Ann. Intern. Med. 2012, 156, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Dolnikowski, G.; Seyoum, E.; Harris, S.S.; Booth, S.L.; Peterson, J.; Saltzman, E.; Dawson-Hughes, B. Vitamin D(3) in fat tissue. Endocrine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, L.K.; Livingstone, M.B.; Barnes, M.S.; Horigan, G.; McSorley, E.M.; Bonham, M.P.; Magee, P.J.; Hill, T.R.; Lucey, A.J.; Cashman, K.D.; et al. Effect of adiposity on vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older Irish adults. Br. J. Nutr. 2012, 107, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gil, K.; Perez, C.M.; Joshipura, K. Determinants of vitamin D status among overweight and obese Puerto Rican adults. Ann. Nutr. Metab. 2012, 60, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kremer, R.; Campbell, P.P.; Reinhardt, T.; Gilsanz, V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J. Clin. Endocrinol. Metab. 2009, 94, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; de las Heras, J.; Chen, T.C.; Lee, S.; Holick, M.F.; Arslanian, S.A. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J. Clin. Endocrinol. Metab. 2011, 96, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- National Rural Resources Development Institute, Rural Development Administration. Food Composition Table, 7th ed.; National Rural Resources Development Institute, Rural Development Administration: Suwon, Korea, 2006. [Google Scholar]
- Touvier, M.; Deschasaux, M.; Montourcy, M.; Sutton, A.; Charnaux, N.; Kesse-Guyot, E.; Assmann, K.E.; Fezeu, L.; Latino-Martel, P.; Druesne-Pecollo, N.; et al. Determinants of vitamin D status in Caucasian adults: Influence of sun exposure, dietary intake, sociodemographic, lifestyle, anthropometric, and genetic factors. J. Investig. Dermatol. 2015, 135, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, T.; Rajan, S.; Keller, B. Probability of Vitamin D Deficiency by Body Weight and Race/Ethnicity. J. Am. Board Fam. Med. 2016, 29, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Tanagra, S.; Koutsikas, K.; Nikolaidou, A.; Androutsos, O.; Manios, Y. Association between serum 25-hydroxyvitamin D levels and body composition in postmenopausal women: The postmenopausal Health Study. Menopause 2009, 16, 701–707. [Google Scholar] [CrossRef] [PubMed]
- McKinney, K.; Breitkopf, C.R.; Berenson, A.B. Association of race, body fat and season with vitamin D status among young women: A cross-sectional study. Clin. Endocrinol. 2008, 69, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Kim, M.; Lee, S.M.; Lee, J.P.; Kim, S.; Joo, K.W.; Lim, S.S.; Kim, Y.S.; Kim, D.K. Association between body fat and vitamin D status in Korean adults. Asia Pac. J. Clin. Nutr. 2014, 23, 65–75. [Google Scholar] [PubMed]
- Gilsanz, V.; Kremer, A.; Mo, A.O.; Wren, T.A.; Kremer, R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J. Clin. Endocrinol. Metab. 2010, 95, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Byrne, M.E.; Chang, E.; Jiang, Y.; Donkin, S.S.; Buhman, K.K.; Burgess, J.R.; Teegarden, D. 1alpha,25-Dihydroxyvitamin D hydroxylase in adipocytes. J. Steroid Biochem. Mol. Biol. 2008, 112, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Scala, L.; Cigolini, M.; Zenari, L.; Falezza, G.; Arcaro, G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kull, M.; Kallikorm, R.; Lember, M. Body mass index determines sunbathing habits: Implications on vitamin D levels. Intern. Med. J. 2009, 39, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S.; Dawson-Hughes, B. Reduced sun exposure does not explain the inverse association of 25-hydroxyvitamin D with percent body fat in older adults. J. Clin. Endocrinol. Metab. 2007, 92, 3155–3157. [Google Scholar] [CrossRef] [PubMed]
- Caron-Jobin, M.; Morisset, A.S.; Tremblay, A.; Huot, C.; Legare, D.; Tchernof, A. Elevated serum 25(OH)D concentrations, vitamin D, and calcium intakes are associated with reduced adipocyte size in women. Obesity 2011, 19, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Sulistyoningrum, D.C.; Green, T.J.; Lear, S.A.; Devlin, A.M. Ethnic-specific differences in vitamin d status is associated with adiposity. PLoS ONE 2012, 7, e43159. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, P.; Verrusio, W.; Viscogliosi, G.; Summa, M.L.; Gueli, N.; Cacciafesta, M.; Albanese, C.V. Relationship between vitamin D and body fat distribution evaluated by DXA in postmenopausal women. Nutrition 2016, 32, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Engelman, C.D.; Langefeld, C.D.; Hairston, K.G.; Haffner, S.M.; Bryer-Ash, M.; Norris, J.M. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J. Clin. Endocrinol. Metab. 2009, 94, 3306–3313. [Google Scholar] [CrossRef] [PubMed]
- Tzotzas, T.; Papadopoulou, F.G.; Tziomalos, K.; Karras, S.; Gastaris, K.; Perros, P.; Krassas, G.E. Rising serum 25-hydroxy-vitamin D levels after weight loss in obese women correlate with improvement in insulin resistance. J. Clin. Endocrinol. Metab. 2010, 95, 4251–4257. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Armstrong-Moore, D.; Liang, Z.; Sweeney, J.F.; Torres, W.E.; Ziegler, T.R.; Tangpricha, V.; Gletsu-Miller, N. Contribution of adipose tissue to plasma 25-hydroxyvitamin D concentrations during weight loss following gastric bypass surgery. Obesity 2011, 19, 588–594. [Google Scholar] [CrossRef] [PubMed]
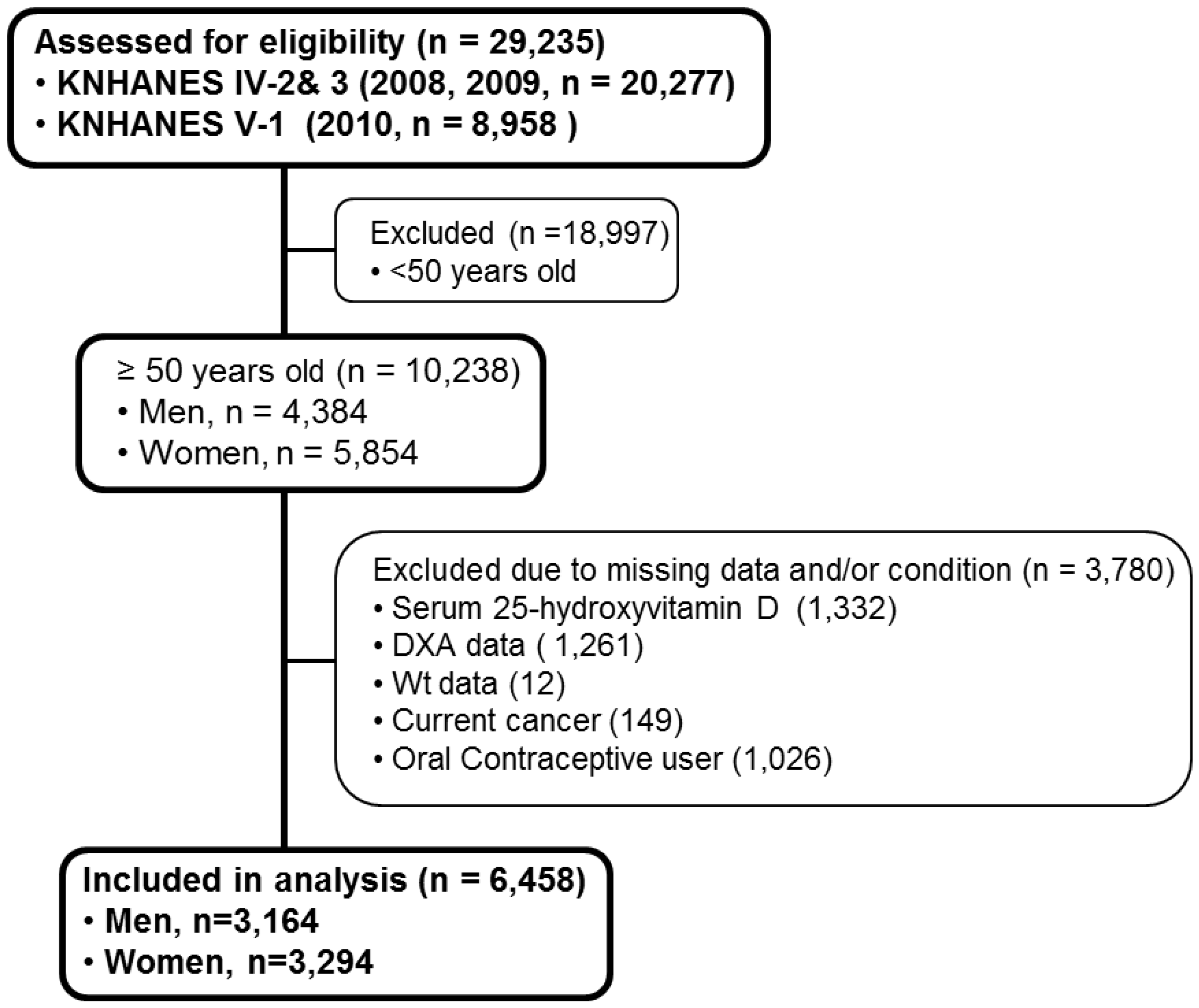
| Total (n = 6458) | Men (n = 3164) | Women (n = 3294) | |
|---|---|---|---|
| Age (years) | 61.5 (0.2) | 60.8 (0.2) | 62.3 (0.2) |
| Body weight (Kg) | 62.1 (0.2) | 66.8 (0.3) | 56.9 (0.2) |
| Height (cm) | 160.6 (0.1) | 167.1 (0.1) | 153.4 (0.1) |
| BMI (kg/m2) | 24.0 (0.1) | 23.9 (0.1) | 24.2 (0.1) |
| Total body fat (Kg) | 17.17 (0.11) | 15.04 (0.15) | 19.56 (0.13) |
| Trunk fat (Kg) | 9.46 (0.07) | 8.51 (0.09) | 10.53 (0.08) |
| Limb fat (Kg) | 6.77 (0.05) | 5.53 (0.05) | 8.17 (0.05) |
| Lean body mass (Kg) | 44.44 (0.16) | 51.12 (0.18) | 36.86 (0.11) |
| 25(OH)D (ng/mL) | 20.2 (0.2) | 21.6 (0.3) | 18.6 (0.2) |
| Energy intake (kcal) | 1883.7 (16.7) | 2166.7 (21.7) | 1566.4 (16.1) |
| Serum 25(OH)D Men (n = 3164) | Serum (25(OH)D Women (n = 3294) | |
|---|---|---|
| Body weight | −0.075 ** | −0.073 ** |
| Height | −0.052 * | −0.023 |
| BMI | −0.058 * | −0.071 ** |
| Waist | −0.043 * | −0.045 * |
| Total body fat | −0.154 ** | −0.116 ** |
| Trunk fat | −0.142 ** | −0.108 ** |
| Limb fat | −0.164 ** | −0.111 ** |
| Lean body mass | 0.023 | 0.013 |
| Men Body Wt | Women Body Wt | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | T1 (n = 1047) | T2 (n = 1064) | T3 (n = 1053) | p Value | T1 (n = 1099) | T2 (n = 1096) | T3 (n = 1099) | p Value |
| Age (years) | 64.2 (0.4) | 60.6 (0.3) | 58.4 (0.3) | <0.001 | 65.6 (0.5) | 61.0 (0.4) | 60.4 (0.3) | <0.001 |
| Body weight (Kg) | 55.6 (0.2) | 65.7 (0.1) | 76.5 (0.2) | <0.001 | 47.8 (0.1) | 56.3 (0.1) | 66.0 (0.2) | <0.001 |
| Height (cm) | 163.3 (0.2) | 166.9 (0.2) | 170.3 (0.2) | <0.001 | 150.0 (0.2) | 153.5 (0.2) | 156.5 (0.2) | <0.001 |
| BMI (kg/m2) | 20.9 (0.1) | 23.6 (0.1) | 26.4 (0.1) | <0.001 | 21.3 (0.1) | 24.0 (0.1) | 27.0 (0.1) | <0.001 |
| Total body fat (Kg) | 10.70 (0.16) | 14.74 (0.12) | 18.72 (0.20) | <0.001 | 14.78 (0.15) | 19.23 (0.12) | 24.38 (0.15) | <0.001 |
| Trunk fat (Kg) | 5.73 (0.10) | 8.32 (0.08) | 10.89 (0.12) | <0.001 | 7.70 (0.10) | 10.33 (0.08) | 13.38 (0.10) | <0.001 |
| Limb fat (Kg) | 4.06 (0.06) | 5.43 (0.05) | 6.77 (0.08) | <0.001 | 6.27 (0.06) | 8.03 (0.06) | 10.08 (0.07) | <0.001 |
| Lean body mass (Kg) | 44.48 (0.17) | 50.39 (0.13) | 57.00 (0.20) | <0.001 | 32.74 (0.10) | 36.59 (0.11) | 40.99 (0.15) | <0.001 |
| 25(OH)D (ng/mL) | 22.0 (0.4) | 21.7 (0.3) | 21.3 (0.3) | 0.164 | 19.2 (0.3) | 18.3 (0.3) | 18.4 (0.3) | 0.088 |
| Energy intake (kcal) | 2017.1 (30.0) | 2134.5 (30.0) | 2313.3 (39.3) | <0.001 | 1481.3 (22.6) | 1589.1 (27.5) | 1622.2 (25.8) | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeum, K.-J.; Dawson-Hughes, B.; Joo, N.-S. Fat Mass Is Associated with Serum 25-Hydroxyvitamin D Concentration Regardless of Body Size in Men. Nutrients 2018, 10, 850. https://doi.org/10.3390/nu10070850
Yeum K-J, Dawson-Hughes B, Joo N-S. Fat Mass Is Associated with Serum 25-Hydroxyvitamin D Concentration Regardless of Body Size in Men. Nutrients. 2018; 10(7):850. https://doi.org/10.3390/nu10070850
Chicago/Turabian StyleYeum, Kyung-Jin, Bess Dawson-Hughes, and Nam-Seok Joo. 2018. "Fat Mass Is Associated with Serum 25-Hydroxyvitamin D Concentration Regardless of Body Size in Men" Nutrients 10, no. 7: 850. https://doi.org/10.3390/nu10070850
APA StyleYeum, K.-J., Dawson-Hughes, B., & Joo, N.-S. (2018). Fat Mass Is Associated with Serum 25-Hydroxyvitamin D Concentration Regardless of Body Size in Men. Nutrients, 10(7), 850. https://doi.org/10.3390/nu10070850




