Abstract
Sperm cells are highly sensitive to reactive oxygen species (ROS), which are produced during cellular oxidation. In normal cell biology, ROS levels increase with a decreasing antioxidant response, resulting in oxidative stress which threatens sperm biology. Oxidative stress has numerous effects, including increased apoptosis, reduced motion parameters, and reduced sperm integrity. In this regard, green tea polyphenols (GrTPs) have been reported to possess properties that may increase the quality of male and female gametes, mostly via the capability of catechins to reduce ROS production. GrTPs have antioxidant properties that improve major semen parameters, such as sperm concentration, motility, morphology, DNA damage, fertility rate, and gamete quality. These unique properties of green tea catechins could improve reproductive health and represent an important study area. This exploratory review discusses the therapeutic effects of GrTPs against infertility, their possible mechanisms of action, and recommended supportive therapy for improving fertility in humans and in animals.
1. Introduction
Infertility affects about 15 to 30% of couples that are trying to conceive. In approximately half of these cases, the male partner is the sole contributing factor [1,2]; therefore, infertility remains a controversial problem worldwide. Male infertility is caused by numerous anatomical abnormalities, such as seminal tube obstruction or neurological disorders, resulting in abnormal spermatogenesis which weakens the function of sperm [3,4]. Many environmental factors can cause infertility, such as nutritional deficiencies and oxidative stress caused by pesticides and industrial chemicals, tobacco smoking, excessive alcohol consumption, and heat exposure to the testes, which can damage semen quality [5,6,7]. Factors such as radiation or urinary tract infections also contribute significantly to fertility. When the production of reactive oxygen species (ROS) exceeds the body’s antioxidant capacity, oxidative stress (OS) occurs [8]. This OS initiates lipid oxidation, which damages membrane integrity and increases its permeability, leading to the inactivation of cellular enzymes, resulting in cell apoptosis and structural DNA damage and ultimately, leading to decreased fertility [9,10,11].
Tea is a pleasant, common, communally accepted, and safe drink that was originally used as a medicine and is now recognized as a significant industrial and pharmaceutical raw material [12]. Green tea polyphenols (GrTPs), especially epigallocatechin gallate (EGCG), have several beneficial properties, including anti-cancer [13,14,15], antioxidant, anti-diabetic, anti-hypertensive, anti-microbial, and anti-metabolic syndrome effects [16] as well as improving fertility in humans and animals [17]. Regular consumption of green tea is associated with a decreased risk of ovarian cancer in women [18]. GrTPs act as free radical scavengers, protecting spermatozoa against OS [19]. The seminal plasma comprises many enzymatic and non-enzymatic antioxidants that form a defense mechanism against the loss of semen cytoplasmic enzymes [20,21]. Studies in animals have shown that the antioxidant capacity of semen decreases under high ROS levels, and can lead to infertility-related issues [22,23]. Clinically, ROS production causes DNA damage and alters the properties of the mitochondrial membrane [24]. Green tea is considered as a dietary source of antioxidant compounds, mainly comprising polyphenolic components like catechins and gallic acid, as illustrated in Figure 1. Green tea also contains numerous other factors, such as vitamin C, carotenoids, and tocopherols; minerals, such as Cr, Mn, Se, or Zn; and certain phytochemical compounds [25]. These compounds might enhance the GrTP’s antioxidant activity [26,27]. Studies have revealed that green tea significantly increases the antioxidant capacity in plasma as well as spermatozoa after consumption of 2–6 cups/day which may lead to a decreased oxidative damage of lipids and DNA [28,29,30]. Erba and colleagues [31] proposed that green tea increases the antioxidation level and protects against oxidative damage in humans. Therefore, GrTPs regulate defensive mechanisms against oxidative damage. In this review, we discuss the possible factors responsible for infertility and the role of antioxidant activity in regulating ROS-induced infertility, and present possible solutions to increase fertility using green tea.
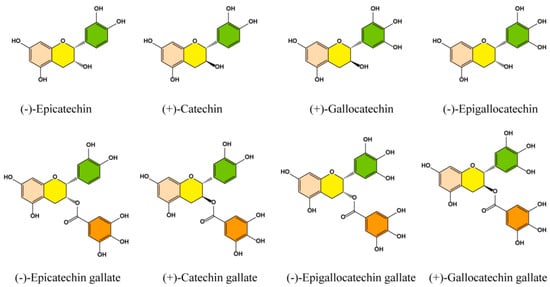
Figure 1.
Chemical structure of the main catechin components in green tea polyphenols.
Recently, several studies have focused on the effects of OS, the etiology of human and animal infertility, and the role of GrTP supplements in improving semen properties to increase fertility [32]. Thus, the aim of this review was to evaluate the effects of GrTPs, their possible mechanisms in semen motility and sperm concentration, and how their use as supplements can support the treatment of infertility, through their strong capability to neutralize ROS, regulate DNA damage, and improve fertility.
2. Factors Affecting Fertility
Various factors affect fertility traits directly or indirectly. One of the most significant factors contributing to poor semen quality is OS [33]. Oxidative stress is a condition that induces cellular damage via ROS production [34,35]. Many in vivo experiments have been conducted to study the pathophysiology and impact of OS on different fertility disorders [36,37]. Recent research has shown that OS causes damage to semen [38], for example, through DNA structural damage and cell apoptosis, which leads to failure to conceive or arrests the development of the embryo. If the production of ROS increases in seminal plasma, OS will affect sperm motility by targeting important components of the cell, such as lipids, nucleic acids, proteins, and sugars [39,40].
The sperm membrane comprises polyunsaturated fatty acids which are highly susceptible to a chain reaction of ROS known as lipid peroxidation. Lipid peroxidation results in the loss of membrane integrity which disrupts cell function and damages sperm motility by inducing apoptosis [33,41]. The addition of metal ions, like Fe2+, accelerates lipid peroxidation, disrupting semen function and motility [42]. During the long-term storage of semen, lipid peroxides are freely generated in the semen membrane which induces DNA damage and reduced fertility. Lipid peroxidation also significantly reduces the capability of sperm to bind with the heterologous zona pellucida [43].
Different growing cells generate ROS when incubated in aerobic conditions. ROS is a term used to describe oxygen radicals which impact on fertility and reproduction [44]. Different sources of ROS can activate leukocytes which have harmful effects on semen morphology, concentration, and motility via acrosomal damage, DNA damage, hyperactivation, and oocyte penetration [43,45]. The induction of apoptosis in antral follicles is initiated by ROS which play a vital role in primary follicle death [46]. ROS are highly reactive and oxidize other molecules that may cause structural and functional changes leading to cellular damage [47]. The different sources of ROS that impair the function of semen internally and externally are shown in Figure 2. The exogenous sources are smoking, alcohol, toxins, and radiation, while the endogenous sources are immature sperm, leukocytes, varicoceles, and cryptorchidism, as well as oocytes, embryo, ovary, and the uterine cavity [48]. These factors can alter semen parameters, induce lipid peroxidation, modify sperm proteins, and ultimately, cause sperm DNA damage, resulting in male infertility. In females these factors cause ovarian endometrium, hyperandrogenaemia, hyperinsulinemia, and placental dysfunction, which decrease fertility rate [49]. These abnormalities induce the onset of endometriosis and polycystic ovarian syndrome, causing defects in the endometrium and embryo, reducing fertility, and causing infertility [50].
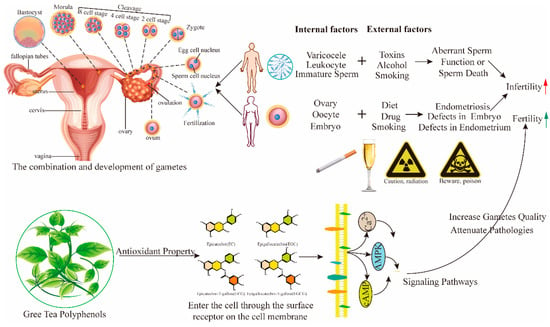
Figure 2.
Action of green tea polyphenols on major fertility reducing factors. Various internal and external factors impair sperm function and/or cause sperm death, whereas in females, they disrupt gamete and embryo development. AMPK, adenosine monophosphate-activated protein kinase; cAMP, cyclic adenosine monophosphate; Ca2+, calcium ion.
3. Antioxidants and Infertility
Previous studies have confirmed the significant roles of antioxidants in male and female infertility and other pathological conditions [51]. Antioxidant supplementation has been specified as a possible approach to treat reproductive diseases and improve fertility. In 2017, it was proven, for the first time, that polyphenols found in significant amounts within the seminal plasma act as natural antioxidants [52].
3.1. Vitamin E
Several studies have reported that vitamin E is a key chain-breaking antioxidant in the seminal plasma membranes because it favors the motility of semen by resisting the peroxidation of lipids [53]. It also deactivates free radicals, protects the cellular membrane against O2 free radicals, and prevents the production of ROS [54]. During cryopreservation, when vitamin E reacts with cryoprotectants, it can efficiently preserve the semen [55,56,57]. Vitamins E and C collectively enhance the intra-cytoplasmic semen injection (ICSI) success rate in patients with semen DNA damage and decrease the level of DNA impairment [58]. Glutathione (GSH) has a protective effect on sperm motility. Parinaud and colleagues [59] compared GSH with Tyrode solution and observed increased recovery of sperm motility with GSH.
3.2. L-Carnitine
Studies have revealed that L-carnitine has an impact on animal and human infertility. Lenzi and coworkers [60] identified a positive relationship between L-carnitine, L-acetyl carnitine, and semen motility in infertile men. When patients received a placebo for more than 3 months with L-carnitine therapy (2 g/day), their sperm showed higher motility. Short-term administration of L-carnitine had a positive effect on the sperm count, leading to successful pregnancy [61]. In addition, albumin successfully avoids the proliferation of peroxidative impairment in sperm by acting as an antioxidant [62].
3.3. Co-Enzymes
Co-enzyme Q10 and catalase act as antioxidants that preserve and increase semen motility [63,64,65,66]. Co-enzyme Q10 is associated with semen parameters, such as concentration, morphology, and motility [6]. Thakur and colleagues [67] proposed that regular supplementation of co-enzyme Q10 at 150 mg increases sperm parameters in infertile males; however, it was not shown to improve the live birth or pregnancy ratio [68].
3.4. Superoxide Dismutase
Superoxide dismutase (SOD) is an important antioxidant enzyme that has a significant role in scavenging ROS, such as hydrogen peroxide (H2O2) and superoxide anion, thereby protecting sperm from peroxidation. For example, high concentrations of SOD prevent the loss of semen motility (p < 0.005) [69]. Negri and coworkers [70] showed that a SOD-based antioxidant supplementation, hydroxytyrosol and carnosol, improves sperm DNA integrity. SOD prevents ROS generation and provides complete protection when added with catalase (0.008 mg/mL) to semen suspensions [71]. Catalase and SOD also have positive effects against sperm intracellular (mitochondrial and plasma membrane) and extracellular (leukocytes) ROS, and promote sperm motility [72,73].
3.5. Selenium
Selenium (Se) is a vital trace element that is important for many physiological processes, such as immune and reproductive systems, the metabolism of thyroid hormones, and antioxidant protection [74]. Selenium plays a vital role in the creation of testosterone and in semen biosynthesis. Selenium and N-acetylcysteine treatment significantly enhances semen motility. In clinical trials [75], 30 weeks of treatment with selenium and N-acetylcysteine in 468 infertile males successfully decreased follicle-stimulating hormone; however, it increased the semen inhibin B and testosterone levels. Rezaeian et al. [76] suggested that 5 mg/mL selenium could improve sperm parameters and sperm vitality after freezing and thawing procedures which could be utilized in clinics to treat the infertility of sub-fertile men. Some placebo trials in different countries have confirmed that selenium administration successfully increases motility, morphology, and sperm counts in infertile males [77]. In a descriptive study conducted by Nenkove et al. [78], a high level of Fe and a low level of Se were the major causes of sperm damage. Variation in trace mineral concentrations in animals’ seminal plasma may be associated with semen quality because trace minerals are involved in the maintenance of the pro-antioxidative balance during ejaculation. It is suggested that antioxidants and micronutrients may be used to protect spermatozoa from the high production of ROS that occurs in many clinical circumstances [79].
4. Green Tea Polyphenols Improve Fertility
Tea is derived from the camellia sinensis plant in the form of green, black, and Oolong tea, a popular beverage consumed globally, which accounts for about 3 billion kilograms (kg) of annual production. Green tea is common and preferred in China, Japan, and certain other Asian countries. Green tea polyphenols (GrTPs) are potentially effective in ameliorating inflammatory bowel disease (IBD) and related disorders via their known anti-inflammatory, antioxidative, and anti-bacterial properties. GrTPs have been shown to reduce inflammatory reactions by targeting several signaling pathways [80]. GrTPs downregulate IκB kinase (IKK), c-Jun N-terminal kinase-mitogen-activated protein kinase (JNK-MAPK) [81], nuclear factor-Kappa B (NF-kB), cytokine-like tumor necrosis factor-α (TNF-α), cyclooxygenase-2 (Cox-2), and B-cell lymphoma (Bcl-2), to protect against hepatic disorders and numerous chronic and inflammatory disorders [82,83]. Moreover, GrTPs also act on extracellular signal-regulated kinases (ERK) and Akt signaling pathways as anti-itch therapies in other skin diseases [84,85]. Green tea contains high quantities of polyphenols which generate oxidized proteins that act as natural antibodies [86]. The antioxidant properties of polyphenols comprise scavenging and destroying free radicals. Testicular tissue is predisposed to suffer from the action of free radicals and OS because of its high cell division rate, oxygen consumption rate, and low oxygen pressure, debilitated vessels, and high levels of unsaturated fatty acids [87]. In addition to its antioxidant properties, green tea may also decrease inflammation, reduce DNA fragmentation, and increase the motility and viability of semen [88]. Other potential benefits of polyphenols include improved egg viability and reduced cellular damage of reproductive organs. In addition, its antioxidative concentration correlates with sperm levels and motility [89].
Normally, sperm cells produce significant amounts of ROS during their physiological metabolism. Testicular tissue comprises a high quantity of unsaturated fatty acids that are very sensitive to ROS. ROS have devastating effects via lipid peroxidation which damages the structure of the lipid matrix in semen membranes and reduces intracellular levels of adenosine triphosphate (ATP) which may lead to reduced sperm viability, axonemal injury, and an increase in mid-piece morphological defects [90]. A study in rats indicated that testicular torsion-detorsion causes OS which may lead to decrease semen concentrations in the testes [91]. GrTPs are water-soluble components, comprised of (−)-epigallocatechin gallate (EGCG), (−)-epicatechin gallate (ECG), (−)-epicatechin (EC), and (−)-epigallocatechin (EGC). EGCG prevents spontaneous mutation, low-density lipoprotein (LDL) oxidation, and chromosomal impairment induced by ROS in somatic cells [92] (Figure 3). Therefore, GrTPs may exert their antioxidative effects on spermatozoa. Roy and coworkers [93] found that supplementation of a Tris-egg yolk extender with polyphenols (0.5, 0.75, or 1 mg/mL) improved the motility and capability of semen. However, the addition of EGCG substances in cooling medium for stallion sperm storage is not useful [94]. The semen antioxidant system is made up of many components, including enzymatic, non-enzymatic, and low molecular weight molecules that provide maximum protection against ROS. An important component in green tea is the catechins, which have higher antioxidant activity than that of vitamin C. In rats, catechins were observed to reduce ROS levels, regulate gene expression, and maintain glucose levels [95,96]. Semen motility in infertile humans is affected by seminal ROS [97]. An increased level of seminal ROS may be associated with an increase in sperm DNA fragmentation [98]. Supplementation of semen storing media with GrTP extract has shown a dose-dependent increase in sperm capability, which has aided cases of idiopathic infertility.
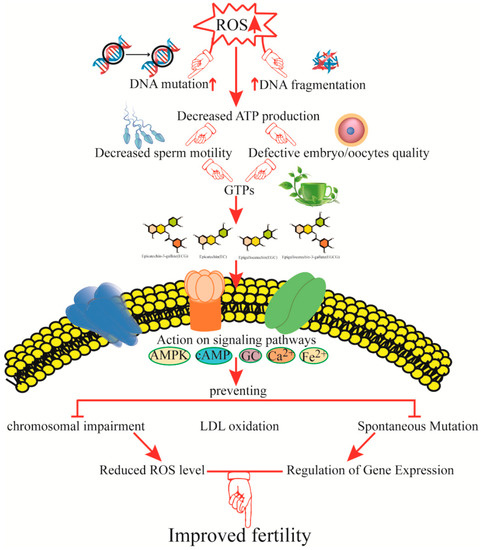
Figure 3.
Mechanisms by which green tea catechins improve fertility and reproductive function. The figure shows potential mechanisms of action of green tea catechins in different pathways and proposes that green tea polyphenols (GrTPs) are capable of improving fertility by improving sperm and embryo quality. ROS, reactive oxygen species; ATP, adenosine triphosphate; AMPK, adenosine monophosphate-activated protein kinase; cAMP, cyclic adenosine monophosphate; Ca2+, calcium ion; Fe2+, ferric iron; LDL, low-density lipoprotein.
Many previous studies have lacked randomization and placebo-controlled arms. The kind, amount, and period of antioxidant treatment has also varied significantly. Some researchers studied a few antioxidants, while others altered the duration of the therapy or used different combinations. Some common antioxidants that have been studied include vitamin C, vitamin E, selenium, zinc, glutathione, L-carnitine, and N-acetylcysteine [99,100]. When comparing EGCG with other components, very low (2 μM and 20 μM) concentrations could improve sperm motility and its capacitation [101]. The use of EGCG at different concentrations (10, 20, and 60 μM) significantly improved the number of semen bound to the zona pellucida (ZP) up to control levels, suggesting that GrTPs are able to decrease the damage caused by rotenone [102]. The addition of 25, 50, and 100 μM EGCG to thawed sperm for one hour did not have any effect on sperm viability and acrosome integrity but increased the in vitro penetration rate and the efficiency of fertilization [103]. GrTPs are expected to have impacts on the treatment and management of idiopathic infertility. An animal model study conducted by Awoniyi and colleagues [104] examined the modulation of ROS by GrTPs in rat semen. They found that GrTP supplementation significantly increased the sperm count and motility. GrTPs have also been shown to decrease lipid peroxidation, protein carbonylation, and DNA damage [105]. The proliferation of semen lipid peroxidation significantly reduces sperm motility [106]. Semen DNA damage is promoted by the enhanced generation of ROS [107]. If semen is treated with polyphenols (0.01 mol/L), the fertility rate significantly increases [108,109]. Adding albumin at 0.3–10% might enhance DNA protection and prevent DNA damage from defusing peroxides formed during lipid peroxidation [110].
GrTPs can protect fertility and provide some degree of protection from reproductive disorders, as shown in animal studies. Green tea extract provided significant protection to the growing fetus against the toxic effects of a high dose of Indomethacin [111]. It also prevented the parental mortality that is associated with this drug and showed ameliorative effects on all parameters associated with reproductive performance and OS. Green tea extract contains vigorous medical ingredients, representing a good source of natural antioxidants that improve fertility, as summarized in Figure 3.
5. Possible Combination of GrTPs with Different Extracts to Improve Fertility
Research on animal and human fertility and its interactions with diet has increased significantly in recent years. The dietary intake of folic acid has been consistently linked to a reduced incidence of infertility, lower risk of pregnancy loss, and better management of infertility [112]. The combination of different nutrients with GrTPs can successfully increase the motility, quality, and quantity of sperm as well as the chances of pregnancy. Numerous natural antioxidants related to both enzymatic and non-enzymatic groups can remove excess ROS and prevent OS.
5.1. Aspalathus Linearis
Rooibos extracts (Aspalathus linearis) in combination with GrTPs may defend against induced oxidative damage by increasing the antioxidant protective mechanisms, thereby, improving the semen quality and function [104].
5.2. Vitex Agnus Castus
Along with GrTPs, another recognized fertility herb is Vitex Agnus castus or chasteberry. This herb is believed to be useful for women with hormonal imbalances, irregular cycles, or reduced luteal stages (especially in the second half of the cycle) [113]. Vitex combined with GrTPs stimulates and balances the hormones that govern the menstrual cycle. It also helps to regulate pituitary gland functions which may help to balance the release of progesterone and estrogen hormone levels as well as enhancing fertility, representing a novel treatment to increase the pregnancy rate [114].
5.3. Pu-Erh
Camellia sinensis var. Assamica combined with Pu-erh black tea was observed to have no adverse effects in terms of reproductive and developmental toxicity at a level of 700 mg/kg/day [115]; however, its potential toxicity when administered at a high dose as a concentrated extract has not been completely investigated.
Thus, in the near future, combinations of green tea with different herbs or nutrients will be developed as novel therapies to improve the fertility rate and treat infertility. More information regarding the treatment of infertility using GrTPs will be gained from well-planned observational studies in the future.
6. Conclusions and Future Perspective
Polyphenols exhibit various effects, including increasing antioxidant (e.g., GSH and cysteine) levels in vital organs [116,117,118,119,120]. GrTP administration at low concentrations could reduce OS and ultimately improve fertility in humans and animals; however, they can exert the opposite effect at higher concentrations. A consensus is still required regarding the type and quantity of GrTPs required to gain the maximum effect. Although GrTPs have demonstrated utility in treating male and female infertility and alleviating OS their precise actions and mechanisms remain to be determined. At the molecular level, GrTPs may represent a complementary treatment to improve fertility and treat infertility or related diseases. It should also be determined whether polyphenols or their composite extracts have supplementary effects on fertility. Furthermore, future studies should combine GrTPs with different extracts or herbs to determine their effects on semen motility and the pregnancy rate as key outcomes.
Author Contributions
S.U.R. prepared and wrote the manuscript. X.W. drafted the first version of the manuscript. Y.H. provided the figures. L.Z., S.F., I.M.K., J.W., and Y.L. read and commented on the manuscript.
Acknowledgments
The present work was supported by the National Key Research and Development Program of China Funds (No. 2016YFD0501009) and Postdoctoral Science Fund of Anhui Province (No. 2016B117). We wish to thank anonymous reviewers for their kind advice.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. J. Urol. 2002, 167, 2138–2144. [Google Scholar] [CrossRef]
- Sharpe, R.M. Environmental causes of testicular dysfunction. In Male Hypogonadism. Contemporary Endocrinology; Huhtaniemi, W.S., Ed.; Humana Press: Cham, The Netherlands, 2017; pp. 281–304. [Google Scholar]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Barrat, C.L.R.; Bjorndahl, L.; Lamb, D.J.; Osorio, M.F.; Mclachlan, R.; Oates, R.D.; Sigman, M.; Sokol, R.; John, B.; Sigman, M.; et al. The diagnosis of male infetility: An analysis of the evidence to support the development of global WHO guidence-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- David, J.M. Male infertility: Lifestyle factors and holistic, complementary, and alternative therapies. Asian J. Androl. 2016, 18, 410–418. [Google Scholar]
- Devi, A. Oxidative stress on male reproductive toxicitys. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 143–147. [Google Scholar]
- Tremellen, K. Oxidative Stress and Male Infertility: A Clinical Perspective. Hum. Reprod. Update 2012, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Schill, W.B. Sperm separation in patients with urogenital infections. Andrologia 1998, 30, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sanocka-Maciejewska, D.; Ciupińska, M.; Kurpisz, M. Bacterial infection and semen quality. J. Reprod. Immunol. 2005, 67, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Syan, N.; Mathur, P.; Choudhary, S. Pharmacological profile of green tea and its polyphenols: A review. Med. Chem. Res. 2012, 21, 3347–3360. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liang, X.; Liang, J.; Zhang, C.; Yang, J.; Wang, C.; Kong, D.; Sun, H. ROS-Responsive Capsules Engineered from Green Tea Polyphenol-Metal Networks for Anticancer Drug Delivery. J. Mater. Chem. B 2018, 6, 1000–1010. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Phu, H.T.; Cossu, A.; Giordo, R.; Fois, M.; Dtb, T.; Piga, A.; Sotgia, S.; Zinellu, A.; Carru, C.; et al. Oxidative stress-induced Akt downregulation mediates green tea toxicity towards prostate cancer cells. Toxicol In Vitro 2017, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Alleviation of Metabolic Syndrome by Green Tea Polyphenol EGCG. Master’s Thesis, The State University of New Jersey, Rutgers, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Jin, D.; Hui, W.; Zhen-Biao, W.; Jie, Z.; Shun, Z.; Wei, L. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 92, 1–13. [Google Scholar]
- Lee, A.H.; Su, D.; Pasalich, M.; Binns, C.W. Tea consumption reduces ovarian cancer risk. Cancer Epidemiol. 2013, 37, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Lin, T.Y.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Improvement of green tea polyphenol with milk on skin with respect to antioxidation in healthy adults: A double-blind placebo-controlled randomized crossover clinical trial. Food Funct. 2016, 7, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol. Clin. N. Am. 2002, 29, 817–827. [Google Scholar] [CrossRef]
- Smith, R.; Vantman, D.; Ponce, J.; Escobar, J.; Lissi, E. Andrology: Total antioxidant capacity of human seminal plasma. Hum. Reprod. 1996, 11, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Sukcharoen, N.; Keith, J.; Irvine, D.S.; Aitken, R.J. Predicting the fertilizing potential of human sperm suspensions in vitro: Importance of sperm morphology and leukocyte contamination. Fertil. Steril. 1995, 63, 1293–1300. [Google Scholar] [CrossRef]
- Aitken, R.J.; Irvine, D.S.; Wu, F.C. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am. J. Obstet. Gynecol. 1991, 164, 542–551. [Google Scholar] [CrossRef]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z.H. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran. J. Reprod. Med. 2015, 13, 525–532. [Google Scholar] [PubMed]
- Hashim, F.; Tvrdá, E.; Massányi, P.; Stawarz, R.; Lukáč, N. Effects of biological active substances to the spermatozoa quality. J. Microbial. Biotechnol. Food Sci. 2016, 5, 263–267. [Google Scholar] [CrossRef]
- Mckay, D.L.; Blumberg, J.B. The Role of Tea in Human Health: An Update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, B.H.; Jeong, J.M. Antioxidant, Antimutagenic and Chemopreventive Activities of a Phyto-extract Mixture Derived from Various Vegetables, Fruits, and Oriental Herbs. Food Sci. Biotechnol. 2003, 12, 631–638. [Google Scholar]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.; Heber, D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates With the Antioxidant Capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Erba, D.; Riso, P.; Bordoni, A.; Foti, P.; Biagi, P.L.; Testolin, G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005, 16, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Benjamin, J.C. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 180, 464–468. [Google Scholar]
- Moazzam, A. Oxidative Stress Induced Infertility in Varicocele. Andrology 2016, 5, 2167–2250. [Google Scholar]
- Showell, M.G.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2011, 1, CD007411. [Google Scholar]
- Balogun, A.M.; Charles-Davies, M.A.; Chikezie, I.C.; Okoli, S.U. Relationship between testosterone, oxidative stress biomarkers and antioxidant levels in male auto-mechanics in Ibadan, Nigeria. Afr. J. Biomed. Res. 2016, 19, 191–197. [Google Scholar]
- Ciattei, A.P. Micronutrients and reduction of oxidative stress in spermatozoas. Int. J. Nutrol. 2016, 9, 153–159. [Google Scholar]
- Desai, N.R.; Mahfouz, R.; Sharma, R.; Gupta, S.; Agarwal, A. Reactive oxygen species levels are independent of sperm concentration, motility, and abstinence in a normal, healthy, proven fertile man: A longitudinal study. Fertil. Steril. 2010, 94, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Effect of ferrous sulphate and ascorbic acid on motility, viability and lipid peroxidation of crossbred cattle bull spermatozoa. Animal 2008, 2, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 40, 183. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab. J. Urol. 2017, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.E.; Agarwal, A.; Mahfouz, R.; Plessis, S.S.D.; Schatzl, G. The association between leukocytes and sperm quality is concentration dependent. Reprod. Biol. Endocrinol. 2010, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, F.F.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil. Steril. 2000, 73, 459–464. [Google Scholar] [CrossRef]
- Luderer, U. Ovarian toxicity from reactive oxygen species. In Vitamins & Hormones; Academic Press: New York, NY, USA, 2014; Volume 94, pp. 99–127. [Google Scholar]
- Guérin, P.; El, M.S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Camlin, N.J.; Sobinoff, A.P.; Sutherland, J.M.; Beckett, E.L.; Jarnicki, A.G.; Vander, R.L.; Hansbro, P.M.; Mclaughlin, E.A.; Hold, J.E. Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biol. Reprod. 2016, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advance sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Qureshi, M.S.; Akhtar, S.; Ali, I. Fertility improvement in cross breed dairy cows through supplementation of vitamin E as antioxidant. Pak. J. Zool. 2016, 48, 923–930. [Google Scholar]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative Impact of Oxidative Stress on the Functional Competence and Genomic Integrity of Human Spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Hashim, F.; Tvrdá, E.; Greifová, H.; Lukáč, N. Effect of vitamins on the quality of insemination doses of bulls. J. Microbial. Biotech. Food Sci. 2018, 7, 242–247. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Nashtaei, M.S.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mazjoub, A.; Agarwal, A.; Esteves, S.C. Antioxidant for elevated sperm DNA fragmentation: A mini review. Trans. Androl. Urol. 2017, 6, S649–S653. [Google Scholar]
- Askari, H.A.; Check, J.H.; Peymer, N.; Bollendorf, A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch. Androl. 1994, 33, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Parinaud, J.; Le, L.D.; Vieitez, G.; Griveau, J.F.; Milhet, P.; Richoilley, G. Enhancement of motility by treating spermatozoa with an antioxidant solution (Sperm-Fit) following ejaculation. Hum. Reprod. 1997, 12, 2434–2436. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Sgrò, P.; Salacone, P.; Paoli, D.; Gilio, B.; Lombardo, F.; Santulli, M.; Agarwal, A.; Gandini, L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil. Steril. 2004, 81, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Maiorino, M.; Roverato, A.; Roveri, A.; Ursini, F.; Foresta, C. Oral carnitine supplementation increases sperm motility in asthenozoospermic men with normal sperm phospholipid hydroperoxide glutathione peroxidase levels. Fertil. Steril. 2005, 83, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Lu, X.; Wang, Y.W.; Sun, J.; Tao, J.W.; Yin, F.H.; Cheng, H.J. Short-term medication of L-carnitine before intracytoplasmic sperm injection for infertile men with oligoasthenozoospermia. Zhonghua Nan Ke Xue 2012, 18, 253–256. [Google Scholar] [PubMed]
- Hong, C.Y.; Lee, M.F.; Lai, L.J.; Wang, C.P. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia 1994, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Rajasekaran, M.; Hellstrom, W.J.; Sikka, S.C. Antioxidant potential of human serum albumin: Role in the recovery of high quality human spermatozoa for assisted reproductive technology. J. Androl. 1998, 19, 412–419. [Google Scholar] [PubMed]
- Lewin, A.; Lavon, H. The effect of coenzyme Q10 on sperm motility and function. Mol. Asp. Med. 1997, 18, 213–219. [Google Scholar] [CrossRef]
- Baker, H.W.G.; Brindle, J.; Irvine, D.S.; Aitken, R.J. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil. Steril. 1996, 65, 411–419. [Google Scholar] [CrossRef]
- Thakur, A.S.; Littarru, G.P.; Funahashi, I.; Painkara, U.S.; Dange, N.S.; Chauhan, P. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J. Clin. Diagn. Res. 2015, 9, BC01. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, R.; Gonzálezcomadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Miyazaki, T.; Natori, M.; Nozawa, S. Protective role of superoxide dismutase in human sperm motility: Superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991, 6, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Benaglia, R.; Monti, E.; Morenghi, E.; Pizzocaro, A.; Levi, P.S. Effect of superoxide dismutase supplementation on sperm DNA fragmentation. Arch. Ital. Urol. Androl. 2017, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Iwasaki, A.; De, L.E.; Kovalski, N. Reactive oxygen species and human spermatozoa. Ann. N. Y. Acad. Sci. 1991, 637, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kovalski, N.N.; De, L.E.; Gagnon, C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil. Steril. 1992, 58, 809–816. [Google Scholar] [CrossRef]
- Flohe, L. Selenium in mammalian spermiogenesis. Biol. Chem. 2007, 388, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, E2209. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2008, 181, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, Z.; Yazdekhasti, H.; Nasri, S.; Rajabi, Z.; Fallahi, P.; Amidi, F. Effects of selenium on human sperm parameters after freezing and thawing procedures. Asian Pac. J. Reprod. 2016, 5, 441–446. [Google Scholar] [CrossRef]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003, 49, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of Trace Elements for Oxidative Status and Quality of Human Sperm. Balkan Med. J. 2017, 34, 343. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Condorelli, R.A.; Russo, G.I.; Vignera, S. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed. Res. Int. 2017, 2017, 4650182. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, D.; Hu, Z.; Zhao, S.; Zheng, Z.; Wei, L. Protective Effects of Green Tea Polyphenol Against Renal Injury Through ROS-Mediated JNK-MAPK Pathway in Lead Exposed Rats. Mol. Cells 2016, 39, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of inflammatory bowel disease via green tea polyphenols: Possible application and protective approaches. Inflammopharmacology 2018, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, K.F.; Marinovic, M.P.; Morandi, A.C.; Bolin, A.P.; Otton, R. Green tea polyphenol extract in vivo attenuates inflammatory features of neutrophils from obese rats. Eur. J. Nutr. 2016, 55, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, F.M.; Su, C.J.; Liu, T.T.; Zhou, Y.; Fan, L.; Wang, Z.H.; Liu, X.; Huang, Y.; Liu, T.; et al. Epigallocatechin-3-gallate attenuates acute and chronic psoriatic itch in mice: Involvement of antioxidant, anti-inflammatory effects and suppression of ERK and Akt signaling pathways. Biochem. Biophys. Res. Commun. 2018, 496, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hatasa, Y.; Chikazawa, M.; Furuhashi, M.; Nakashima, F.; Shibata, T.; Kondo, T.; Akagawa, M.; Hamagami, H.; Tanaka, H.; Tachibana, H.; et al. Oxidative Deamination of Serum Albumins by (−)-Epigallocatechin-3-O-Gallate: A Potential Mechanism for the Formation of Innate Antigens by Antioxidants. PLoS ONE 2016, 11, e0153002. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieiankopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01. [Google Scholar] [CrossRef] [PubMed]
- Sreejaya, P.; Nirmala, P. Evaluation of anti-oxidant effect of green tea extract in cryo preserved human semen samples. Int. J. Curr. Res. 2016, 8, 27270–27274. [Google Scholar]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and Polyphenols: Concentrations and Relation to Male Infertility and Treatment Success. Oxid. Med. Cell. Longev. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R. The impact of oxidants on sperm function. Andrologia 2005, 37, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, D.; Parlaktas, B.; Uluocak, N.; Gencten, Y.; Erdemir, F.; Ozyurt, H.; Erkorkmaz, U.; Aslan, H. Pomegranate (Punica granatum) juice reduces oxidative injury and improves sperm concentration in a rat model of testicular torsion-detorsion. Exp. Ther. Med. 2014, 8, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Chakrabarty, S.; Sinha, D.; Bhattacharya, R.K.; Siddiqi, M. Anticlastogenic, antigenotoxic and apoptotic activity of epigallocatechin gallate: A green tea polyphenol. Mutat. Res. 2003, 523–524, 33–41. [Google Scholar] [CrossRef]
- Wittayarat, M.; Ito, A.; Kimura, T.; Namula, Z.; Luu, V.V.; Do, L.T.K.; Sato, Y.; Taniguchi, M.; Otoi, T. Effects of green tea polyphenol on the quality of canine semen after long-term storage at 5 degrees C. Reprod. Biol. 2013, 13, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.; Spinaci, M.; Mislei, B.; Gadani, B.; Rizzato, G.; Love, C.; Tamanini, C.; Galeati, G.; Mari, G. Epigallocatechin-3-gallate (EGCG) and green tea polyphenols do not improve stallion semen parameters during cooling at 4 °C. Reprod. Domest. Anim. 2017, 52, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, M.M.; Khatoon, N.; Azmi, M.A.; Rajput, M.T.; Zaidi, S.I.; Azmi, M.A.; Perveen, R.; Naqvi, S.N.; Rashid, M. Report: Effects of Camellia sinensis L. (green tea) extract on the body and testicular weight changes in adult Wistar rate. Pak. J. Pharm. Sci. 2015, 28, 249. [Google Scholar] [PubMed]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Sabanegh, E.S. The Role of Over-the-Counter Supplements for the Treatment of Male Infertility—Fact or Fiction? J. Androl. 2012, 33, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Amicis, F.D.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Plaza, D.M.; Bucci, D.; Galeati, G.; Peña, F.J.; Mari, G.; Giaretta, E.; Tamanini, C.; Spinaci, M. Epigallocatechin-3-Gallate (EGCG) Reduces Rotenone Effect on Stallion Sperm-Zona Pellucida Heterologous Binding. Reprod. Domest. Anim. 2015, 50, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 2017, 90, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Awoniyi, D.O.; Aboua, Y.G.; Marnewick, J.; Brooks, N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phytother. Res. 2012, 26, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, M.M.; Manfredini, V.; Brum, D.D.S.; Vargas, L.M.; Spiazzi, C.C.; Soares, M.B.; Izaguirry, A.P.; Santos, F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015, 2, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R.J. Adverse effects of peroxidized lipid on human spermatozoa. Proc. R. Soc. Lond. B 1978, 201, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A.; Hafez, T.; Comhaire, F. Evaluation of the role of reactive oxygen species in male infertility. Hum. Reprod. 1995, 10, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lu, S.C.; Chen, A. Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis. Mol. Pharmacol. 2008, 73, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Kitaji, H.; Ookutsu, S.; Sato, M.; Miyoshi, K. Preincubation with green tea polyphenol extract is beneficial for attenuating sperm injury caused by freezing-thawing in swine. Anim. Sci. J. 2015, 86, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Jurisicova, A.; Sun, J.G.; Casper, R.F. Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. J. Urol. 1998, 160, 896–900. [Google Scholar] [CrossRef]
- Mahmood, S.; Jawd, S.M.; Jwad, S.M. The Ethanolic Extract of Green Tea Ameliorates Oxidative Stress Parameters and Female Reproductive Performance Regression Induced by Indomethacin in Pregnant Rats. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 549–563. [Google Scholar]
- Gaskins, A.J.; Chavarro, J.E. Diet and Fertility: A Review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Seidlova-Wuttke, D.; Wuttke, W. The premenstrual syndrome, premenstrual mastodynia, fibrocystic mastopathy and infertility have often common roots: Effects of extracts of chasteberry (Vitex agnus castus) as a solution. Clin. Phytosci. 2017, 3, 6. [Google Scholar] [CrossRef]
- Batool, H.R.; Maryam, N. Effects of Vitex agnus-castus extract on the secretory function of pituitary-gonadal axis and pregnancy rate in patients with premature ovarian aging (POA). J. Herb. Med. 2017, 10, 24–30. [Google Scholar]
- Di, W.; Jie, M.; Hui, G.; Kunlong, X.; Rong, X.; Ying, Z.; Xiao, L.; Ping, Y.; Hong, Y.; Liu, L. Evaluation of reproductive and developmental toxicities of Pu-erh black tea (Camellia sinensis var. assamica) extract in Sprague Dawley rats. J. Ethnopharmacol. 2013, 148, 190–198. [Google Scholar]
- Twigg, J.; Fulton, N.; Gomez, E.; Irvine, D.S.; Aitken, R.J. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: Lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum. Reprod. 1998, 13, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.; De, V.W.J.S. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; Mcclain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Ebersole, J.L. Application of prodrugs to inflammatory diseases of the gut. Molecules 2008, 13, 452–474. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).