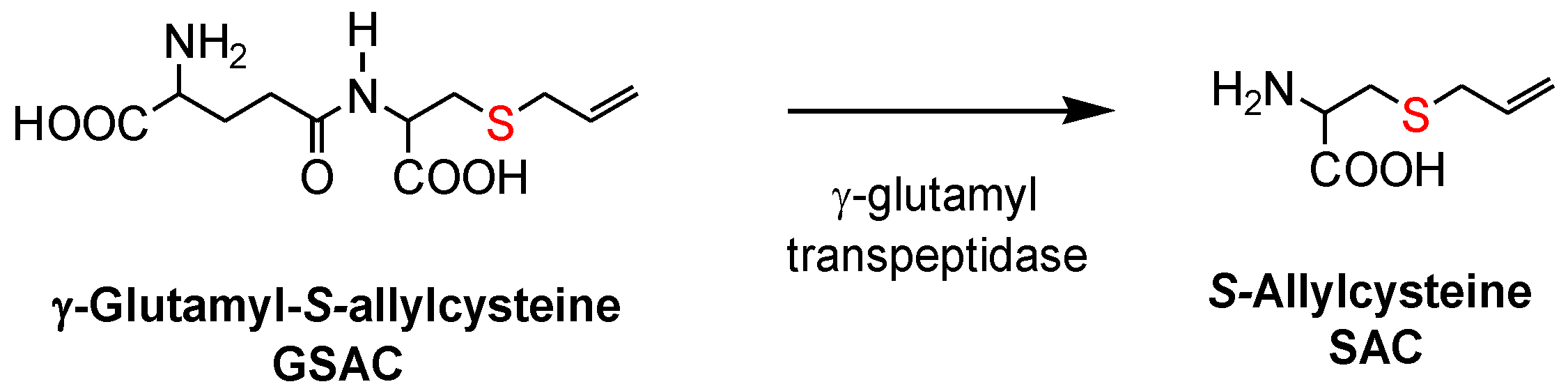1. Introduction
Garlic supplements, mainly dried and pulverized whole clove supplements, have been used in a large number of controlled clinical trials since the mid-1980s, focusing primarily on serum cholesterol and blood pressure [
1,
2,
3]. The effects on blood pressure have been moderately consistent for hypertensive subjects, while the effects on serum lipids have been inconsistent, even for persons with high baseline cholesterol levels [
1,
2,
4,
5,
6]. Of the 23 qualifying trials on serum cholesterol with a garlic powder product, 43% found no effect [
1]. Due to the inconsistencies, the trials have been the subject of several meta-analyses (nine on serum lipids and eight on blood pressure), with the overall conclusions being conservative [
7]. Authors of the meta-analyses most frequently cite the high heterogeneity among the trials (high variation in dose, variable product types, identification of active compounds, standardization concerns, and unknown bioavailability) as the reason for caution in recommending garlic products for treatment of hypercholesterolemia and hypertension [
1,
4,
5,
7,
8,
9,
10,
11]. A recent review of the mostly in vitro antimicrobial effects of allicin concluded that determination of allicin bioavailability from various products is necessary before proper clinical studies can be conducted [
12]. Hence, it is clear that attention needs to be given to the bioavailability and standardization of garlic’s active compounds under a variety of processing conditions, especially because of known or suspected potential issues with their formation, stability, metabolism, and detection in the body.
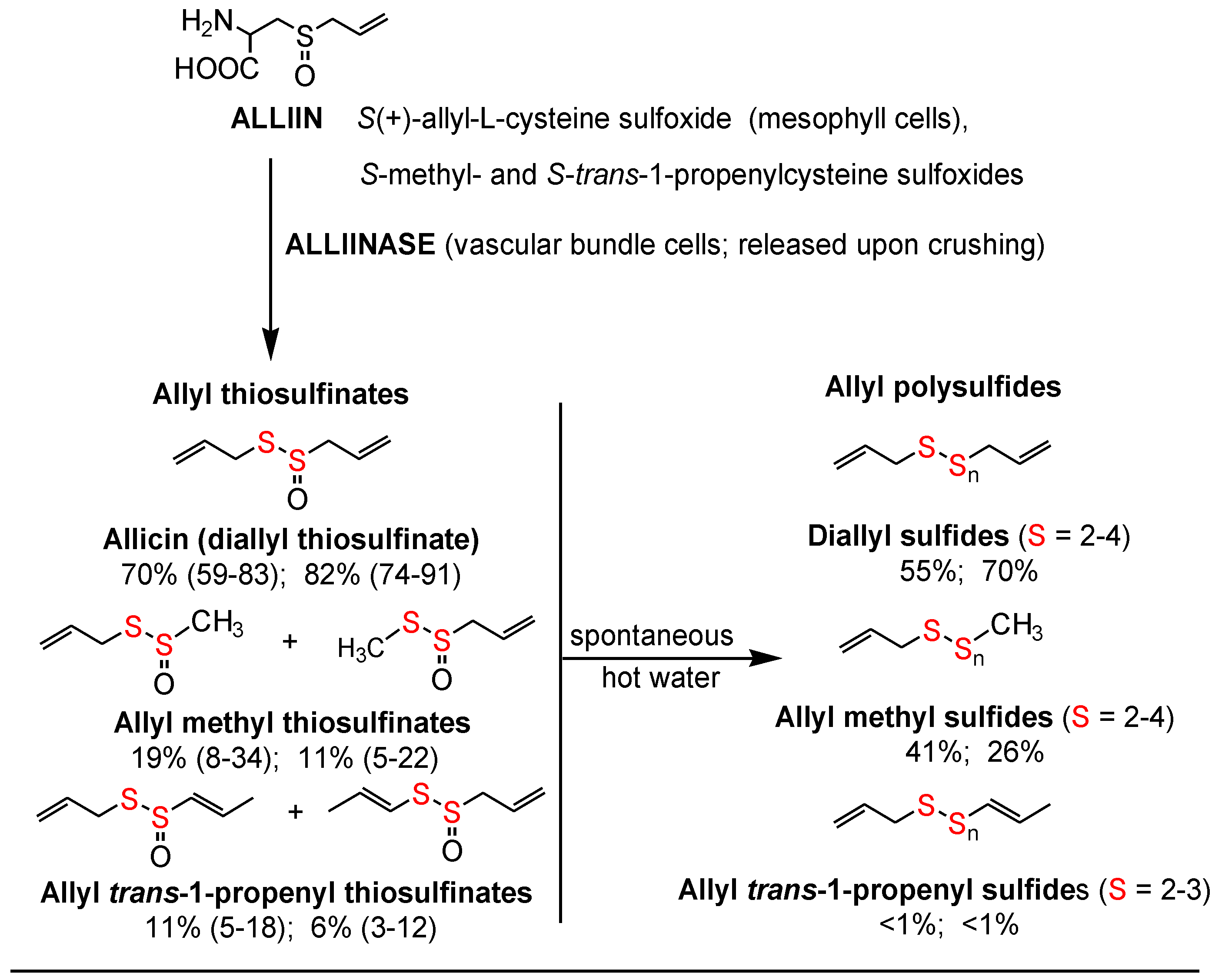
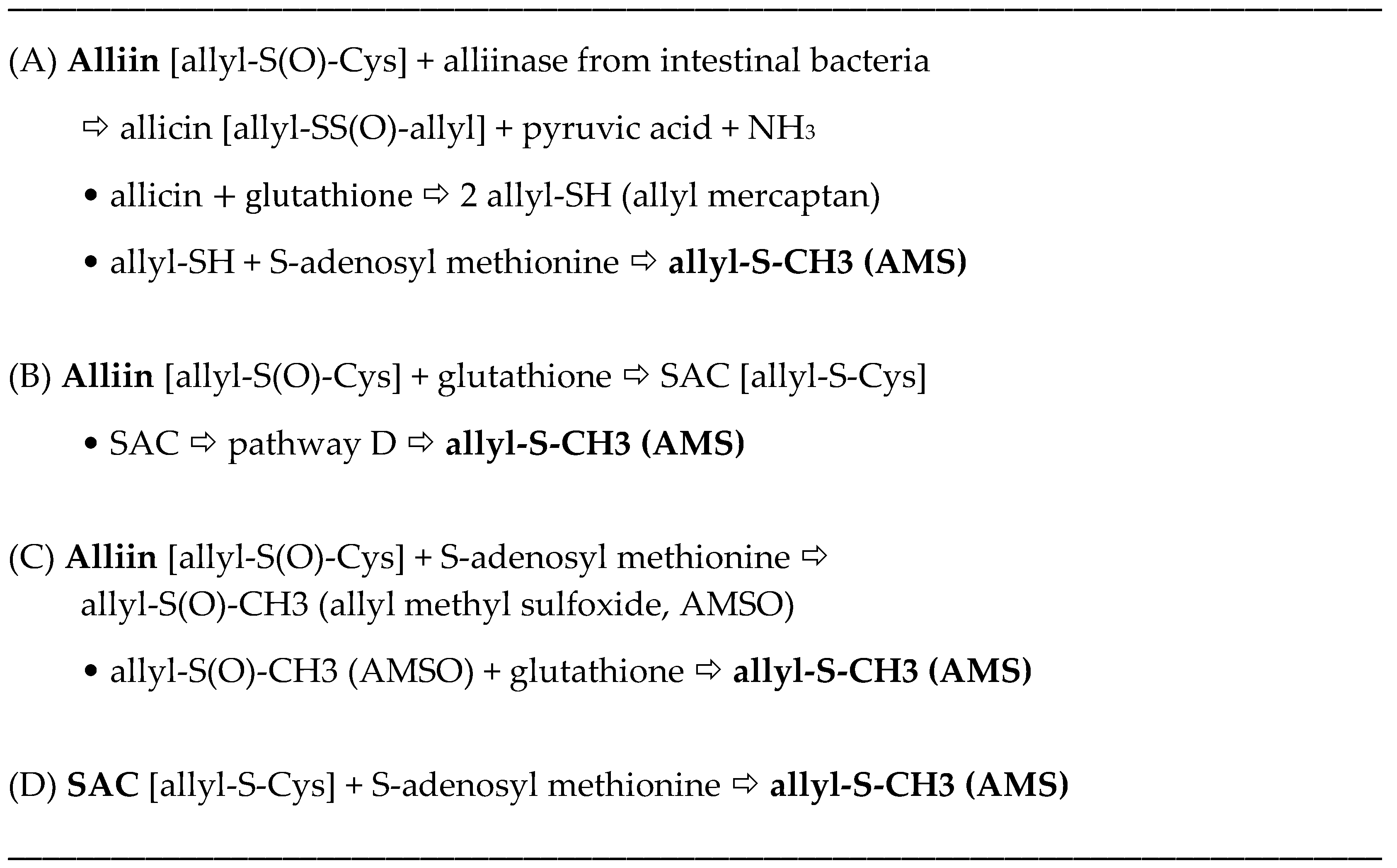
The allyl thiosulfinates, of which allicin (diallyl thiosulfinate) is the most abundant and most studied member (
Figure 1), are enzymatic products of alliin,
S(+)-allyl-
l-cysteine sulfoxide, and alliinase. They are rapidly formed when raw garlic cloves undergo cell rupture (
Figure 1) or when dried and pulverized cloves (garlic powder) become wet [
13,
14]. The allyl thiosulfinates have been shown to be responsible for most of the pharmacological activity of crushed raw garlic cloves. Beginning in 1944 it was shown that allicin is responsible for the antibacterial activity of garlic and that selective removal of allicin also removed all activity [
15,
16]. Considerable evidence suggests that the allyl thiosulfinates, or their spontaneous transformation compounds (allyl polysulfides), or their common metabolite (allyl methyl sulfide, AMS),
Figure 1 and
Figure 2, are responsible for most of the lipid-lowering, antioxidant, anti-atherosclerotic, and anticancer effects of whole garlic, as observed in animals and humans [
13,
17]. Both allicin and γ-glutamyl-
S-allylcysteine (GSAC), as the source of
S-allylcysteine (SAC) (
Figure 1), appear to be responsible for the hypotensive effects of garlic [
18]. Indeed, no other compound has yet been shown to have significant activity at levels present in a normal human dose (3–5 g) of crushed raw garlic. The majority of the clinical trials on the possible cardiovascular effects of garlic have used supplements that are standardized on alliin or allicin potential [
1,
2].
Due to the abundance of alliinase [
25], complete formation of allicin takes place in 0.5 min (5 min for the allyl methyl thiosulfinates) when water is added to garlic powder [
14]. However, their formation in the body after consumption of garlic powder supplements is questionable because alliinase is inactive at pH 3.5 or below [
14,
26], a pH range commonly found in the stomach, although a moderate to high-protein meal can briefly raise the pH to 4.4 or higher [
27,
28], a range in which alliinase is active. Hence, many brands of garlic supplements have been enteric-coated to prevent disintegration in the stomach, and the U.S. Pharmacopeia (USP) has established a monograph for estimating allicin formation and release from such products under simulated gastrointestinal dissolution conditions [
29]. However, 21 of 24 enteric brands subjected to this dissolution allicin release test yielded less than 20% of their allicin potential (the maximum yield of allicin from alliin upon activation of alliinase; the USP monograph refers to it as “potential allicin”), due to low tablet alliinase activity and to slow tablet disintegration [
30]. Similarly, dissolution allicin release from non-enteric Kwai (Lichtwer Pharma, Berlin) tablets, the most commonly used garlic supplement in cardiovascular clinical trials [
1,
2], has also been found to be problematic: 44% for tablets made before 1993 and only 15% for tablets made from 1994–1997 [
31].
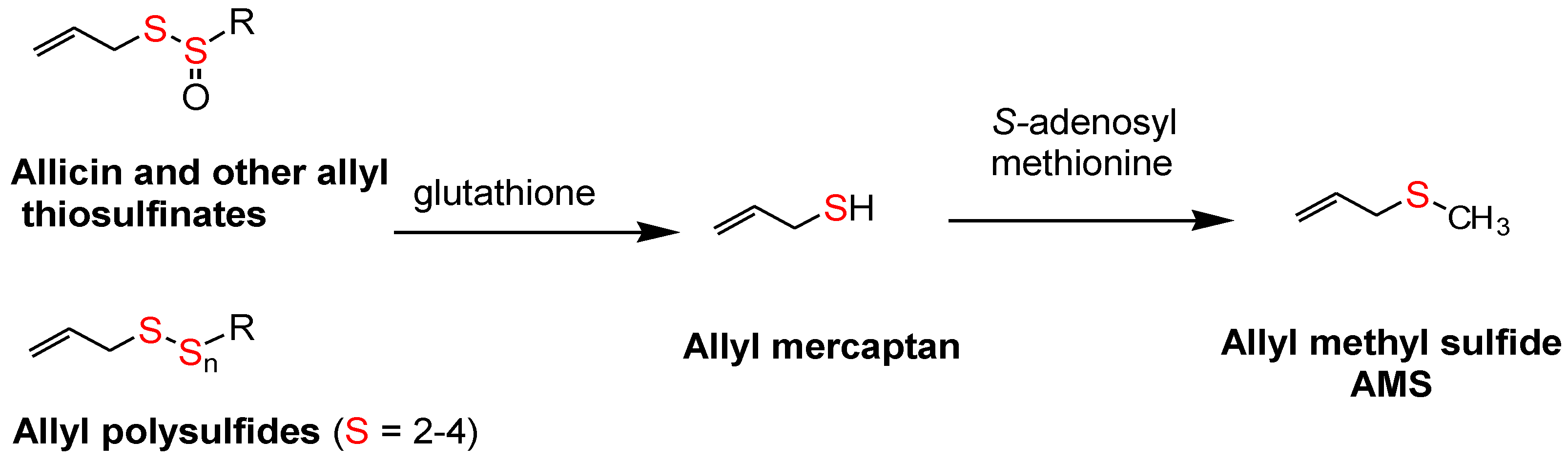
The surprisingly low allicin release found under these standardized in vitro conditions indicates either that allicin release in the body from most garlic supplements is very low or that the U.S.P. dissolution test is not accurate. This dilemma highlights the need to determine the allicin bioavailability in vivo from garlic products, but attempts to do so have been troublesome. Allicin has been shown to be metabolized rapidly (half-life <1 min) to allyl mercaptan (allyl thiol) when added to whole blood [
32], but neither allicin nor its transformation compounds (
Figure 1) nor allyl mercaptan were found in the blood, urine or stool after volunteers consumed a large amount (25 g) of chopped raw garlic [
33] (p. 152). However, it has been known for some time that AMS is a component of human breath after consuming raw garlic and that its rate of appearance and decline in the breath indicated it to be a product of systemic metabolism [
34,
35,
36]. Lawson and Wang [
17] conducted studies on human breath AMS and showed that (a) the area under the 32-h breath AMS concentration curve (AUC) is linearly proportional to the amount of allicin consumed; (b) AMS is the main breath metabolite of allicin, accounting for at least 90% of the allicin consumed; (c) allyl mercaptan is a temporary intermediate in the formation of AMS from allicin; (d) allicin-derived diallyl disulfide and diallyl trisulfide are also metabolized mainly to AMS; and (e) AMS is an active metabolite, responsible for the ability of allicin to increase breath acetone levels. By the use of a sulfur-selective detector, the sensitivity of the method was improved for the consumption of small amounts of garlic [
37]. Hence, a validated method for determining the bioavailability of allicin from any garlic product has been established.
The term “allicin bioavailability” is being used to represent the sum of three processes: enzymatic (garlic alliinase) formation of allyl thiosulfinates (mainly allicin) from alliin, usually in the gastrointestinal tract, followed by their absorption and metabolism to a quantifiable metabolite, AMS. Allicin absorption is known to be highly efficient [
38,
39], although partial metabolism to the rapidly absorbed intermediate, allyl mercaptan, may occur during absorption [
17]. The term “allicin bioequivalence” refers to the metabolic formation of the allicin metabolite, AMS, from any
S-allyl compound, without the assistance of garlic alliinase, including allyl polysulfides, alliin and possibly other
S-allyl compounds, such as GSAC and SAC; the term is being used in particular for products in which garlic alliinase is inactive. Together, the terms are referred to as allicin bioavailability or bioequivalence (ABB).
Although garlic supplements have been used in a large number of clinical trials, garlic is most commonly consumed as a food, usually as a cooked food and often as a commercial product suspended in an acid, such as whole cloves (pickled garlic) or small pieces (minced garlic), but no known clinical trials have been conducted with cooked or acidified garlic foods. However, the general public often wants to know if the results of clinical trials with supplements apply to garlic as a food, especially cooked garlic. In the U.S., cooked garlic is commonly prepared by boiling (soups) or roasting [
40,
41]. Because cooking or suspending garlic in acid fully inactivates alliinase [
14,
26], any allicin-related health benefits found with garlic supplements will probably be negligible in such foods. However, this logic assumes that the body itself—in the absence of active garlic alliinase—does not have the ability to metabolize alliin to allicin or allicin metabolites. Now that a method exists for determining the ABB of any garlic product, the validity of this assumption can be tested for alliinase-inhibited garlic foods.
The primary objective of this study has been to determine the problematic and hitherto unknown bioavailability of allicin from a variety of commonly consumed garlic supplements and garlic foods, in order to provide clinical trial researchers, manufacturers, and consumers with an improved knowledge basis when considering the possible health benefits of garlic products. The study has answered the following questions:
Can allicin bioavailability from garlic powder supplements be as high as that from crushed raw garlic?
Is the allicin bioavailability of the main garlic powder supplement (Kwai) used in clinical trials significantly less than that of raw garlic?
Is the allicin bioavailability of enteric garlic supplements greater than that of non-enteric ones?
Does the protein content of the meal being consumed with the supplement influence the allicin bioavailability?
How much supplement alliinase activity is necessary to achieve high allicin bioavailability?
What standards are recommended for the quality, content, and allicin bioavailability of garlic supplements being used in clinical trials?
Is the in vitro USP. dissolution allicin release test accurate for estimating allicin bioavailability from garlic supplements?
Do cooked garlic or acidified commercial garlic products have significant allicin bioequivalence? If so, how much of these foods would one need to consume to obtain the same ABB as raw garlic or a garlic powder supplement used in clinical trials?
Do garlic compounds other than alliin-derived compounds have significant allicin bioequivalence?
2. Materials and Methods
2.1. Standards
l(+)- and
l(±)-
S-allylcysteine sulfoxides (natural and racemic alliin),
S-allylcysteine, diallyl disulfide, and diallyl trisulfide (each ≥98%) were purchased from LKT Laboratories, Inc. (St. Paul, MN, USA). Allyl methyl sulfide and diallyl sulfide (each 99%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). γ-Glutamyl-
S-allylcysteine (99%) was purchased from U.S. Pharmacopeia (Rockville, MD, USA). Allicin (98%) was prepared by oxidation of diallyl disulfide with hydrogen peroxide, followed by purification, as previously described [
30]. Its concentration was validated with a second allicin standard prepared from alliin and crude alliinase [
29]. Allyl methyl thiosulfinates, allyl-1-propenyl thiosulfinates, diallyl tetrasulfide, allyl methyl disulfide, allyl methyl trisulfide, and allyl methyl tetrasulfide were identified and quantified based on their relative retention times and relative extinction coefficients, compared to allicin and diallyl trisulfide, as previously described [
19,
21].
2.2. Control
The control for 100% ABB was prepared by homogenization of 500 g of peeled raw garlic cloves (California Late variety, Garlic World, Gilroy, CA, USA; 39.5% dry weight), after addition of water at 0.60 mL/g, in an Osterizer blender at the highest speed for 2 min. This allowed alliinase to transform all of the alliin to known amounts of allicin and other allyl thiosulfinates. The homogenate was divided into 50-mL jars and stored at −80 °C, under which condition the allyl thiosulfinates have been shown to be stable for at least 24 months [
37]. Prior to consumption, the homogenate was thawed overnight at 4 °C, at which temperature the allyl thiosulfinates have been shown to be stable for at least 3 days [
30]. After thawing, the homogenate was further macerated with a high speed Polytron homogenizer (Brinkmann, Kinematica AG, Luzern, Switzerland) with a 12-mm generator for 1 min at speed 6 of 10. This made it possible for the viscous homogenate to pass through the modified tip (drilled to 3 mm, inside diameter) of a 10-mL disposable syringe. At the time of consumption, a specific weight of the homogenate was transferred by syringe into the bottom of one or more size 0 hypromellose (hydroxypropyl methylcellulose) capsules (vegetarian capsules). The bottom of the size 0 capsule rested inside the bottom of a size 00 capsule to prevent aqueous allicin from coming in contact with the throat. The capsule was then closed with the top of a size 00 capsule. Under these conditions, the outer capsule remained firm and tasteless for 6 min. The standard control was 1.4 g of garlic homogenate, which was made from 0.88 g of raw garlic (0.35 g dry wt).
2.3. Supplements
All garlic supplements used in the study were purchased in 2009 at local stores or from online distributors and stored at ambient temperature. Only C1 was purchased directly from the manufacturer. All were tested by May 2011, within their claimed expiration dates (typically 2 years from the manufacture date, except 3 years for N1 and N4), with two intended exceptions (E6 and N4).
Table 1 identifies the garlic supplements used in the study and includes the claims for garlic powder content (sometimes called garlic extract content or garlic dry weight), the actual tablet or capsule weights (average of at least 10 units), standardization claims, if any, and the recommended dose. All labels stated that the products should be consumed with a meal, except for N3.
Table 2 gives the complete list of other ingredients for the supplements, with indications for the likely enteric coating agents when not specifically stated. The 13 supplements contained 51 different ingredients other than garlic powder.
2.4. Commercially Prepared Garlic Foods
Three common (purchased at grocery stores) and one less common (black garlic, purchased online) commercially prepared garlic foods were tested for ABB. The manufacturing procedures for all the products inactivates alliinase. Spicy Pickled Garlic (referred to as pickled garlic), manufactured by G.L. Mezzetta, Inc. (Napa Valley, CA, USA) consisted of whole raw cloves suspended in a medium of water, vinegar, crushed chili, and sodium bisulfite; cloves constituted 65% of the total volume. The pH of a 1:10 aqueous extract of the cloves was 4.3; the pH of the undiluted medium was also 4.3. Minced Garlic (referred to as acid-minced garlic), manufactured by Spice World, Inc. (Orlando, FL, USA) consisted of finely minced raw cloves mixed with a minimal amount of water (almost no standing liquid) and phosphoric acid; the pH of a 1:10 aqueous extract was 3.6. Chopped Garlic (referred to as oil-chopped garlic), manufactured by Christopher Ranch (Gilroy, CA, USA), consisted of finely chopped raw garlic mixed with soybean oil, olive oil (almost no standing liquid) and citric acid; the pH of a 1:10 aqueous extract was 4.3. The manufacturer’s web site calls it Chopped Garlic in Oil. Black garlic, manufactured in South Korea and distributed by Black Garlic, Inc. (Hayward, CA, USA), consisted of peeled whole cloves that were black (no additives).
2.5. Kitchen-Prepared Garlic Foods (Cooked and Raw Diced)
Roasted. Garlic (California Early variety, Garlic World, Gilroy, CA, USA) was roasted at two different temperatures commonly used for cooking (actual air temperature): 160 °C for 30 min and 215 °C for 60 min. Three garlic bulbs were placed on a rack inside a pre-heated Nesco 6 L electric roaster oven (Walmart). Wire probes from a calibrated Fluke 52II digital thermometer (Fluke Corp., Everett, WA, USA) were used to measure the temperature (recorded every 5–10 min) of the air inside the oven and the temperature inside the cloves of two different bulbs. The average temperature inside the cloves, after the first 10 min, was 92 °C for the 160 °C preparation and 101 °C for the 215 °C preparation. Roasting caused 5% and 25% loss of total weight, respectively. After cooling, the cloves were peeled and the soft contents thoroughly mashed and mixed. All of the contents of the 160 °C cloves were soft, but about half of the weight of the 215 °C cloves was hard and unusable. Roasting decreased the moisture content of the cloves from 63% to 58% and 55%, respectively.
Boiled. Unpeeled garlic cloves (California Early variety, Garlic World, Gilroy, CA, USA) were boiled for 4 min or for 45 min, by adding 24 cloves (80 g for 4 min or 110 g for 45 min) from six bulbs to 1.5 L of boiling water. The water resumed boiling about 15 s after the cloves were added. After boiling, the cloves were cooled, peeled, and thoroughly mashed and mixed. Preliminary studies showed that the temperature inside the cloves reached 95 °C (boiling point 96 °C) by 4 min if the clove weights were under 5 g or by 6 min if the clove weights were 5.5 to 7 g. It was also shown that alliinase activity was completely inhibited in 2 min for cloves weighing under 5 g. Hence, for the 4 min preparation, only cloves weighing 1.2 to 5.0 g were used, while cloves for the 45 min preparation weighed 1.2 to 9 g. The total weight of the cloves boiled for 4 min did not change, while the weight of the cloves boiled for 45 min increased by 5%. To reduce variation, cloves from each of the six bulbs were split into the two groups used for boiling.
Diced (cubed) raw. Raw garlic cloves (California Early variety, Garlic World, Gilroy, CA, USA) were peeled, then placed on a plastic grid of 3-mm lines, and cut with a razor blade into cubes measuring approximately 3 mm on each side (40 cubes per gram). This size is similar to that of commercial minced garlic. Two cloves from each of the same bulbs used for the boiling preparations were used. The cloves were carefully peeled and cut in order to minimize allicin formation.
2.6. Special Garlic Extracts
Protein-free, high-alliin garlic extract (PFHA). A garlic extract was prepared in which 97% of the soluble protein was removed, without loss of alliin. Garlic cloves (California Early variety, Garlic World, Gilroy, CA, USA) were peeled (100 g), boiled for 15 min in 1.5 L water (giving 14 mL water/g clove after evaporation loss), transferred to an Osterizer blender, homogenized at the highest speed for 1 min, filtered through cheesecloth, centrifuged at 1300× g for 20 min, subjected to 11 cycles of freezing at −22 °C and thawing, and finally centrifuged at 16,000× g. The soluble protein content decreased from 12.5 mg/g clove (before boiling) to 0.80 mg/g clove (before freeze-thawing) to 0.42 mg/g clove. The final clear extract contained 0.034 mg protein/mL.
Alliin-free, high-GSAC extract (AFHG). A garlic extract void of alliin and alliin-derived compounds was prepared to examine the effects of GSAC on breath AMS. Cloves (85 g), from the same 2-kg batch of bulbs that were used to prepare PFHA, were peeled, homogenized (2 min) in an Osterizer blender after addition of water at 14 mL/g (converted all of the alliin to allyl thiosulfinates), filtered through cheesecloth, boiled for 100 min, (causing all of the allyl thiosulfinates and resultant allyl sulfides to evaporate), and centrifuged at 1300× g for 20 min. Additional water was added as needed during boiling to make up for most of the evaporation loss. The soluble protein content decreased from 12.5 mg/g clove (before homogenization) to 2.4 mg/g clove (81% loss of protein). The final clear extract contained 0.25 mg protein/mL.
Alliin-free, high-SAC extract (AFHS). Product AFHG was treated with γ-glutamyl transpeptidase to convert 95% of the GSAC to SAC to learn if the two compounds have equal effects on breath AMS. A portion of AFHG (200 mL) was adjusted to pH 8.5 with 0.1 N NaOH, followed by the addition of 100 units of equine kidney γ-glutamyl transpeptidase (9.1 units/mg) (Sigma-Aldrich) and incubation at 37 °C for 3.5 h.
2.7. Participants
A total of 13 self-described healthy persons (6 female, 7 male) were recruited for the study in May–June 2009, all of whom continued throughout the 22 months of the study. Persons who had an intestinal disease, used tobacco, or frequently consumed alcohol, were excluded from the study.
The participant age range was 26–62 year (averages: total 35, men 34, women 36), with a body mass index (BMI) range of 22–35 kg/m2 (averages: total 26, men 28, women 25). The study was a series of bioassays; hence, it was not blind, nor was there a placebo or randomization Participants were always informed which garlic product they were consuming prior to consumption. Twenty-three types of garlic products were used in 43 tests, with usually 7–13 participants per test. A single set of bioavailability tests required two days to conduct; they were usually conducted two times per week and included 3–4 participants per set. Participants usually participated in two tests per month and were paid for each test. A record of all interactions with the subjects during a test, along with any report of side effects, was maintained on set consumption records. All bioavailability tests with garlic products and garlic extracts, along with the consent form and research protocol, were approved by the Western Institutional Review Board, Olympia, Washington, USA, on 18 March 2009 (study number 1105434).
2.8. Standard Breakfast Meals and Garlic Product Consumption
All garlic supplements, the homogenized raw garlic (control), and the extracts, were consumed with either a standardized low-protein meal (LP) or a standardized high-protein meal (HP). The LP meal consisted of two slices of toasted white bread, 19 g of butter, 30 g of jam, a banana, and 200 mL of water; it contained 5.5 g of protein and 17 g of fat. The HP meal consisted of a whole wheat tuna sandwich and 200 mL of whole milk, including two slices of bread, 54 g of pressed canned albacore solid white tuna, and 34 g of garlic-free light mayonnaise; it contained 31 g of protein and 19 g of fat. The meals were isocaloric (460 kcal). The gastric pH is lowest before a meal (av 1.9) and quickly rises (av 14 min), depending upon the meal protein content, to a maximum pH of 4.4–6.7 after starting a meal [
28,
42]. Hence, the supplements were consumed immediately before the LP meal, to allow exposure to approximately the lowest gastric pH attainable when they are consumed with a meal—or immediately after the HP meal, to allow exposure to a substantially higher gastric pH. The control, in capsules, was always consumed immediately after the LP or HP meals to reduce the chance of stomach disturbance. For the control, gastric pH was not a concern, as alliinase had been activated before consumption. Garlic foods (cooked garlic, etc.) and their control were consumed inside the LP meal, with the garlic placed between the two slices of buttered bread and the jam omitted. The special extracts (clear aqueous liquids) were consumed with the LP meal.
2.9. Diet Restrictions
It was essential to prevent dietary interference with the bioavailability test by restricting the consumption of garlic and onions for two days before each test and for the 1.5 days of each test. The restrictions were as follows: two days before a test, only modest amounts of garlic; one day before the test and during the test, no foods containing garlic or onion, with some exceptions. Some prepared foods listing garlic were found to contain too little garlic to cause breath AMS production: Kraft Miracle Whip, Pace Chunky Salsa, and Doritos Cool Ranch Chips. A small amount of cooked onion caused no interference. Prepared foods, such as ketchup, mustard, salad dressing, tomato sauce, and chips, which listed onion as a spice, but not garlic, caused no interference. Participants were discouraged from eating restaurant food unless they were sure garlic and onion were absent from their choices.
On the day of each test, participants were asked to not eat or drink until arriving at the facility, except, if necessary, up to 150 mL water one hour before arrival. After consuming the standard breakfast meal and garlic product, participants were asked to not eat or drink again until two hours later. However, if they felt discomfort during the two hours, small amounts of non-protein food were permitted. Participants were occasionally asked if they had adhered to these restrictions.
Compliance with the diet restrictions was determined by the absence of AMS and the presence of no more than a modest amount of onion sulfides in a breath sample taken immediately before the test product was consumed. Non-compliance after the test began was monitored by a sudden increase in breath AMS concentration at 2–3 h after consumption of the subsequent meals consumed during the test. If non-compliance was determined, participants were asked to stop the test and repeat it at a later date.
2.10. Breath Sampling Procedure
Participants breathed into 1.2-L Tedlar® gas sampling bags (Grace Davison Discovery Sciences, Waukegan, IL, USA), fitted with a septum port, a barbed nickel-plated twist-valve, and a 4-cm length of Tygon® tubing, as a mouthpiece, attached to the valve. They were instructed to exhale a normal breath, starting at the top of the breath (avoiding the natural tendency to first take a deep breath), into the bag, then to empty the bag by flattening it, followed by exhaling a second breath until the bag was at least mostly full and closing the valve. After the flattening, the mouthpiece was placed against the tongue or lip to prevent air from entering the bag until the participant was ready to exhale the second breath. The speed at which one breathes into the bag was found to have no influence on the breath AMS concentration. Participants recorded their name and breath time on each bag.
Whole breath, rather than alveolar breath, was used throughout the study. Although alveolar breath has been shown to contain 18% higher AMS than whole breath [
17], the procedure for sampling alveolar breath was considered too difficult for participants to use away from the laboratory, where most of the 17 breaths per test needed to be collected. Because all tests were conducted in the same manner and because only relative amounts are important, using whole breath was considered sufficient.
2.11. Breath Bag Quality
The average rate of disappearance of AMS from the Tedlar® bags was 0.4%/h (range 0.05–1.05%/h). The disappearance rate for each bag was determined over a 48-h period and only the better bags (<0.50% loss/h) were used for the breaths that needed to be stored overnight before analysis. Except for the overnight breaths, breaths were usually analyzed within 4–6 h after sampling. Following these guidelines, losses were considered insignificant and not corrected. Moisture accumulation in the bags was found to have no effect on the AMS concentration.
2.12. Test Protocol
After an overnight fast, participants came to the facility, breathed into a bag and then consumed the standardized breakfast meal and the garlic product. After consuming the garlic product, participants left the facility with several empty bags and provided breath samples every hour for the next eight hours, then every two hours, except during sleep, until breath AMS was undetectable (typically 24–30 h), but not longer than 32 h. Participants returned to the facility to deliver breath samples and obtain more bags at about noon and 5 pm of the first day and at about 8 am and 2 pm of the second day.
2.13. Breath Analysis for Allyl Methyl Sulfide (AMS)
Breath samples (5 mL) were injected directly (manually with a 5-mL gas-tight syringe fitted with a 0.63 mm side-port needle), one time, into a gas chromatograph (Agilent 6890) fitted with a model 5380 sulfur-selective pulsed flame photometric detector (PFPD, OI Analytical, College Station, TX, USA), PFPD zero-output setting of 10, and a 30 m × 0.32 mm × 4 μm SPB-1 Sulfur (bonded polydimethylpolysiloxane) capillary column (Sigma-Aldrich #24158). Helium was the carrier gas (1.6 mL/min, constant flow). The column temperature was programmed from 45 °C (0.2 min) to 200 °C (1.2 min) at 50°/min, with a hold time of 2 min, giving a retention time of about 4.0 min, a run time of 5 min, and a re-injection time of 11 min. The injection port contained a straight 4 mm borosilicate liner (Agilent #19251-60540) and was operated at 175 °C in the splitless mode, with a purge flow of 45 mL/min for 0.8 min. The detector was operated at 250 °C, with the following flow rates: hydrogen 9.5 mL/min, air 18.5 mL/min, helium (make-up) 5.5 mL/min. Due to injection of an overload volume of 5 mL and a rapid temperature gradient, the baseline did not become stable until 2.9–3.0 min after each injection. Because inserting the column into the detector requires disassembly of the detector and several hours of equilibration time, a 40-cm piece of the column (extension) was attached to the detector, with the other end attached to an Agilent Ultimate Union (G3182-61580). The remainder of the column was then attached to the extension at the union. This allowed for quick removal and replacement of the column for other uses of the gas chromatograph. The PFPD peak area is the square root of the detector response to sulfur. This detector gave about 20 times greater sensitivity (detection limit, 40 area units or 3 ng/L or 1 ppb at s/n = 2) than the FID (flame ionization detector) detector used in prior allicin bioavailability studies [
17], which made it possible to measure the AUC after consuming small amounts of garlic products. AMS is normally absent from human breath [
43].
2.14. AMS Standard Curve
The stock allyl methyl sulfide vapor standard (266 ng/L) was prepared by adding 13.0 μL of a solution of 88 μg allyl methyl sulfide/mL in methanol to duplicate 4.3-L glass jugs with lids fitted with septum ports. The concentration in the jugs reached stability by 2–3 h and remained stable for 24–30 h. Dilutions of this standard gave a curvilinear linear response down to 6 ng/L. Dilutions were prepared by adding various volumes of the stock (0.25–3 mL) and air (4.75–2 mL) to a 5-mL syringe and injecting the 5-mL. For volumes less than 1 mL, the stock was added to the 5-mL syringe using a 1-mL syringe. An air-blank was injected daily. When eight standard curves, created over a 14-month period (see examples in
Figure 3), were applied to a single set of breath data, the resultant AUC was found to vary by only 5% (RSD, relative standard deviation), indicating the stability of the PFPD detector response.
2.15. AMS Concentration Curve (AUC)
The AUC was measured in ng-h/L by determining the AMS concentration of each breath, based on AMS peak areas and interpolated concentration values (Spline Lowess regression) from the standard curve, followed by determining the AUC over 32 h. All of the calculations were determined using GraphPad Prism 5.0 (San Diego, CA, USA) software.
2.16. Allicin Bioavailability or Bioequivalence
The ABB for each product was based on response factors (RF, RF2, RF3) and calculated as relative response factors (RRF, RRF2, RRF3). RF is the ratio of AUC to the μmol of alliin and alliin-derived dithioallyl compounds (AADD, see
Figure 1) consumed. RF2 is the ratio of AUC to the μmol of total known
S-allyl compounds (TKSA) consumed; TKSA is AADD plus GSAC and SAC (G/SAC). RF2 was especially useful when AADD was very low and G/SAC were dominant. RF3 is the ratio of AUC to the weight (g) of product consumed; it was used mainly for “aged” garlic products, such as black garlic, in which most of the alliin has been converted to unidentified
S-allyl compounds. The relative response factors were calculated as 100% times the response factors for the products divided by the response factors for the standard control. When products contained alliinase activity (the supplements and diced raw garlic), allicin bioavailability was determined based on AADD. When the alliinase of garlic products had been inhibited (cooked, acidified), allicin bioequivalence was determined based either on AADD or TKSA. The minimum AUC that could be accurately determined was 15 ng-h/L, when there was a relatively sharp decline in AMS after Tmax was reached (typical when the product had active alliinase, such as with the control and N1). It was about 45 ng-h/L when there was a gradual decline after the Tmax (typical when alliinase was absent, such as with PHFA, AFHG, and alliin). When the product contained active alliinase, ABB as low as 0.7–11% could be determined when the AADD consumed was 320–20 μmol, respectively. When the product did not contain active alliinase, ABB as low as 2–32% could be determined when the AADD consumed was 320–20 μmol.
2.17. Dissolution Allicin Release
The formation and release of allicin from garlic tablets and capsules under simulated gastrointestinal conditions was determined according to the USP-NF (U.S. Pharmacopeia-National Formulary) dissolution method for delayed-release garlic tablets [
29]. Using a model VK 700 dissolution apparatus (Agilent/Varian, Palo Alto, CA, USA) equilibrated at 37 °C, 2–6 tablets or capsules (enough to provide 20–30 mg alliin) were placed into each of two covered 1-L round bottom glass vessels containing 750 mL of 0.1 N HCl and paddle-stirred at 100 rpm for 2 h, after which 250 mL of 0.2 M Na
3PO
4 was added and the pH slightly adjusted, if necessary, giving 1000 mL at pH 6.80 ± 0.05. After stirring the buffered medium for 60 min, 1 mL was added to 0.05 mL of 210 mM (final 10 mM) carboxymethoxylamine (CMA) (Sigma-Aldrich) alliinase inhibitor, followed by high-performance liquid chromatography (HPLC) analysis of allicin. For tablets that had not completely disintegrated in 60 min of buffer, an additional 1 mL aliquot was taken upon complete tablet disintegration. Capsules were loosely wrapped with three winds of plastic-covered wire to keep them from floating. The time to achieve complete disintegration was determined by observation during the dissolution test. The percent dissolution allicin release (DAR) was calculated as the amount of allicin released during the dissolution tests divided by the amount of allicin released when pulverized tablets or capsule contents were incubated 30 min in water to allow complete allicin formation (allicin potential).
2.18. Analysis of Garlic Products
Garlic supplements (tablets and capsule contents) were mortar ground, extracted with 20 mM CMA alliinase inhibitor (50–100 mL/g) for analysis of alliin, GSAC, and SAC, or extracted with 70% acetonitrile (ACN)/30% 40 mM CMA (10 mL/g) for analysis of allyl sulfides. For water activation of alliinase to generate the allyl thiosulfinates, ground tablets or the contents of capsules were mixed with water at 50 mL/g, rotated rapidly for 10 min, centrifuged for 5 min at 1000×
g, followed by addition of one volume of ACN (precipitates protein), centrifuged for 3 min at 16,000×
g, and kept at 4 °C or lower until analyzed [
37]. Commercial garlic foods were extracted with a Polytron homogenizer at speed 6 for 15 s, in the presence of either 20 mM CMA (10 mL/g) for analysis of alliin, GSAC, and SAC or in the presence of 75% ACN/25% water (10 mL/g) for analysis of allyl thiosulfinates and allyl sulfides. The control (raw garlic homogenate) was extracted with 20 mM CMA (20 mL/g) for analysis of alliin, GSAC, and SAC, or with 50% ACN (10 mL/g) for analysis of allyl thiosulfinates, or with 70% ACN (10 mL/g) for analysis of allyl sulfides. Diced raw garlic was extracted with a Polytron homogenizer (speed 6, 15 s) in the presence of 20 mM CMA (25 mL/g) for analysis of alliin, GSAC, SAC, and, after addition of 1 volume of ACN, for analysis of allyl thiosulfinates and allyl sulfides. Roasted or boiled garlic were extracted with a Polytron homogenizer (speed 6, 15 s) in the presence of water (25 mL/g) for analysis of alliin, GSAC, and SAC, or in the presence of 75% ACN (10 mL/g) for analysis of allyl thiosulfinates and allyl sulfides.
Alliin, GSAC, and SAC were analyzed by ion-pair HPLC (250 mm × 4.6 mm, Agilent/Varian Microsorb-MV 100-5 C18 250 mm × 4.6 mm column) at 208 nm by modification of the method of Arnault et al. [
44]. Solvent A consisted of 20 mM sodium heptanesulfonate (ion-pair agent) (Sigma-Aldrich) and 20 mM sodium phosphate monobasic, pH adjusted to 2.1 with concentrated phosphoric acid. Solvent B was ACN. The column was heated at 38 °C with a flow rate of 1.0 mL/min. Gradient: from 0% B to 15% B in 5 min, then to 22% B by 15 min and hold until 17 min, then back to 0% B by 18 min and hold until 24 min. Typical retention times:
l(−)-alliin 8.5 min,
l(+)-alliin 8.8 min, isoalliin 9.1 min, γ-glutamyl-
S-methylcysteine 11.0 min,
S-allylcysteine 13.7 min, γ-glutamyl-
S-allylcysteine 15.9 min, γ-glutamyl-
S-
cis-1-propenylcysteine 17.3 min, γ-glutamyl-
S-trans-1-propenylcysteine 17.5 min, γ-glutamylphenylalanine 18.5 min. The allyl sulfides and allyl thiosulfinates (when their abundance was small) were analyzed, using the same column, isocratically with 70% ACN/30% water at 210 nm, as previously described [
21] (1991 sulfides pub). When the allyl thiosulfinates were abundant, such as with the control, or when the dissolution allicin release and USP potential allicin (allicin potential) were determined, they were analyzed isocratically with 45% ACN/55% water at 240 nm, as previously described [
37].
2.19. Alliinase Assay
Alliinase activity, as μg allicin produced min
−1 g
−1 garlic powder (dry garlic matter), was determined as previously described [
30]. Briefly, the capsule contents or mortar-pulverized tablets, without sieving, were added to water at a concentration of 1 g garlic powder content to 800 mL water, followed by immediate and vigorous shaking for 7–8 s and removal of 1 mL aliquots at 15, 30, 60, and 120 s to 0.05 mL of 210 mM CMA to stop the reaction. After analysis of allicin, the alliinase activity was determined from the time point giving the highest rate of allicin production. If the activity was found to be less than 500, it was redetermined at 200 mL/g garlic powder. The garlic powder content for tablets and capsules was based on label claims.
2.20. Protein Assay
The protein content of extracts PFHA and AFHG were assayed according to the procedure of Bradford [
45], using Brilliant Blue G (Sigma-Aldrich) in methanol as the binding dye and bovine serum albumin (Sigma-Aldrich) as the protein standard (standard curve 10–100 μg/mL).
2.21. Peptide Hydrolysis
To reveal any possible peptide-bound alliin or SAC, both AFHG extract and roasted garlic prepared at 160 °C (homogenized with 10 mL water/g) were incubated while stirring at pH 8.5 at 37 °C for one hour with a large excess of either protease from
Bacillus licheniformis (9.6 units/mg, Sigma-Aldrich) or alkaline protease from
Bacillus subtilis (400 PC units/mg, Bio-Cat, Inc., Troy, VA, USA), followed by one hour of incubation with porcine kidney γ-glutamyl transpeptidase (3.4 units/mg, Sigma-Aldrich). Aliquots were centrifuged at 16,000×
g and directly assayed for alliin, SAC, and GSAC as described in
Section 2.18.
2.22. Statistical Analysis
Statistical analyses were conducted using Microsoft Excel Analysis ToolPak software (Redmond, WA, USA). Student’s t-Test (paired two sample for means; two-tail) was used to determine differences between groups. In a few cases, the two-sample t-Test was used when n was small (≤4). p-Values < 0.05 were considered to be significant. Data are presented as means ± standard deviation (SD).
3. Results
3.1. AMS Peak Resolution and Interference from Onion Sulfides
AMS (retention time 4.0 min) was well-resolved from other volatile sulfur compounds derived from garlic: allyl mercaptan (3.3 min), diallyl sulfide (4.9 min), and diallyl disulfide (6.9 min), although none of these compounds were detected when the breath was sampled at ≥0.5 h after garlic product consumption. AMS was almost baseline separated from XMS (4.2 min), the only volatile sulfur compound detected after consuming 5 g cooked onion. Upon consuming raw onion, only two peaks were found: XMS and YMS (4.1 min). AMS was not well-separated from YMS; hence, it was important to restrict study participants from eating raw onion. Consumption of small amounts of cooked onion did not cause interference. No sulfur compounds were detected in the breath unless garlic or onion had been consumed.
The identities of XMS and YMS were not confirmed by gas chromatography–mass spectrometry (GC–MS); however, XMS is probably
trans-1-propenyl methyl sulfide, which is often found in human breath [
43], and YMS co-eluted with pure n-propyl methyl sulfide (Alfa Aesar, Tewksbury, MA, USA). For cooked onion, these sulfides would be metabolites of isoalliin (
S-
trans-1-propenylcysteine sulfoxide), the main sulfur compound found in onion, and propiin (
S-n-propylcysteine sulfoxide), which is only 3–10% as abundant [
46,
47]. For raw onion, they would be the metabolites of the respective 1-propenyl and propyl thiosulfinates. In contrast to the much greater abundance of isoalliin than propiin in whole raw onion, consumption of raw onion resulted in 6-fold more YMS than XMS. This probably occurred because, after the action of alliinase and lachrymatory factor synthetase upon crushing raw onion, isoalliin is mainly (80–90%) transformed to highly volatile lachrymatory factor (propanethial
S-oxide, CH
3CH
2CH=
S-O) and only a small amount (5–10%) to 1-propenyl thiosulfinates, while propiin is transformed only to propyl thiosulfinates [
24,
46,
47]. It is also possible that 1-propenyl thiosulfinates are metabolized to the methyl sulfides less efficiently than are the propyl thiosulfinates. A small amount of XMS was often seen after consuming garlic products, probably due to the presence of a small amount isoalliin in garlic and the subsequent 1-propenyl thiosulfinates found in crushed raw garlic [
19,
48].
3.2. Example Breath AMS Concentration Curves
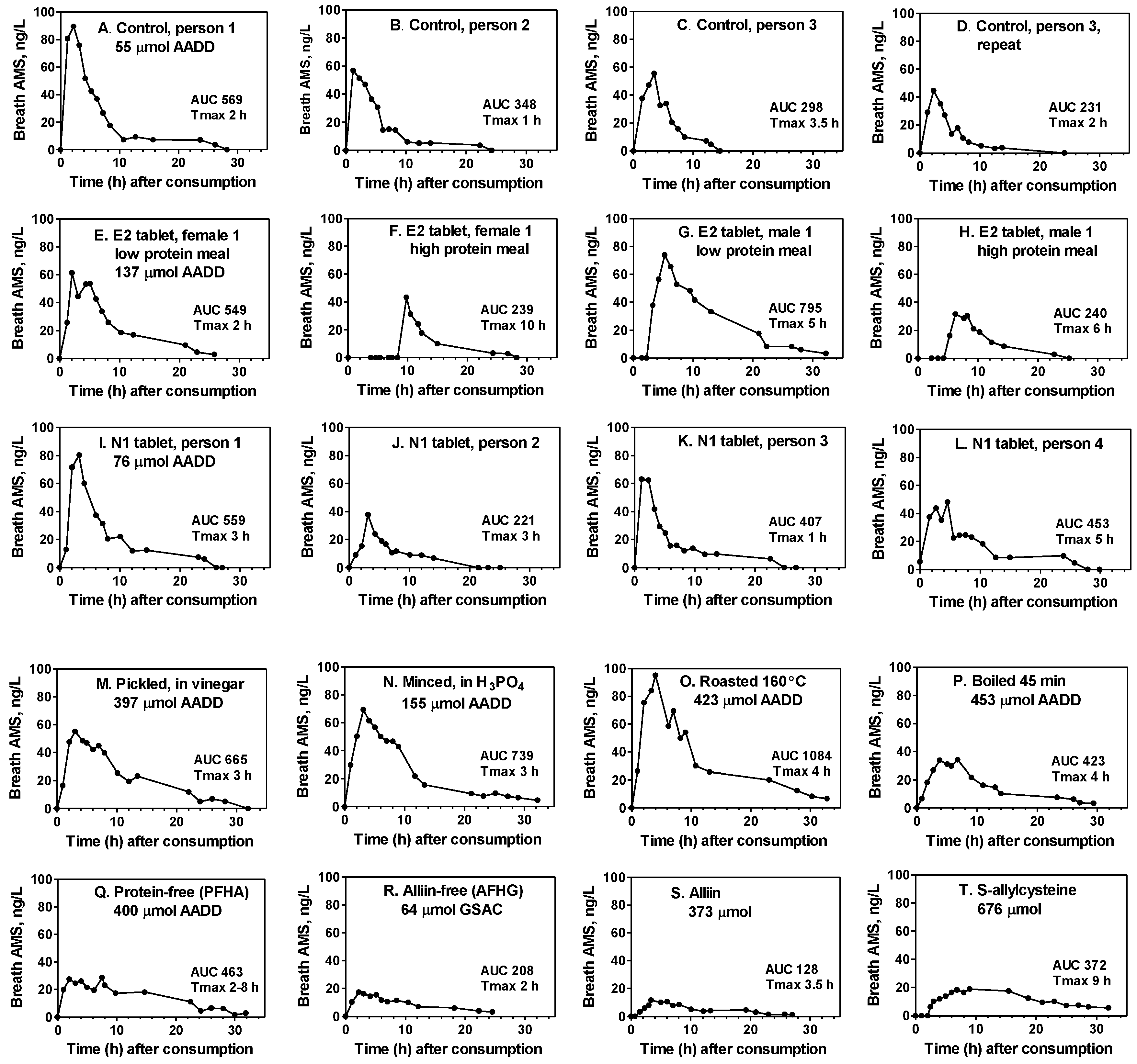
Typical breath AMS concentration curves for several of the products consumed in the study are illustrated in
Figure 4. They depict hourly fluctuations, the Tmax (time to achieve maximum breath concentration), the AUC relative to the amount consumed (AADD), and delays in AMS production (F, G, H, T). They also demonstrate the variation between persons for the standard control (A–C) and N1 tablets (I–L), the variation between duplicate tests by the same person (C and D), and the effect of a high meal protein content on reduced AUC and delayed AMS production when enteric-coated tablets are consumed (E–H). The substantial production of breath AMS, even when active garlic alliinase is not present, is shown for both acid-processed and cooked garlic foods (M–P) as well as for extracts and pure
S-allyl compounds (Q–T).
3.3. Dose Response
The response of the breath AMS-AUC to consumption of 0.35, 0.70, 1.4, and 2.8 g of the raw homogenate control (0.22–1.76 g raw garlic) under high-protein meal conditions was determined for all 13 participants (
Figure 5). The allicin content of the control was 0.87–6.94 mg per dose and was fully present before consumption. The response was dose-dependent (r
2 = 0.9989) and gave high linearity through 0.35 g. Under low-protein meal conditions, only two doses were used, 0.35 and 1.4 g, which closely paralleled the result with the high-protein meal. A dose of 1.4 g with the low-protein meal was chosen as the standard control condition. N1 tablets, where allicin was absent until made in the body, were tested at two doses with a high-protein meal. The response was dose-dependent (r
2 = 0.9975) and moderately linear.
3.4. Experimental Variation
The experimental variation is summarized in
Table 3. The variation (RSD) in the AMS peak area between triplicate injections of the same breath was 3.4% (
n = 12), with no significant difference (
p > 0.4) between breaths containing a high AMS concentration (88 ng/L, peak area 2110 au), a moderate concentration (42 ng/L, peak area 860 au), or a low concentration (10 ng/L, peak area 180 au). The variation between triplicate breaths taken 1 min apart was a little greater, 5.6%, as determined for four persons, and also includes the variation between replicate injections of the same breath. The average variation in the AUC between all 13 persons consuming the same product was 42 ± 7.5% (range 32–63%) for 21 tests with garlic supplements and the control. However, this variation decreased to 32 ± 9.9%, a significant (
p < 0.001) 24% decrease, by comparing the AUC for a product to the control AUC value found for each person, the relative response (AUC/AUC
control). The variation was independent of meal type and supplement type.
The variation in the AUC for duplicate consumption of a garlic product by the same person was determined three times for all 13 participants, which revealed an overall average within-person variation of 16.9%. When 1.4 g of the raw homogenate control was consumed with a low-protein meal, the variation was 14.4 ± 11.5%. When 1.4 g of the raw homogenate control was consumed with a high-protein meal, the variation was 17.4 ± 15.4%. When three N1 tablets were consumed with a high-protein meal, the duplicate variation was 18.7 ± 14.0%. The variation in the average AUC for duplicate sets of tests with the same product, same dose, and same meal type for the 13 persons was 4.7%. Duplicate test sets were conducted three times: 1.4 g of control with the low-protein meal (1.2% RSD), 1.4 g of control with the high-protein meal (3.9% RSD), and three N1 tablets with the high-protein meal (9.0% RSD).
3.5. Composition
The TKSA content of the garlic products consumed in the study is described in
Table 4. Alliin was absent from the homogenized raw garlic used as the control because it had been converted to allicin and other allyl thiosulfinates during homogenization; however, the original alliin content of the cloves—calculated from the allyl thiosulfinates produced—was 154 μmol/g dry wt (60.9 μmol/g fr wt) or 27.3 mg/g dry wt (10.8 mg/g fr wt), which values are close to the average values reported for 46 samples: 156 ± 33 μmol/g dry wt (54.7 μmol/g fr wt) or 27.7 ± 5.8 mg/g dry wt (9.7 ± 2.0 mg/g fr wt) [
49,
50,
51]. Among the garlic supplements and foods, alliin was usually the most abundant
S-allyl compound, typically accounting for 65 to 85% of the total, but it was lower, 30–40%, for products E5, N2, and oil-chopped garlic, while being undetectable in black garlic. The alliin concentration in the garlic powders used in the supplements varied 7.5-fold (6.5–49; 21.0 ± 12.2 mg/g) (
Table 4, second set of alliin values). This range is substantially broader than the 2-fold range consistently found for garlic bulbs among sets of 13, 9, and 24 cultivars [
49,
50,
51]; this indicates variable loss of alliin upon manufacture of garlic powders and validates the need for the allicin potential or alliin standardization label claims found for most brands (
Table 1).
Alliin consists of both the l(+)-isomer and the l(−)-isomer. The l(−)-isomer, which tends to increase with age, constituted <6% of the alliin for most of the products, the exceptions being E4 (8%), E5 (14%), E6 (24%), N1 (8%), N2 (10%), N4 (19%), C2 (9%), C3 (15%), pickled (11%), acid-minced (18%), and oil-chopped (24%). Second in abundance was GSAC, which accounted for 8–35% of the total S-allyl, with E5 and N2 being exceptionally high (57–58%). SAC accounted for 2–6% of the total, except 13% for C3 and 86% for black garlic. Of the total SAC (GSAC + SAC), SAC typically accounted for 1–15%, exceptions being E3 (36%), pickled garlic (44%), and black garlic (87%).
As intended, alliin, allyl thiosulfinates and allyl polysulfides were absent from the AFHG and AFHS extracts, and treatment of AFHG with γ-glutamyl transpeptidase to produce AFHS converted 95% of the GSAC to SAC. Allicin and other allyl thiosulfinates were absent from all supplements because alliinase had not been activated. Upon activation in water (
Section 2.18), the following average yields and ranges were found (
n = 13, not shown in
Table 4): (a) as mg/g garlic powder (powder weight claims are in
Table 1)—allicin 7.8 ± 4.8 (2.4–16.8), allyl methyl thiosulfinates 1.7 ± 1.1 (0.5–4.6), allyl
trans-1-propenyl thiosulfinates 0.3 ± 0.4 (0.01–1.4); (b) as mg/tablet or capsule—allicin 3.6 ± 2.9 (0.3–10.8), allyl methyl thiosulfinates 0.8 ± 0.6 (0.05–2.1), allyl
trans-1-propenyl thiosulfinates 0.2 ± 0.2 (0.002–0.6). The allyl thiosulfinates were absent from homogenized commercial garlic foods because alliinase had been inhibited by the processing acids (vinegar, phosphoric acid, citric acid). They were abundant in the homogenized control, where allicin accounted for 80.3
S-allyl mol % or 70.3 wt % of the allyl thiosulfinates, and they were slightly present in the roasted, boiled, and diced raw garlic preparations. Dicing cloves into 3 mm cubes caused only about 3% of the alliin to be transformed to thiosulfinates, while homogenization in water caused complete transformation.
The allyl polysulfides, which are metabolized to AMS to the same extent as allicin [
17], were significant (>5% of the AADD) only in E5 (14%), acid-minced garlic (14%), and oil-chopped garlic (51%). Oil-chopped garlic uniquely contained cyclic vinyldithiins, 2-vinyl-4
H-1,3-dithiin and 3-vinyl-4
H-1,2-dithiin (5.4 μmol/2.5 g), which are not metabolized to AMS, and a small amount of ajoene (0.1 μmol/2.5 g), which is metabolized to AMS [
17]. Of these products, the diallyl sulfides comprised 82–94% of the total allyl sulfides, with diallyl trisulfide always dominant, as detailed in the footnotes of
Table 4. AMS itself was not found in any product except in oil-chopped garlic (0.7% of the AADD).
3.6. Control: Allicin Bioavailability or Bioequivalence
The response factor (RF) for 1.4 g of the control was used for determining the ABB of all other products. Hence, it was important to establish its variation between duplicate tests, its variation with respect to meal types, its variation with the mode of consumption, and its variation over the duration of the study. The results for the individual duplicate tests and their averages for consumption with the high-protein or low-protein meals are shown in
Table 5. The variation in the AUC and RF between duplicate tests was low for both the high-protein meal (RSD 3.9%) and the low-protein meal (RSD 1.2%). Compared to the low-protein meal, consuming the control with a high-protein meal significantly decreased (
p = 0.023) the RF by 18% (7.37 vs. 6.25), delayed the Tmax by 0.7 h and decreased the Cmax by 25%. Apparently, some of the allicin binds to or reacts with the milk protein or tuna protein or other components in the high-protein meal without forming a reaction product that can be transformed to AMS. The RF was not significantly different between men and women for either the low-protein tests (
p = 0.56) or the high-protein tests (
p = 0.78).
The control was also consumed in the same two modes in which the various garlic products were consumed. Similar to the consumption of supplements, it was consumed in capsules, giving the gastrointestinal tract initial contact with allicin and other garlic compounds. Garlic foods were consumed inside a low-protein sandwich, giving the mouth initial contact with the garlic compounds; hence, the control was also consumed inside the same sandwich. The RF for the control consumed inside the sandwich (
Table 5, test 3) was not found to be significantly different (
p = 0.48) than consumption in capsules (7.31 vs. 6.99). As expected, consumption in a bread sandwich significantly decreased the Tmax from 2.6 h to 1.4 h and doubled the breath AMS concentration at 1 h after consumption (% of Cmax at 1 h, an evaluation of Tmin).
It was critical to the validity of the bioavailability control to consume it under conditions that allowed for maximum allicin availability (maximum RF). Based on the factors presented in
Table 5, the standard control RF used was the value found upon consumption of the control in capsules with the low-protein meal (test 2). Tests 2a and 2b were conducted just prior to the tests with the supplements. Because of the long duration needed to conduct most of the supplement tests, 10 months, an additional standard control test (test 2c) was conducted 9 months after the initial tests. The average RF for all three standard tests (test 2, final = 6.99 ± 2.39) was used for determining ABB for all the garlic products, regardless of the type of meal with which they were consumed.
3.7. Garlic Supplements: Allicin Bioavailability
3.7.1. Dose Basis
The dose used for the supplement bioavailability tests was not based on manufacturer recommendations (
Table 1), which varied from 1–6 units per day (garlic powder range of 0.3 to 2.0 g, average 1.0) or from 1–3 units per meal (garlic powder range of 0.1–1.5 g, average 0.6 g). Rather, it was based on the amount needed to give an AUC value within or near the tested linear range that was found for the control (100–750 ng-h/L,
Figure 5a), as determined from a preliminary test for each product with a person who gave a typical response. It was also desired to consume at least two units to obtain a better average response. In one case, N3, only 1 tablet was consumed because of its very large size (1.8 g) and high AUC. If there was low alliinase activity, a higher dose was used. Typically, the dose consumed was 1–2 times the recommended daily dose or 2–3 times the recommended dose per meal.
3.7.2. Enteric-Coated Tablets
Under low-protein meal conditions, allicin bioavailability from brands E1, E4, and E5 was not significantly different from that of the 100% bioavailability control, but the bioavailability for brands E2 and E3 was significantly lower, especially for E3 (
Table 6). Four of the five brands gave medium to high allicin bioavailability (>65%), while E3 gave moderate bioavailability (35–65%). Even brand E6, which was 12 years old, also gave moderate bioavailability. The delay in tablet disintegration and allicin formation caused by the enteric coatings is indicated by both the Tmax and the Tmin (the first measured time for AMS to appear in the breath), relative to the garlic homogenate control that contained allicin before consumption. However, because the first breath was not sampled until one hour after consumption, the true Tmin is not known for any garlic product with a Tmin of less than 1 h. The true Tmin for the control was determined for one person by taking breath samples every 6–8 min for the first hour and was found to be 26 min if consumed immediately after the low-protein meal or 15 min if consumed immediately before the same meal. As learned from the % of Cmax at 1 h, which was 35–50% among products with a designated Tmin of less than about 1 h, it is apparent that the true Tmin for these products is indeed less than 1 h. The Tmin for the low-protein meal was delayed, relative to the control, by about 1.5–2.0 h for brands E1 and E2, with similar delays for the Tmax. However, for brands E3, E4, and E5, the Tmin and Tmax were not significantly different from the control, indicating that the coatings of these products did not delay tablet disintegration in vivo, even though that was their intended purpose.
Three of the EC brands, E1, E2, E3, were also consumed with the high-protein meal. All three gave significantly lower bioavailability than the low-protein control and significantly lower bioavailability than the same product consumed with the low-protein meal. Brands E2 and E3 gave low bioavailability (<35%) with the high-protein meal. The Tmin and Tmax for E1 and E2 were increased (delayed) by 4–8 h relative to the control and by 3–5.6 h compared to consumption with the low-protein meal. Even for product E3, which gave no delay in the Tmin and Tmax for the low-protein meal, the high-protein meal caused a 1.5 h delay in the Tmin and a 2 h delay in the Tmax. For E1, the decreased bioavailability and greatly delayed (by 7 h) tablet disintegration time caused by the high-protein meal (54 g tuna in whole wheat sandwich plus milk, 31 g total protein), were not caused by the low-protein meal (no tuna, 5.5 g total protein). Prior studies with E1 showed that these effects were not caused by moderate protein meals (25 g tuna in whole wheat sandwich plus water, 13 g total protein; or 280 g yoghurt, 10 g total protein), where allicin bioavailability was 90–94%, the Tmax was 6–6.3 h and the delay in Tmax, relative to the control, was 2.9–3.1 h [
17,
37].
The alliinase activity of each product is also given in
Table 6. For the six brands of enteric tablets consumed with the low-protein meal, there was no correlation between alliinase activity (26-fold range) and allicin bioavailability (2.9-fold range) (r
2 = 0.07) or the Tmax (1.9-fold range) (r
2 = 0.02). The products with both the highest (E1 and E4) and the lowest (E5) alliinase activity also gave the highest bioavailability, possibly indicating that the lowest observed alliinase activity was sufficient to allow for maximum conversion of alliin to allyl thiosulfinates. When E1, E2, and E3 were consumed with the high-protein meal, there was a moderate correlation (r
2 = 0.92) between alliinase activity and allicin bioavailability, but it was not significant (
p = 0.094). A correlation between type of enteric coating agent (
Table 2) and allicin bioavailability could not be established. Brands E1 and E6 did not list their coating agents. Sodium alginate was used by the brands that gave both the highest (E5) and lowest (E3) bioavailability. Hence, the variation found among brands of enteric tablets appears to be more related to unknown manufacturing procedures than to coating agents or alliinase activity.
3.7.3. Normal (Non-Enteric) Tablets
Allicin bioavailability from all of the normal tablets was found to be high, even though they disintegrated rapidly and their alliinase activity varied 30-fold (
Table 6). Only the N1 tablets, when consumed with the high-protein meal, gave significantly lower bioavailability (73%) than the control; however, when an additional 225 mL of water was consumed with the meal, the bioavailability increased insignificantly to 85%, and the difference from the control disappeared. Even N4 tablets (same brand as N1), which were consumed 19 years after their manufacture, gave high bioavailability. Two brands, N1 and N2, were consumed with both the low-protein and high-protein meals. Unlike with enteric tablets, the high-protein meal did not significantly decrease allicin bioavailability. Normal tablets rapidly achieved maximum allicin production. The Tmin was not significantly different than the controls for any of the tests with normal tablets, while the Tmax was significantly longer only for N1 consumed with the high-protein meal and for the old N4, but the delay was only 0.6–1.3 h.
3.7.4. Capsules
Although capsules were expected to give very low allicin bioavailability (<5%), due to their assumed rapid disintegration in stomach acid at a low pH, the three brands tested gave a broad range of bioavailability (26–109%,
Table 6). For capsules containing fine particles (C1 and C2,
Table 1), the difference in bioavailabilities (54% and 26%) appears to be due to differences in alliinase activity (10,840 and 210). However, products C2 and C3 had similar alliinase activity (210 and 250) with large differences in bioavailability (26% and 109%), even though they were made by the same manufacturer and had almost the same listed ingredients (
Table 1 and
Table 2). The only known difference between C2 and C3 is that C3 was substantially more coarse and darker in color (
Table 1). It appears that the larger particle size of C3 offered more protection against gastric acid inhibition of alliinase, although such a dramatic protection seems unexpected. The results also indicate that alliinase activity as low as about 250 μg allicin min
−1 g
−1 garlic powder, if protected, is sufficient to allow for maximum possible allicin formation from alliin. Consumption of C1 and C2 with the high-protein meal, rather than with the low-protein meal, had no effect on allicin bioavailability. Capsules disintegrated and produced allicin almost as rapidly as the controls, with the Tmax values being an average of 0.35 h longer with the low-protein meal and 0.77 h longer with the high-protein meal.
3.8. Garlic Supplements: Gender Comparisons
No significant gender differences for allicin bioavailability or the Tmax were found for the raw homogenate control or for any of the supplements, with two exceptions: the Tmax when enteric E1 and E2 were consumed with a high-protein meal (
Table 7). The Tmax is a close measure of their disintegration times, as breath AMS levels were undetectable for enteric tablets at 1–2 h before the Tmax was reached. Very long disintegration times (Tmax 11–24 h) were found with the high-protein meal for 4 of 6 women (av 18 h) and 1 of 7 men (11 h) consuming E1 and for 2 of 6 women (av 15 h) and 0 of 7 men consuming E2. When E1 and E2 were consumed with the low-protein meal, very long disintegration times were found only for 1 woman (18 h) consuming E1. Similarly, consumption of enteric-coated aspirin tablets with a meal (22 g protein, estimate) has been found to give substantially longer gastric residence times (8.4 h vs. 3.5 h) and plasma salicylate Tmin (10.8 h vs. 5.0 h) for women than for men (Tmax not reported). However, when the aspirin tablets were simply consumed with water and no food, both the gastric residence time (0.8 h), Tmin (2.7 h), and Tmax (8.3 h) were much shorter and showed no gender differences [
52].
3.9. United States Pharmacopeia (USP) Dissolution Allicin Release (In Vitro) Compared to Actual Allicin Release (In Vivo)
3.9.1. Enteric-Coated Tablets
The dissolution test (
Table 8) revealed that the quality of the coating of all tested brands of enteric tablets was sufficient to prevent tablet disintegration after 2 h in acid, as required in the USP monograph [
29]. In fact, all brands had the appearance of being undisturbed at the end of the 2-h acid stage, except that E4 tablets were swollen and blistered and E6 had one small blister per tablet. After entering the buffer stage (pH 6.8), three brands (E1, E2, E5) disintegrated rapidly, within the time specified (1 h) in the USP monograph, while the other brands (E3, E4, E6) disintegrated much slower, requiring 2.5–5.7 h. However, the average Tmax for breath AMS with the low-protein meal was similar for both sets of tablets (~4 h), indicating that they disintegrated at similar average rates in the body. Considerably higher dissolution allicin release (%) was found for brands with rapid disintegration times than for those with slow disintegration times (88 ± 5% vs. 5 ± 2% at 1 h in buffer or 88 ± 5% vs. 18 ± 9% at the time of complete disintegration), even though the average alliinase activity for both groups was similar. However, in vivo, there was a smaller non-significant difference between the same groups (88 ± 18% vs. 53 ± 18%,
p = 0.07). Only one brand (E1) met the USP requirement of releasing at least 90% of the label claim (
Table 1) for potential allicin.
For the rapidly disintegrating tablets (E1, E2, E5), there was moderately good agreement between percent dissolution allicin release and percent allicin bioavailability, when consumed with the low-protein meal (ratio near 1). Overall, the USP in vitro dissolution test was partly accurate for predicting in vivo results when enteric tablets were consumed with a low-protein meal, but not when consumed with a high-protein meal (
Table 6). That is, when dissolution allicin release, as percent of allicin potential, was high (>80%), allicin bioavailability was medium to high (69–104%, av 88%) with the low-protein meal and moderate (32–57%, a 45%,
Table 6) with the high-protein meal. Furthermore, for the slowly disintegrating tablets (E3, E4, E6), the actual allicin bioavailability (36–71%, av 53%) was about 11 times greater than that predicted (av 5%) by the USP dissolution method. Hence, the USP dissolution method is not reliable for predicting disintegration times in vivo or allicin bioavailability from enteric tablets.
3.9.2. Normal (Non-Enteric) Tablets
Although the USP dissolution method was designed for enteric (delayed-release) tablets, it was prepared under the assumption that only acid-resistant enteric tablets would be able to provide high allicin release in the body. The method does present the general simulated gastrointestinal conditions to which all supplements are exposed. Hence, the same conditions were applied to normal tablets and capsules, where two hours of acid exposure should strongly inhibit alliinase activity and substantially decrease allicin release. As expected, all of the brands tested under dissolution conditions (N1, N2, N3) completely disintegrated in the 2 h acid stage (1.5–1.75 h) and dissolution allicin release was small (6–17%), even though alliinase activity ranged from low to high (
Table 8). However, the in vivo allicin bioavailability averaged 95% with the low-protein meal, an average of eight times greater than expected based on the dissolution test.
3.9.3. Capsules
Because capsules contain loose powder and shells that dissolve quickly, they were expected to undergo rapid disintegration, resulting in nearly complete inactivation of alliinase, and very low dissolution allicin release. The results for the three brands tested were partly unexpected (
Table 8). Only C3 disintegrated rapidly in the acid stage (0.25 h), perhaps because it uniquely consisted of coarse particles, while C2 required the full 2 h in acid and C1 required the acid stage and 1 h of the buffer stage. Hence, C3 and C2 fully disintegrated in the acid stage, resulting in alliinase inhibition and undetectable levels of allicin release, as expected, while C1 released nearly half of its potential allicin.
Actually, the shells of C1 and C2 did disintegrate rapidly in the acid stage, but in both cases the capsule contents, rather than immediately disintegrating upon loss of the shell, congealed into a solid mass that disintegrated slowly. Three other brands of garlic powder capsules were also observed for their disintegration patterns in the acid stage (not shown); only one disintegrated rapidly (5 min; this product contained predominantly rice powder), while the other two also formed congealed masses that disintegrated slowly, by 30 and 60 min in acid. Hence, congealing of the garlic powder in the acid stage is a consistent pattern among garlic powder capsules comprising mainly fine garlic powder and is independent of other ingredients in the capsule; C1 listed no other ingredient (
Table 2), yet it congealed the most. When tested in water, all four of the capsule products that had congealed in acid disintegrated about 4-fold faster; hence, the congealing is probably the effect of acid on the protein of fine garlic powder. In vivo, however, congealing appears to not occur. This conclusion is based on observation that similar times (5–12 min) were found for the occurrence of side effects associated with allicin release after capsule disintegration (garlic burp, heart burn, gastric disturbance) after consuming capsules C1 and C3.
There was not a consistent correlation between dissolution allicin release and allicin bioavailability of the capsules. Although the 42% allicin release for C1 agreed well with the 53% allicin bioavailability, the brands that gave undetectable allicin release gave 26–109% bioavailability. A large difference in disintegration times between C1 and C3 was found in vitro, but no difference was found in vivo, based on Tmax times for the high-protein meal.
3.10. Garlic Foods: Allicin Bioequivalence
Garlic foods prepared in the kitchen are usually made by the cooking of raw cloves or bulbs, such as by roasting or boiling or stir-frying, all methods of which completely inhibit alliinase. It is also common to simply dice (cut into small cubes) raw garlic and add it to various foods, a method which does not inhibit alliinase. Commercial garlic foods are normally treated with an organic acid or phosphoric acid, which acidity (pH 3.6–4.3) fully inactivates alliinase [
14], especially under long-term storage. However, for garlic products that are cut into small pieces (the acid-minced and oil-chopped products), alliinase is active during chopping process, allowing for small to modest amounts of allyl thiosulfinates to form, depending upon the extent and coarseness of the chopping. After formation, the allyl thiosulfinates are gradually transformed at ambient temperature to allyl polysulfides.
An attempt was made to predict the amount of AMS produced by the body after consuming these garlic foods, especially when alliinase has been inhibited. It has been shown that allyl thiosulfinates account for >90% of the AMS produced when crushed raw garlic is consumed [
17]. This was demonstrated by comparing the breath AMS produced upon consuming crushed raw garlic (all of the alliin previously converted to allyl thiosulfinates) with the AMS produced upon consuming pure allicin. Furthermore, it has been shown that allyl thiosulfinates and allyl polysulfides produce equimolar amounts of breath AMS [
17]. Hence, it should be possible to predict the breath AMS-AUC for any garlic product, including alliinase-inhibited garlic, based on the content (
Table 4) or expected yield (if alliinase is active) of allyl thiosulfinates and allyl polysulfides, relative to the raw garlic homogenate control. These predictions assume, as indicated from crushed raw garlic, that no other
S-allyl compound present in garlic is metabolized to significant amounts of AMS. They also assume that the body does not contain significant endogenous alliinase activity. However, these predictions were found to be incorrect (
Table 9). Typical concentration curves are found in
Figure 4M–P.
As expected, nearly all of the alliinase-inhibited garlic foods produced substantially less AMS (smaller RF) than did the homogenized raw garlic control; the exceptions were oil-chopped and acid-minced garlic, due to their relatively high content of allyl polysulfides (
Table 4). Nevertheless, the amount of AMS (AUC) produced from alliinase-inhibited garlic foods was 5–25 times higher than predicted (see ratio: actual/predicted)—except for oil-chopped garlic—giving an allicin bioequivalence of 14–34% for cooked garlic and 19–79% for acidified commercial products. Overall, these results demonstrate that the body can produce substantial amounts of AMS from
S-allyl compounds in garlic by a mechanism that is independent of garlic’s alliinase activity, but this mechanism appears to be significant only when garlic’s alliinase activity is inhibited.
3.10.1. Cooked Garlic
The intensity and duration of cooking made little difference in the allicin bioequivalence. Roasting at 215 °C (internal temperature 101 °C) for 60 min did not produce significantly less AMS than roasting at 160 °C (internal temperature 92 °C) for 30 min. Similarly, boiling (internal temperature 95 °C) for 45 min reduced allicin bioequivalence from 18% to only 14% (
p < 0.05), compared to boiling for 4 min. However, the method of cooking made a difference. The roasted preparations gave about two times (
p < 0.01) as much allicin bioequivalence as either of the boiled preparations. This was unexpected, as the internal temperature for roasting at 215 °C was higher than that which was found upon boiling. The difference is probably due to the higher content of allyl polysulfides in roasted garlic (
Table 4).
3.10.2. Acidified Garlic Products
The acid-minced and the oil-chopped commercial garlic products gave high allicin bioequivalence (66–79%) because of major tissue disruption before alliinase was inhibited by acidification. Both products were minced by an industrial process that was probably harsh (unlike the careful use of a razor blade for the diced raw garlic in
Table 9). Based on visual examination, the oil-chopped garlic product also appears to have been partially crushed. These industrial procedures gave the products a desired and substantial garlic flavor. The comparatively low levels of alliin and high levels of allyl polysulfides in acid-minced and oil-chopped products (allyl polysulfides were 14% and 50% of the AADD, respectively, compared to <5% for pickled garlic, cooked garlic, and most of the supplements,
Table 4) validate the substantial amount of tissue disruption that occurred in the preparation of these products. However, when undisturbed whole cloves were suspended in acid (pickled garlic), both allyl polysulfides and allicin bioequivalence were low.
3.10.3. Tmin and Tmax
Although the Tmin for the alliinase-inhibited garlic foods could not be determined (less than the first measure, 1 h), the % of Cmax achieved at 1 h provides an estimate of Tmin. To improve the accuracy of comparison to the control, the control values for % Cmax at 1 h and Tmax shown in
Table 9 are for the “in sandwich” control (
Table 5, test 3), rather than for the “in capsules” control (the standard for AUC and RF), because the garlic foods were also consumed inside sandwiches; the % Cmax at 1 h and Tmax are dependent on the initial time of body contact, which is delayed when consumed in capsules. The % Cmax at 1 h for the garlic foods was found to be 19–31%, indicating that AMS production began about 30 min after consumption. The Tmax for almost all of the garlic foods (3.1–4.4 h) was significantly longer than that of the in-sandwich control (1.3–1.8 h,
Table 9). This difference was expected, due to (a) the lack of allicin and alliinase activity in the garlic foods, (b) the immediate contact of allicin with the body (starting in the mouth) for this control, and (c) the fact that allicin is lipophilic, readily passing through epithelial cell membranes, in contrast to the lipophobic alliin and γ-glutamyl-
S-allylcysteine, the main
S-allyl sources in the garlic foods. The similarity among the Tmax values for the alliinase-inhibited garlic foods indicates that they all have a similar and moderately effective mechanism by which AMS is produced.
3.10.4. Diced Raw Garlic
For finely diced raw garlic, where active alliinase and about 97% of the initial alliin content (prior to cutting) were still present, predicting the amount of AMS production is not as straightforward as it is with crushed or homogenized raw garlic. This is because of the unpredictable amount of allyl thiosulfinates produced by alliinase upon chewing the diced garlic (about 60 cubes) in the bread and butter sandwich. It appears that consuming the sandwich caused only a small percent of the garlic cells to have ruptured, exposing alliin to alliinase. When the seven participants were asked if they felt a “garlic burn” in the throat, which burn is caused by allicin, only one person reported a noticeable burn, one that was mild. In a separate test, when a single cube was chewed without other food, there was an immediate and strong burn. Hence, only minor crushing of the garlic cubes occurred during consumption of the sandwich.
The significantly longer Tmax for the diced garlic, compared to the control (3.1 vs. 1.3 h), and its high allicin bioavailability, demonstrate that garlic alliinase was active enough to convert most of the alliin to allyl thiosulfinates after entering the stomach. This was possible because the gastric pH was likely elevated to at least 4.0 for about 0.5 h by the buffering effect of the meal [
28]; alliinase has some activity at pH 4.0 and maximum activity at pH 4.5 [
14]. The amount of AMS produced upon consuming the diced garlic was close to that which was predictable based on the assumption that all of the alliin was converted to allyl thiosulfinates (ratio: actual AUC/predictable AUC = 0.8,
Table 9). This confirms prior evidence that alliin-derived compounds account for nearly all of the AMS produced from garlic products in which active alliinase is present [
17].
3.10.5. Black Garlic
Black garlic is produced by incubating garlic bulbs at medium heat (60–90°C) and high humidity (80–90%) for 30–40 days, a process loosely referred to as “fermenting” or “aging,” during which the cloves turn black due to the Maillard reaction [
53,
54]. The process eliminates the sharp taste (mainly due to allicin) associated with raw garlic and replaces it with a sweet taste due to the reactions between sugars and free amino acids. Because of the virtual absence of AADD in black garlic (
Table 4), the relative production of AMS from this garlic food gave an impossibly high value for RRF (285/7.2 = 3960%; RF = 228/0.8 = 285). If the allicin bioequivalence for black garlic is based on the remaining known
S-allyl compounds in garlic (G/SAC), it would give a high RRF2 value (4.87/6.8 = 72%; RF2 = 228/46.8 = 4.87), which also seems excessive and indicates the presence of unknown
S-allyl compounds. Because whole garlic bulbs were heated for a long time, alliinase was never significantly activated; hence, the absence of alliin in black garlic indicates that alliin was converted to new compounds unrelated to allicin, the identity of which is unknown.
If the new compounds could be identified and quantified, the TKSA value would increase, giving lower values for RF2 and RRF2. It is possible to estimate the amount of new
S-allyl compounds produced, based on the alliin loss. Although the initial content of alliin in the bulbs used to prepare the black garlic is not known, if one assumes it to be the average value among 46 garlic varieties, 55 μmol alliin/g fresh weight [
49,
50,
51], then the amount of new
S-allyl compounds generated from alliin would have been 561 μmol (55 × 10.2 g consumed). Hence, the new value for TKSA would be 608 μmol (47 μmol G/SAC plus 561 μmol new
S-allyl compounds), giving a much lower RF2 (228/608 = 0.38) and much lower allicin bioequivalence of 6% (100% × 0.38/6.8). Furthermore, when allicin bioequivalence was estimated based simply on the weight (RRF3) of black garlic consumed, it was found to give a value of 5% (22.4/448), as calculated from the product RF3 (228/10.2 g = 22.4) and the control RF3 (394/0.88 g = 448). The RRF3 values for both roasted garlic products (35% and 32%, respectively) and both boiled garlic products (17% and 17%) were similar to their RRF2 values (
Table 9), indicating the validity of estimating allicin bioequivalence on a weight basis. The agreement between RRF3 and the new estimated RRF2 indicates that the allicin bioequivalence of black garlic is close to 5% and that the new alliin-derived compounds are only poorly metabolized to AMS.
The new
S-allyl compounds are most likely the products of the Maillard reaction [
53] between reducing sugars and alliin. The Maillard reaction may have also occurred to a lesser extent with G/SAC. A 25% loss of G/SAC has been reported when black garlic is produced [
53]. Further evidence for a decrease in the content of G/SAC during the manufacture of the black garlic used in the current study is that its content (4.5 μmol/g) is below the range found for 13 varieties of garlic (26.2 ± 9.0 μmol/g fresh wt, range 8–38) [
49].
3.10.6. Aged Garlic Extract
Also tested was a commercial aged garlic extract supplement (not shown) that has some similarities to and differences from black garlic. It is sold under the name of Kyolic Aged Garlic Extract and is manufactured by Wakunaga of America, Mission Viejo, California, USA. Both black garlic and Kyolic AGE are promoted as “aged” garlic products; both contain
S-allylcysteine (derived from GSAC) as their main identified
S-allyl compound; neither contains active alliinase; and both contain little or no alliin or alliin-derived thiosulfinates or sulfides. While black garlic consists of whole bulbs incubated in heated, moist air for 30–40 days, Kyolic AGE is made by incubating sliced raw garlic in 20% ethanol for up to 20 months at ambient temperature [
55,
56]. The extract has been used in a large number of experimental and clinical studies.
A single test for allicin bioequivalence was conducted, in which nine tablets of Kyolic Formula 100 were consumed with the standard meal. The product label claims that each tablet contains 300 mg of “Aged Garlic Extract Powder (bulb)”. The composition of the product (same lot number) has been previously reported [
37]. Nine tablets contained 0.86 μmol alliin, 16.8 μmol SAC, and 12.4 μmol GSAC, but no allyl sulfides (<0.2 μmol), giving a TKSA value of 30.1. The breath AMS curve revealed a Tmax of 2 h (Cmax = 8 ng/L; 31% of Cmax at 1 h), a plateau at Tmax that lasted about 10 h before returning to baseline at 20 h, and an AUC of 92 ng-h/L. The RF2 was 3.1 (92/30.1), while that for the control was 8.3 (543/65.5,
Table 10), giving an allicin bioequivalence (RRF2) of 37% (3.1/8.3), which was higher than expected. When allicin bioequivalence was based on the weight of product consumed (RRF3), it was found to be only 2% (34/1551), as calculated from the product RF3 (92/2.7 g dry garlic = 34) and the control RF3 (543/0.35 g dry wt = 1551). As with black garlic, the large discrepancy between RRF2 and RRF3 (37% vs. 2%) indicates that much of the
S-allyl (mainly alliin) lost during the aging process was converted to unidentified
S-allyl compounds that were partly metabolized to AMS.
3.11. Special Garlic Extracts and Pure Compounds: Allicin Bioequivalence
The unexpectedly high results found for the allicin bioequivalence of alliinase-inhibited garlic foods (
Table 9) indicates that the body itself contains either (a) substantial alliinase activity; (b) the ability to partially reactivate inactivated garlic alliinase; or (c) enzymes that can metabolize the
S-allyl compounds in garlic (alliin, GSAC, SAC) to AMS without using allicin as an intermediate. In order to examine these possibilities, AMS production was examined after consuming pure alliin or pure SAC, as well as extracts in which the protein had been removed (PFHA) or the alliin selectively removed (AFHG). The results are summarized in
Table 10.
3.11.1. Alliin
After consuming
l(+)-alliin in a sandwich, breath AMS was detectable at 0.2 h, reached Cmax at 3–3.5 h and gradually decreased almost to baseline by about 27 h (
Figure 4S). About 5% of the alliin was metabolized to AMS (RRF2). This represents the maximum contribution that intestinal bacterial alliinase could make to in vivo production of allicin from alliin. Two tests with synthetic
l(±)-alliin were also conducted with the same person who consumed
l(+)-alliin (not shown). No significant differences in the Tmax, RF2, or RRF2 values were found between natural
l(+)-alliin and synthetic
l(±)-alliin.
3.11.2. S-Allylcysteine (SAC)
After consuming SAC, breath AMS (measured initially every 30 min) was undetectable until 2.5 h, reached Cmax at about 8 h and gradually decreased almost to baseline by about 35 h (
Figure 4T). About 6% of the SAC was metabolized to AMS (RRF2). The difference in Tmin between alliin (0.2 h) and SAC (2.5 h) indicates a difference in metabolic pathways, which is even more prominent in the difference in the Tmax values (3.2 h vs. 8.4 h;
Table 10). In a previous report, breath AMS was not detectable after consuming a similar amount of AMS (730 μmol) because the method used was about 20 times less sensitive [
17].
3.11.3. Diallyl Sulfide (DAS)
Diallyl sulfide (DAS), a monosulfide, is not present in crushed raw garlic [
21,
37]. It is typically less than 3% of the small amount of sulfides found in garlic powder products (
Table 4), and it is only about 2% of the large amount of sulfides found in commercial steam-distilled garlic oil [
21]. Although its abundance is minor in garlic products, it has been included in
Table 10 because it has been used in a large number of experimental studies [
57,
58]. After consuming DAS, breath AMS was undetectable until 4 h, reached Cmax at about 6 h and remained at Cmax (plateau) for about 23 h, and became undetectable at about 35 h (not shown). About 3% of the DAS was metabolized to AMS (RRF2). Possible impurities in DAS that are known to be fully metabolized to AMS are diallyl disulfide and allyl mercaptan [
17]; however, analysis of the DAS used in the study revealed that allyl mercaptan was undetectable (<0.037%) and that the diallyl disulfide content was only 0.04%. Hence, impurities are not responsible for the breath AMS found after DAS consumption. A previous failure to detect AMS in the breath after DAS consumption was due to the use of a less-sensitive detector [
17].
3.11.4. Protein-Free, High-Alliin Garlic Extract (PFHA)
It was shown in
Table 9 that AMS production from alliinase-inhibited garlic products is much greater than expected, based on the amount of alliin-derived dithioallyl compounds present. One possible explanation for these results, although unlikely, is that the body might be partially re-activating alliinase. To examine this possibility, an extract (PFHA) was prepared in which 97% of the protein of boiled garlic was removed, without reducing alliin. Alliinase is among the most abundant proteins in garlic and constitutes about 10% of the total clove protein [
25,
59]; hence, removal of 97% of the protein likely removed virtually all of the alliinase, and any amount remaining would have been inactive. Upon consuming PFHA, breath AMS was detectable in less than 0.25 h, reached 48% of Cmax in 1 h, reached Cmax in 3.6 h and remained at Cmax until about 8 h after consumption, followed by a gradual decline to near zero by 30–32 h (
Figure 4Q). About 15% of the TKSA (RRF2) was metabolized to AMS (
Table 10). Because alliinase and alliin-derived
S-allyl compounds were absent in this product, and because GSAC and SAC have been shown to be metabolized to AMS at equal efficiencies (see product AFHS), the expected AUC, based on the response factors for pure
l(+)-alliin and pure SAC and their content, would be 152 ng-h/L (399 × 0.38) for alliin plus 35 ng-h/L (71.4 × 0.49) for SAC, for a total of 187. In reality, the AUC was found to be 2.5-fold higher (472 ng-h/L). Similar enhanced ratios were found for boiled garlic, roasted garlic and pickled garlic (av 2.4, not shown). The nature of this enhancing factor is unknown, but it is unlikely to be a protein. The similarity found in the RRF2 values for PFHA (14.7) which contains almost no protein, and boiled garlic (14–18,
Table 9), where protein has not been removed, demonstrates that the inactivated alliinase present in these garlic foods was not reactivated in the body to a measurable extent.
3.11.5. Alliin-Free, High Γ-Glutamyl-S-Allylcysteine (GSAC) Extract (AFHG)
An extract (AFHG) void of alliin and alliin-derived compounds was prepared for the purpose of determining the effect of the remaining known
S-allyl compounds (G/SAC) in garlic on breath AMS production. AFHG and PFHA were made from the same small batch of bulbs and extracted at the same ratio (14 mL water/g clove). Different amounts were consumed (57 mL and 68 mL) to obtain the same amount of G/SAC. Similar to consuming PFHA, consumption of AFHG resulted in breath AMS that was detectable in less than 0.25 h, reached 50% of Cmax in 1 h, and reached Cmax in 2.9 h, followed by a gradual decline to near zero at about 24–25 h (
Figure 4R). The loss in AMS production, due to removal of alliin in preparing AFHG, was determined by comparing the allicin bioequivalence (RF3) of PFHA and AFHG on the basis of the weight of garlic clove in the consumed extracts: 4.9 g for PFHA and 4.1 g for AFHG. The RF3 for PFHA was 97 (AUC 472/4.9 g), while the RF3 for AFHG was 50 (AUC 203/4.1 g). Hence, only about 50% (50/97) of the allicin bioequivalence of PFHA was lost upon removal of alliin, demonstrating that about 50% of the AMS production from PFHA was due to G/SAC and that the body is capable of metabolizing significant amounts of G/SAC to AMS when alliinase is inhibited.
Because GSAC and SAC are the only known
S-allyl compounds in this extract, and because they have been shown to be metabolized to AMS at equal efficiencies (see product AFHS), the expected AUC for AFHG was calculated—based on the response factor for pure SAC and the content of G/SAC—to be 35 ng-h/L (70.6 × 0.49). In reality, the AUC was found to be 5.8-fold higher than expected (203 ng-h/L,
Table 10), giving a response factor (RF2) of 2.85 for G/SAC when they are present in garlic, compared to an RF value of 7.2 for the allyl thiosulfinates in the raw homogenate control (
Table 9). As with PFHA and alliin, something is enhancing the metabolism of G/SAC to AMS in the absence of active alliinase, and it is more effective in doing so for G/SAC than it is for alliin.
3.11.6. Alliin-Free, High-SAC Extract (AFHS)
The kinetic difference between AMS production from GSAC or SAC in garlic was examined after converting 95% of the GSAC in AFHG to SAC with added γ-glutamyl transpeptidase. The kinetics of the AMS concentration curve for AFHS was essentially identical to that of AFHG (Tmin <0.25 h, 43% of Cmax reached in 1 h, Cmax reached in 2.5 h, followed by gradual decline to near zero in 23–24 h). Both products gave the same RRF2 value (
Table 10). Hence, GSAC and SAC are metabolized to AMS with equal efficiency, which also indicates that the body is highly efficient at cleaving GSAC to form SAC. Surprisingly, conversion of the GSAC to SAC did not change the Tmax (2.4 h), even though the Tmax for pure SAC was 8.4 h. Hence, the factor that is enhancing the amount of SAC being metabolized to AMS is also enhancing the rate of AMS formation from SAC.
3.11.7. AFHG Plus Added SAC
To test the proposal that something in enzyme-inactivated garlic is enhancing the percent of metabolism of SAC to AMS, pure SAC was added to AFHG, followed by consumption. Because the addition of 141 μmol pure SAC to the AFHG increased the total SAC content three-fold (from 70.6 to 212 μmol), the AUC for this product was expected to be three-fold higher than for AFHG alone, or 636 ng-h/L (3 × 212). In reality, the AUC was found to be only 277 ng-h/L (
Table 10), which is only about 30% higher than for AFHG. This low increase is either due to a highly non-linear dose response or something else is involved.
Although AFHG was consumed at only one dose, consumption of another alliinase-inhibited garlic product, acid-minced garlic (
Table 9), at 7 g and 20 g, gave a fairly linear response (AUC/wt ratio: 1.2) in the AUC range of 740 to 2600 ng-h/L (not shown). Hence, non-linearity is unlikely to be the reason for the low response to the added SAC. The AUC value of 277 ng-h/L for AFHG + SAC is very close to the calculated value of 281 ng-h/L that is found if the added SAC was metabolized to AMS in an unenhanced manner [AUC from AFHG (212) and from added pure SAC (141 μmol × 0.49, RF) = 281)]. Hence, the factor in garlic that enhances the metabolism of SAC to AMS appears to be present only in sufficient quantity to enhance the amount of G/SAC naturally present in garlic, an unlikely situation.
3.11.8. Search for Unknown S-Allyl Compounds
Rather than the proposed presence of an enhancing factor to explain the higher than expected amounts of breath AMS from alliin-free or alliinase-inhibited garlic, another explanation could be the possible presence of substantial amounts of previously undiscovered
S-allyl compounds, such as peptides containing
S-allylcysteine,
S-allylcysteine sulfoxide (alliin), γ-glutamyl
S-allylcysteine, or γ-glutamyl
S-allylcysteine sulfoxide. To explore this possibility, both AFHG extract and roasted garlic (prepared at 160 °C) were incubated with a large excess of either protease or alkaline protease, followed by incubation with γ-glutamyl transpeptidase (see
Section 2.21). Protease alone caused no change in alliin, SAC, or GSAC. Alkaline protease alone or γ-glutamyl transpeptidase alone caused complete depletion of the free GSAC present before incubation and increased SAC only by the amount expected from hydrolysis of the free GSAC, with no change in alliin. Combining the proteases with γ-glutamyl transpeptidase also did not increase the amount of SAC beyond that expected from hydrolysis of the free GSAC, also with no change in alliin. Hence, no hidden or unknown
S-allyl sources were revealed by this hydrolysis protocol. Thus, the real reason for the enhanced metabolism of SAC to AMS in PFHA, AFHG, AFHS, and alliinase-inhibited garlic foods cannot be explained based on the present work and remains unknown.
3.12. Accounting for the AMS Formed upon Consumption of Alliinase-Inhibited Garlic Products
To determine the individual contribution of alliin and G/SAC to the breath AMS content after consuming alliinase-inhibited garlic foods, their in-garlic response factors were determined, in contrast to using the response factors found after consuming the pure compounds (0.38 for alliin and 0.49 for SAC,
Table 10). Based on the results with AFHG, where GSAC and SAC are the exclusive known
S-allyl compounds, the in-garlic response factor for G/SAC, in the absence of active alliinase, was found to be 2.85 (
Table 10), a 5.8-fold enhancement (2.85/0.49). Based on the results with PFHA, where alliin is 85% of the known
S-allyl compounds, the in-garlic response factor for alliin, after correcting for the amount of GSAC and SAC present, was calculated to be 0.67 (
Table 11, footnote 2) when alliinase is inactive, a 1.8-fold enhancement (0.67/0.38), in contrast to a response factor of 7.2 when alliinase is active.
An important concern is whether or not enough is known about the composition and response factors for the
S-allyl compounds in alliinase-inhibited garlic foods to be able to approximately predict the AMS-AUC values. As shown in
Table 11, the average predicted AUC was 93% of the actual, compared to 19%, when only the alliin-derived allyl thiosulfinates and allyl polysulfides (AllylTS/Sx) were included. A range of 72 to 133% indicates that some of the actual AUC may be unaccounted for, especially for roasted garlic and acid-minced garlic, or that it is in the range of experimental or interpretive error. Alternatively, perhaps the in-garlic response factors for G/SAC and alliin are not the same in each of these garlic foods as it is for AFHG or PFHA, due to different methods of preparation. However, a prediction that is at least 72% accurate represents a reasonably good understanding of the
S-allyl sources and their response factors.
Using the in-garlic response factors, it was found that alliin, AllylTS/Sx, and G/SAC accounted for an average of 36%, 19%, and 39% (total 94%) of the actual AUC. If all contribution from G/SAC had been eliminated, alliin and AllylTS/Sx would have accounted for only 55% of the actual AUC, demonstrating that GSAC and SAC are important contributors to breath AMS for alliinase-inhibited garlic products. Additionally, if the response factors found for pure SAC and pure alliin had been applied, G/SAC and alliin would have accounted for only 27%, rather than 75% of the actual AUC. This would have left most of the actual AUC unaccounted for and demonstrates the importance and approximate accuracy of the in-garlic response factors.
3.13. Accounting for the AMS Formed upon Consumption of Alliinase-Active Garlic Supplements
Because G/SAC contributed about 40% of the breath AMS found after consuming alliinase-inhibited garlic foods (
Table 11), it can no longer be assumed that G/SAC makes no contribution to the AMS found after consuming garlic products that have active alliinase, even though SAC is known to not be a substrate for alliinase [
26]. The data in
Table 12, arranged in order of decreasing allicin bioavailability (RRF), attempts to explain the extent to which the various
S-allyl sources contribute to the breath AMS found after consuming garlic supplements containing active alliinase. Determining the contribution of each
S-allyl source is more complex than it is for alliinase-inhibited garlic foods. The complexity is mainly due to the different levels of protection of alliinase from gastric acid among supplements. Alliinase protection appears to vary with coating type, buffering capacity of the garlic powder, the influence of added excipients (
Table 2) [
30], particle size of garlic powders used in capsules (
Section 3.7.4), and reversibility of alliinase activity inhibition when the local gastric pH does not go below 3.0–3.5 [
14]. With enteric-coated tablets, additional variability is caused by the effect of meal protein content on allicin bioavailability (
Table 6). A further significant complication is the unknown extent to which the metabolism of G/SAC to AMS is enhanced when alliinase activity is inhibited to variable degrees, which enhancement has been demonstrated to be 5.8-fold when alliinase is fully inactivated prior to consumption, as with cooked or acid-treated garlic foods (
Section 3.11.5).
The contribution of each
S-allyl source to the observed AMS-AUC has been determined based on the in-garlic response factors for alliin, allyl polysulfides, and G/SAC, but the values for these response factors for alliin and G/SAC are not straightforward. The RF for alliin has been calculated (
Table 12, footnote 2) based on the RF for allyl polysulfides (7.2), which is independent of alliinase activity, and the RF for G/SAC, which value is uncertain, especially when alliinase inhibition is substantial. Two values are proposed in
Table 12 for RF
G/SAC: the unenhanced value of 0.49, which is used when the allicin bioavailability is >50% (above the line in
Table 12), and a four-fold enhanced value of 2.0 when alliinase inhibition is substantial, but not complete, as indicated by allicin bioavailability <40%. The proposed RF
G/SAC value of 2.0 is less than the value found (2.85) when alliinase is completely inhibited. The in-garlic response factor for alliin (RF
alliin) was found to vary for each product, but it generally declined according to the in vivo inhibition of alliinase, as indicated by the percent allicin bioavailability. The RF
alliin can also be used to calculate (
Table 12, footnote 3) the percent conversion of alliin to AMS, which value closely reflects the percent allicin bioavailability.
When the allicin bioavailability was >50% (presumed RF
G/SAC = 0.49,
n = 10), alliin and the small amounts of allyl polysulfides—formed during manufacture of the garlic powders—accounted for an average of 95% of the AMS-AUC. In these cases, the contribution from G/SAC was small (1–10%, average 5%), which was true for all brands of normal tablets, for 4 of 5 brands of enteric tablets consumed with the low-protein meal, and for 2 of 3 brands of capsules. When the allicin bioavailability was <40% (presumed RF
G/SAC = 2.0,
n = 4), the contribution from G/SAC was elevated to 13–21% (av 15%), excluding capsule brand C2, which was exceptionally high at 77%, presumably due to its very low initial alliinase activity (
Table 6), the fineness of the garlic powder, and perhaps to its exceptionally high content of G/SAC (
Table 4). Hence, G/SAC is not a significant contributor to breath AMS from garlic supplements, except when inhibition of alliinase activity substantially reduces allicin bioavailability.
5. Conclusions
The bioavailability of allicin from garlic powder supplements containing alliin and active alliinase can be as high as that from an equivalent amount of crushed raw garlic containing maximum allicin, when consumed with a meal. This was found to be true for all tested brands of normal (non-enteric) tablets, including the main garlic powder supplement, Kwai (N1), which has been used in a large number of clinical trials on the possible health benefits of garlic and validates the results of these trials as representing about 1 g of crushed raw garlic. Compared to non-enteric tablets, allicin bioavailability from enteric tablets was found to be much more variable (36–104%) and to be strongly reduced (22–57%) when consumed with a high-protein meal, due to delayed gastric emptying. The in vitro USP dissolution allicin release test was found to be unreliable for predicting allicin bioavailability from enteric tablets and to be only 20% accurate for predicting allicin bioavailability from non-enteric tablets and capsules, validating the importance of determining allicin bioavailability in vivo. Based on the allicin metabolite equivalence found for the 13 garlic powder supplements tested, a person would need to consume 0.7 to 27 tablets/capsules to obtain the same equivalence as consuming 2 g of crushed raw garlic; however, the recommended daily dose by the supplement manufacturers varied from 1–6 tablets/capsules per day, highlighting the need for more complete standardization. With a more correct understanding of allicin bioavailability, better standards are now being recommended for garlic powder supplements being used in future clinical trials and being consumed by the general public, which standards include a dosage form with sufficient allicin bioavailability and alliin content or allicin potential to represent a daily dose of 2–4 g of typical crushed raw garlic (
Section 4.6).
Consumption of alliinase-inhibited cooked garlic was found to give higher than expected allicin bioequivalence, with AMS formation being about 30% (roasted garlic) or 16% (boiled garlic) that of crushed raw garlic. Hence, any health benefits found for raw garlic or garlic supplements representing raw garlic can probably be achieved by consuming higher amounts of cooked garlic, with the likely exception of antimicrobial effects in the gastrointestinal tract. The mechanism by which AMS can be formed when alliinase is inactive was explored. After selective removal of alliin and alliin-derived compounds from boiled garlic, allicin bioequivalence decreased by only about 50% (AFHG vs. PFHA), indicating that the body possesses the ability to partly metabolize G/SAC to AMS, an ability which is enhanced about 6-fold when alliinase is inhibited. The metabolism of alliin to AMS was found to be enhanced about 2-fold, in the absence of active alliinase, compared to consuming pure alliin. Using in-garlic response factors, it was found that alliin and G/SAC contribute about equally to the breath AMS found after consuming alliinase-inhibited cooked garlic, but when alliinase is active, such as for garlic supplements having high allicin bioavailability, G/SAC accounts for only about 5% of the AMS. Possible mechanisms for the metabolism of G/SAC and alliin to AMS in the absence of active alliinase have been proposed but not experimentally verified. Unanswered questions for future research on the metabolism of alliinase-inhibited garlic include (a) identity of the metabolic pathways from alliin and SAC to AMS when alliinase is inactive and (b) the mechanism by which the metabolism of alliin, and especially SAC, to AMS is enhanced when alliinase is inactive.