Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review
Abstract
1. Introduction
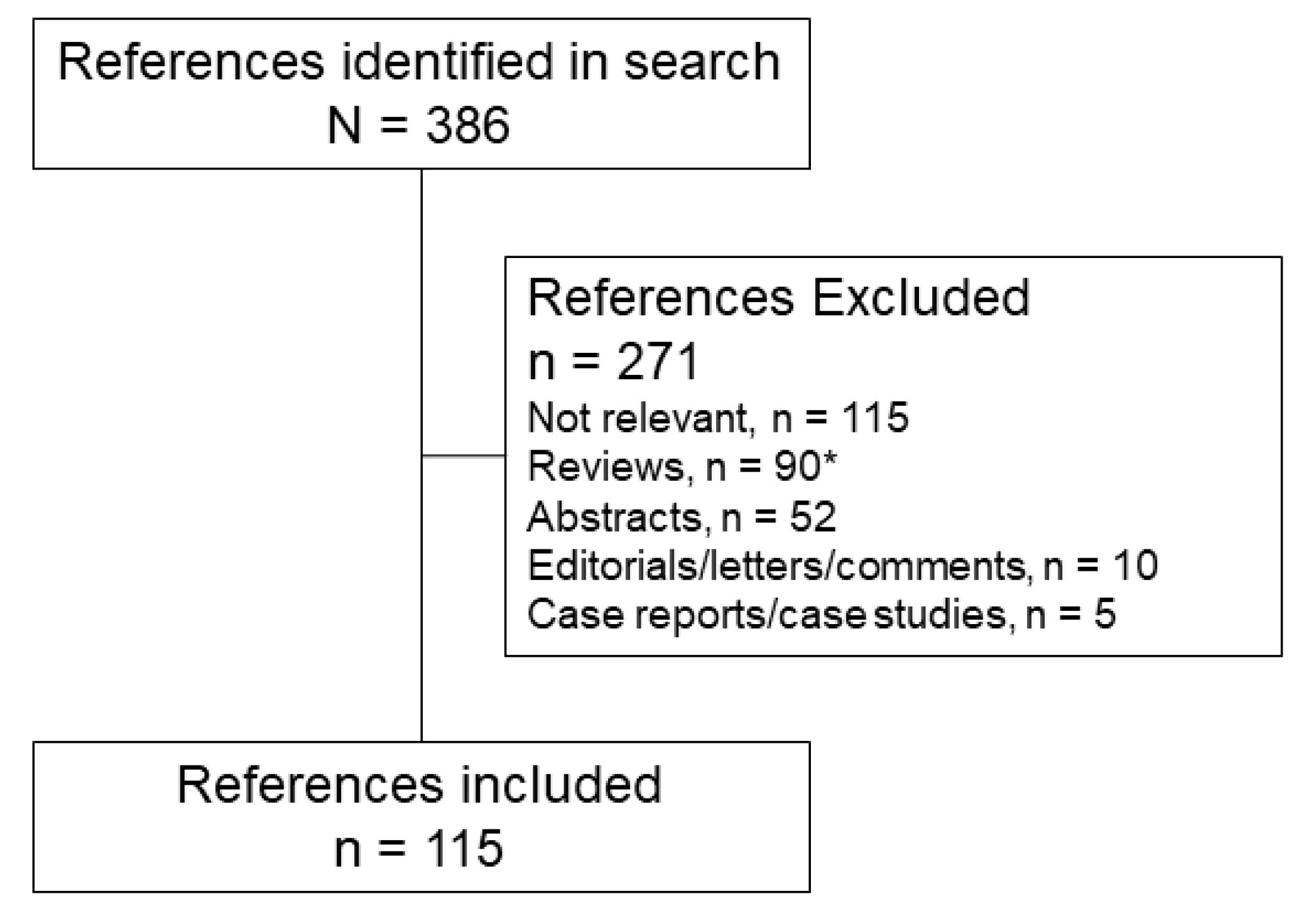
2. Literature Search
2.1. Literature Search Strategy
2.2. Literature Search Results
3. Immune Function
3.1. Immune Response
3.2. Inflammation
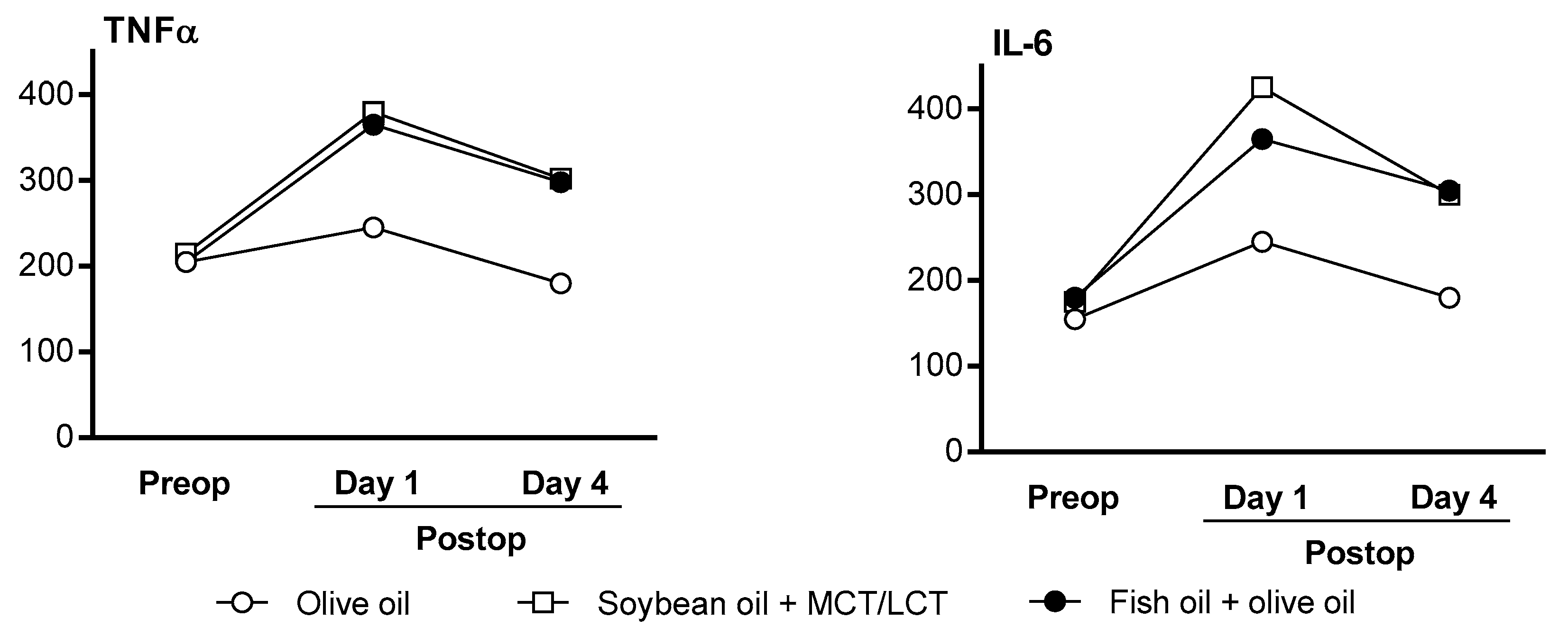
3.2.1. Inflammation Marker Profiles in Adult Clinical Studies
3.2.2. Inflammation Marker Profiles in Pediatric Clinical Studies
3.3. Infections
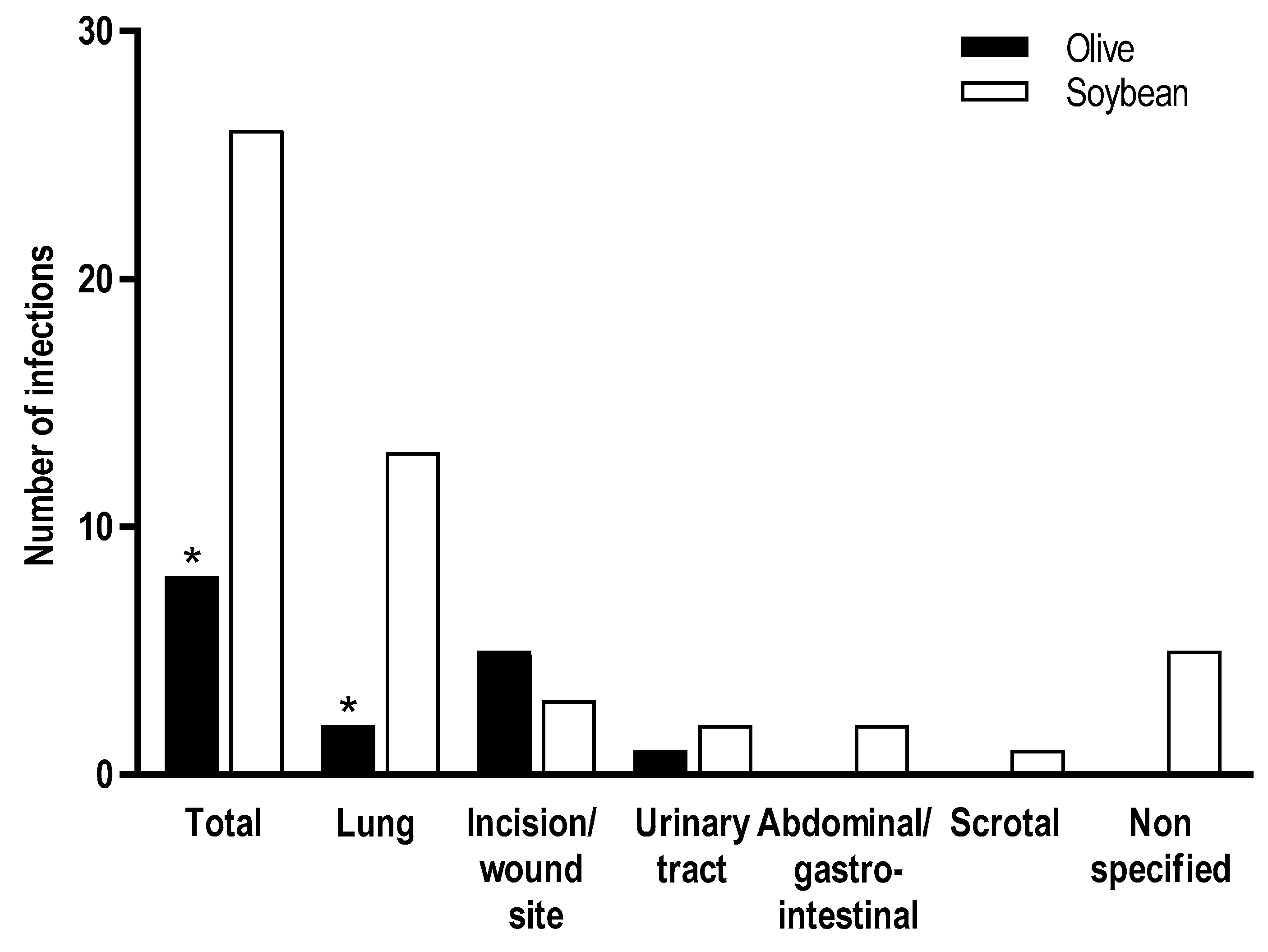
3.3.1. Infection Rates in Adult Clinical Studies
3.3.2. Infection Rates in Pediatric Clinical Studies
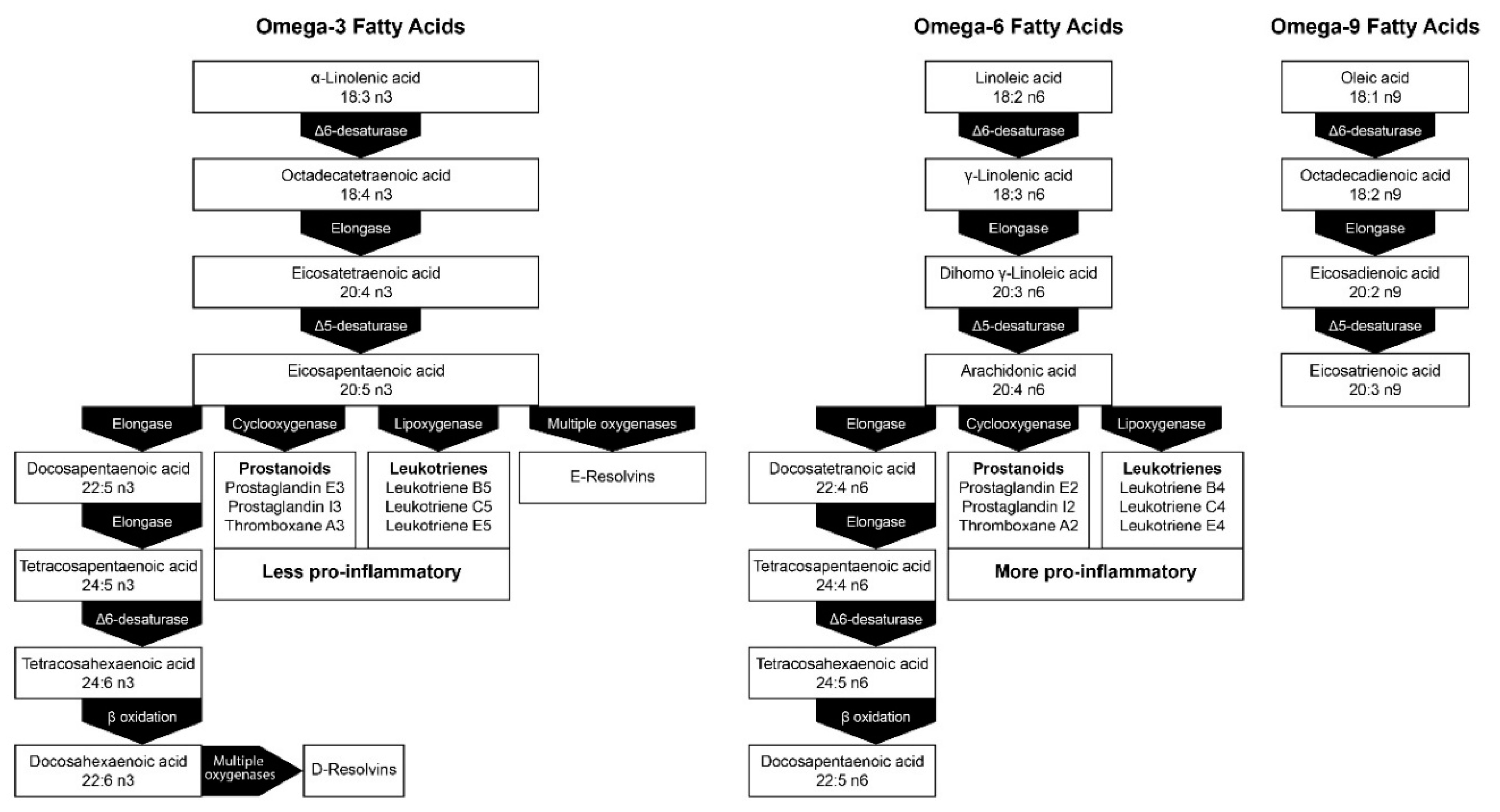
4. Lipid Peroxidation
5. Metabolic Effects
5.1. Lipid Metabolism
5.1.1. Plasma Lipid Levels in Adult Clinical Studies
5.1.2. Plasma Lipid Levels in Pediatric Studies
5.2. Glucose Metabolism
5.3. Emerging Issues Associated with the Fatty Acid Composition of ILEs
6. Liver Function
- Impaired hepatic secretion of fatty acids and triglycerides as VLDL
- Increased synthesis of hepatic triglycerides due to increased intake of n-6 PUFAs and low intake of n-3 PUFAs
- Impaired hepatobiliary secretion leading to cholestasis, possibly resulting from phytosterols present in lipid emulsions and competition of transport owing to differences in phytosterol content
- Impaired hepatobiliary function due to endotoxin entry into the portal circulation or sepsis
- Modulation of oxidative stress and inflammation by peroxidation of PUFAs, increased intake of n-6 PUFAs, and differences in α-tocopherol content
- Lack of enteral nutrition and enteral-stimulated gut growth factors, which may in turn lead to alterations in the gut microbiome.
6.1. Hepatobiliary Function in Adult Studies
6.2. Hepatobiliary Function in Pediatric Studies
7. Endothelial Function
8. Clinical Outcomes
9. Stability
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singer, P.; Berger, M.M.; Van den Berghe, G.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C. ESPEN Guidelines on Parenteral Nutrition: Intensive care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Pontes-Arruda, A. Biological benefits of an oleic acid-rich lipid emulsion for parenteral nutrition. Clin. Nutr. Suppl. 2009, 4, 19–23. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Jew, S.; Jones, P.J. Health benefits and evaluation of healthcare cost savings if oils rich in monounsaturated fatty acids were substituted for conventional dietary oils in the United States. Nutr. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Cavia Mdel, M.; Alonso-Torre, S. Role of oleic acid in immune system; mechanism of action: A review. Nutr. Hosp. 2012, 27, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.; Xu, Z.; Walker, C.; Pavlina, T.; McGrath, S.; Zaloga, G.; Siddiqui, R. Parenteral lipid emulsions in guinea pigs differentially influence plasma and tissue levels of fatty acids, squalene, cholesterol, and phytosterols. Lipids 2014, 49, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Eritsland, J. Safety considerations of polyunsaturated fatty acids. Am. J. Clin. Nutr. 2000, 71, 197S–201S. [Google Scholar] [CrossRef] [PubMed]
- Buenestado, A.; Cortijo, J.; Sanz, M.-J.; Naim-Abu-Nabah, Y.; Martinez-Losa, M.; Mata, M.; Issekutz, A.C.; Marti-Bonmati, E.; Morcillo, E.J. Olive oil-based lipid emulsion’s neutral effects on neutrophil functions and leukocyte-endothelial cell interactions. JPEN J. Parenter. Enter. Nutr. 2006, 30, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Wanten, G.J.; Calder, P.C. Immune modulation by parenteral lipid emulsions. Am. J. Clin. Nutr. 2007, 85, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Wanten, G.J.A. Parenteral lipids in nutritional support and immune modulation. Clin. Nutr. Suppl. 2009, 4, 13–17. [Google Scholar] [CrossRef]
- Xu, Z.; Harvey, K.; Pavlina, T.; Dutot, G.; Zaloga, G.; Siddiqui, R. An improved method for determining medium- and long-chain FAMEs using gas chromatography. Lipids 2010, 45, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Harvey, K.A.; Pavlina, T.; Dutot, G.; Hise, M.; Zaloga, G.P.; Siddiqui, R.A. Steroidal compounds in commercial parenteral lipid emulsions. Nutrients 2012, 4, 904–921. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Harvey, K.A.; Pavlina, T.M.; Zaloga, G.P.; Siddiqui, R.A. Tocopherol and tocotrienol homologs in parenteral lipid emulsions. Eur. J. Lipid Sci. Technol. 2015, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Linseisen, J.; Hoffmann, J.; Lienhard, S.; Jauch, K.W.; Wolfram, G. Liver function and plasma patients receiving TPN with an omega-3-fatty acid-containing lipid emulsion supplemented with alpha-tocopherol. Clin. Nutr. 2000, 19, 177–184. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, R.; Savini, S.; Biagetti, C.; Bellagamba, M.P.; Marchionni, P.; Pompilio, A.; Cogo, P.E.; Carnielli, V.P. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clin. Nutr. 2014, 33, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Ling, P.R.; Andersson, C.; Bistrian, B.R. Hepatic indicators of oxidative stress and tissue damage accompanied by systemic inflammation in rats following a 24-hour infusion of an unstable lipid emulsion admixture. JPEN J. Parenter. Enter. Nutr. 2009, 33, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Antebi, H.; Wolf, C.; Talbotec, C.; Alcindor, L.G.; Corriol, O.; Lamor, M.; Colomb-Jung, V. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: A single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2010, 34, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Grimm, H.; Mertes, N.; Goeters, C.; Schlotzer, E.; Mayer, K.; Grimminger, F.; Furst, P. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur. J. Nutr. 2006, 45, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Antebi, H.; Mansoor, O.; Ferrier, C.; Tetegan, M.; Morvan, C.; Rangaraj, J.; Alcindor, L.G. Liver function and plasma antioxidant status in intensive care unit patients requiring total parenteral nutrition: Comparison of 2 fat emulsions. JPEN J. Parenter. Enter. Nutr. 2004, 28, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.Y.; Yang, J.; Xia, Y.; Tong, D.N.; Zaloga, G.P.; Qin, H.L.; Qiu, Z.; Li, W.; Jiang, X.; Peng, B.; et al. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: A prospective, randomized, multi-center clinical trial in China. Nutr. J. 2015, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P. Monounsaturated fatty acids in parenteral nutrition; evaluation of risks and benefits. Br. J. Nutr. 2005, 94, 867–868. [Google Scholar] [CrossRef] [PubMed]
- Cury-Boaventura, M.F.; Gorjao, R.; de Lima, T.M.; Fiamoncini, J.; Torres, R.P.; Mancini-Filho, J.; Soriano, F.G.; Curi, R. Effect of olive oil-based emulsion on human lymphocyte and neutrophil death. JPEN J. Parenter. Enter. Nutr. 2008, 32, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Blum, S.; Rossle, C.; Le Boucher, J.; Malnoe, A.; Dutot, G. Effects of parenteral lipid emulsions with different fatty acid composition on immune cell functions in vitro. JPEN J. Parenter. Enter. Nutr. 2000, 24, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.; Smiley, D.; Newton, C.; Le, N.-A.; Gosmanov, A.R.; Spiegelman, R.; Peng, L.; Osteen, S.J.; Jones, D.P.; Quyyumi, A.A.; et al. Substitution of standard soybean oil with olive oil-based lipid emulsion in parenteral nutrition: Comparison of vascular, metabolic, and inflammatory effects. J. Clin. Endocrinol. Metab. 2011, 96, 3207–3216. [Google Scholar] [CrossRef] [PubMed]
- Juttner, B.; Kroplin, J.; Coldewey, S.M.; Witt, L.; Osthaus, W.A.; Weilbach, C.; Scheinichen, D. Unsaturated long-chain fatty acids induce the respiratory burst of human neutrophils and monocytes in whole blood. Nutr. Metab. 2008, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Mateu-de Antonio, J.; Grau, S.; Luque, S.; Marin-Casino, M.; Albert, I.; Ribes, E. Comparative effects of olive oil-based and soyabean oil-based emulsions on infection rate and leucocyte count in critically ill patients receiving parenteral nutrition. Br. J. Nutr. 2008, 99, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, K.; Poeschl, J.; Braach, N.; Hudalla, H.; Kuss, N.; Frommhold, D. The olive oil-based lipid clinoleic blocks leukocyte recruitment and improves survival during systemic inflammation: A comparative in vivo study of different parenteral lipid emulsions. Mediat. Inflamm. 2015, 2015, 757059. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Garnacho-Montero, M.C.; Garcia-Garmendia, J.L.; Perez-Paredes, C.; Moyano-Del Estad, M.R.; Barrero-Almodovar, A.; Jimenez-Jimenez, F.J. Effects of three intravenous lipid emulsions on the survival and mononuclear phagocyte function of septic rats. Nutrition 2002, 18, 751–754. [Google Scholar] [CrossRef]
- Versleijen, M.W.; Roelofs, H.M.; te Morsche, R.H.; Simonetti, E.R.; Hermans, P.W.; Wanten, G.J. Parenteral lipids impair pneumococcal elimination by human neutrophils. Eur. J. Clin. Investig. 2010, 40, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Le, H.D.; Meisel, J.A.; de Meijer, V.E.; Gura, K.M.; Puder, M. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2009, 81, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Nanhuck, R.M.; Doublet, A.; Yaqoob, P. Effects of lipid emulsions on lipid body formation and eicosanoid production by human peripheral blood mononuclear and polymorphonuclear cells. Clin. Nutr. 2009, 28, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.-M.; Scheer, O.; Muller, C.D.; Pinna, G.; Duclos, B.; Baumann, R. In vitro modulation of inflammatory cytokine production by three lipid emulsions with different fatty acid compositions. Clin. Nutr. 2004, 23, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Badía-Tahull, M.B.; Llop-Talaveron, J.M.; Leiva-Badosa, E.; Biondo, S.; Farran-Teixido, L.; Ramon-Torrell, J.M.; Jodar-Masanes, R. A randomised study on the clinical progress of high-risk elective major gastrointestinal surgery patients treated with olive oil-based parenteral nutrition with or without a fish oil supplement. Br. J. Nutr. 2010, 104, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Demirer, S.; Sapmaz, A.; Karaca, A.S.; Kepenekci, I.; Aydintug, S.; Balci, D.; Sonyurek, P.; Kose, K. Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann. Surg. Treat. Res. 2016, 91, 309–315. [Google Scholar] [CrossRef] [PubMed]
- García-de-Lorenzo, A.; Denia, R.; Atlan, P.; Martinez-Ratero, S.; Le Brun, A.; Evard, D.; Bereziat, G. Parenteral nutrition providing a restricted amount of linoleic acid in severely burned patients: A randomised double-blind study of an olive oil-based lipid emulsion v. medium/long-chain triacylglycerols. Br. J. Nutr. 2005, 94, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Olthof, E.D.; Roelofs, H.M.J.; Versleijen, M.W.J.; Te Morsche, R.H.M.; Simonetti, E.R.; Hermans, P.W.M.; Wanten, G.J.A. Long-term olive oil-based parenteral nutrition sustains innate immune function in home patients without active underlying disease. Clin. Nutr. 2013, 32, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Olthof, E.D.; Roelofs, H.M.J.; Fisk, H.L.; Calder, P.C.; Wanten, G.J.A. No clinical or biochemical evidence for essential fatty acid deficiency in home patients who depend on long-term mixed olive oil- and soybean oil-based parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2016, 40, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Onar, P.; Yildiz, B.D.; Yildiz, E.A.; Besler, T.; Abbasoglu, O. Olive oil-based fat emulsion versus soy oil-based fat emulsion in abdominal oncologic surgery. Nutr. Clin. Pract. 2011, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Reimund, J.M.; Rahmi, G.; Escalin, G.; Pinna, G.; Finck, G.; Muller, C.D.; Duclos, B.; Baumann, R. Efficacy and safety of an olive oil-based intravenous fat emulsion in adult patients on home parenteral nutrition. Aliment. Pharmacol. Ther. 2005, 21, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Spiegelman, R.; Zhao, V.; Smiley, D.D.; Pinzon, I.; Griffith, D.P.; Peng, L.; Morris, T.; Luo, M.; Garcia, H.; et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit. Care Med. 2012, 40, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Oguz, S.S.; Celik, I.H.; Erdeve, O.; Uras, N.; Dilmen, U. The metabolic effects of two different lipid emulsions used in parenterally fed premature infants—A randomized comparative study. Early Hum. Dev. 2012, 88, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Gawecka, A.; Michalkiewicz, J.; Kornacka, M.K.; Luckiewicz, B.; Kubiszewska, I. Immunologic properties differ in preterm infants fed olive oil vs. soy-based lipid emulsions during parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2008, 32, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Koksal, N.; Kavurt, A.V.; Cetinkaya, M.; Ozarda, Y.; Ozkan, H. Comparison of lipid emulsions on antioxidant capacity in preterm infants receiving parenteral nutrition. Pediatr. Int. 2011, 53, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Savini, S.; D’Ascenzo, R.; Biagetti, C.; Serpentini, G.; Pompilio, A.; Bartoli, A.; Cogo, P.E.; Carnielli, V.P. The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: A randomized clinical trial. Am. J. Clin. Nutr. 2013, 98, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, K.-J.; Tang, Q.-Y.; Hong, L.; Feng, Y.; Lu, L.-N.; Wang, W.-P.; Cai, W. Effect of an olive oil-based lipid emulsion compared with a soybean oil-based lipid emulsion on liver chemistry and bile acid composition in preterm infants receiving parenteral nutrition: A double-blind, randomized trial. JPEN J. Parenter. Enter. Nutr. 2016, 40, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, Y.; Lu, L.-N.; Wang, W.-P.; He, Z.-J.; Xie, L.-J.; Hong, L.; Tang, Q.-Y.; Cai, W. The effects of different lipid emulsions on the lipid profile, fatty acid composition, and antioxidant capacity of preterm infants: A double-blind, randomized clinical trial. Clin. Nutr. 2016, 35, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Cury-Boaventura, M.F.; Gorjao, R.; de Lima, T.M.; Piva, T.M.; Peres, C.M.; Soriano, F.G.; Curi, R. Toxicity of a soybean oil emulsion on human lymphocytes and neutrophils. JPEN J. Parenter. Enter. Nutr. 2006, 30, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, R.; Cavinato, S.; Giangreco, M.; Grana, G.; Centonze, S.; Deiana, M.L.; Biolo, G.; Barbone, F. Peripheral and total parenteral nutrition as the strongest risk factors for nosocomial candidemia in elderly patients: A matched case-control study. Mycoses 2013, 56, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Netto, R.; Mondini, M.; Pezzella, C.; Romani, L.; Lucignano, B.; Pansani, L.; D’Argenio, P.; Cogo, P. Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. JPEN J. Parenter. Enter. Nutr. 2017, 41, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Bruna, E.; Petit, E.; Beljean-Leymarie, M.; Huynh, S.; Nouveloto, A. Specific susceptibility of docosahexaenoic acid and eicosapentaenoic acid to peroxidation in aqueous solution. Lipids 1989, 24, 970–975. [Google Scholar] [CrossRef]
- Goulet, O.; de Potter, S.; Antebi, H.; Driss, F.; Colomb, V.; Bereziat, G.; Alcindor, L.G.; Corriol, O.; Le Brun, A.; Dutot, G.; et al. Long-term efficacy and safety of a new olive oil-based intravenous fat emulsion in pediatric patients: A double-blind randomized study. Am. J. Clin. Nutr. 1999, 70, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Barbosa, V.M.; Calder, P.C. Olive oil in parenteral nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Witting, P.K.; Upston, J.M.; Stocker, R. The molecular action of alpha-tocopherol in lipoprotein lipid peroxidation. Pro- and antioxidant activity of vitamin E in complex heterogeneous lipid emulsions. Subcell. Biochem. 1998, 30, 345–390. [Google Scholar] [PubMed]
- Xu, Z.; Harvey, K.A.; Pavlina, T.M.; Zaloga, G.P.; Siddiqui, R.A. Distribution of tocopherols and tocotrienols in guinea pig tissues following parenteral lipid emulsion infusion. JPEN J. Parenter. Enter. Nutr. 2016, 40, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Carter, L.C.; German, J.B. Docosahexaenoic acid accumulates in cardiolipin and enhances HT-29 cell oxidant production. J. Lipid Res. 1998, 39, 1583–1588. [Google Scholar] [PubMed]
- Fuhrman, B.; Volkova, N.; Aviram, M. Postprandial serum triacylglycerols and oxidative stress in mice after consumption of fish oil, soy oil or olive oil: Possible role for paraoxonase-1 triacylglycerol lipase-like activity. Nutrition 2006, 22, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Lauriti, G.; Zani, A.; Aufieri, R.; Cananzi, M.; Chiesa, P.L.; Eaton, S.; Pierro, A. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: A systematic review. JPEN J. Parenter. Enter. Nutr. 2014, 38, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Antebi, H.; Zimmermann, L.; Bourcier, C.; Le Brun, A.; Giudicelli, A.; Dutot, G.; Colomb, V.; Corriol, O.; Goulet, O.; Ricour, C.; et al. Peroxydation in vitro et effet de l’administration en nutrition parenterale totale d’une emulsion lipidique a base d’huile d’olive sur la peroxydabilite des lipoproteines de dasse densite chez l’enfant. Nutr. Clin. Metab. 1996, 10, 41S–43S. [Google Scholar] [CrossRef]
- Deshpande, G.C.; Simmer, K.; Mori, T.; Croft, K. Parenteral lipid emulsions based on olive oil compared with soybean oil in preterm (<28 weeks’ gestation) neonates: A randomised controlled trial. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 619–625. [Google Scholar] [PubMed]
- Roggero, P.; Mosca, F.; Gianni, M.L.; Orsi, A.; Amato, O.; Migliorisi, E.; Longini, M.; Buonocore, G. F2-isoprostanes and total radical-trapping antioxidant potential in preterm infants receiving parenteral lipid emulsions. Nutrition 2010, 26, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.N.; Hardy, P.; Peterkin, M.; Lee, O.; Shalley, H.; Croft, K.D.; Mori, T.A.; Heine, R.G.; Bines, J.E. Tolerability and safety of olive oil-based lipid emulsion in critically ill neonates: A blinded randomized trial. Nutrition 2008, 24, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Ben-Artzi, E.; Berkowitz, D.; Elhasid, R.; Lajterer, N.; Postovski, S.; Hadad, S.; Shamir, R. Olive oil-based intravenous lipid emulsion in pediatric patients undergoing bone marrow transplantation: A short-term prospective controlled trial. Clin. Nutr. 2009, 28, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Pitkanen, O.M.; Luukkainen, P.; Andersson, S. Attenuated lipid peroxidation in preterm infants during subsequent doses of intravenous lipids. Biol. Neonate 2004, 85, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Simmer, K.; Deshmukh, M.; Mori, T.A.; Croft, K.D.; Kristensen, J. Fish Oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Demirel, N.; Erol, S.; Isik, D.U.; Kulali, F.; Iyigun, F.; Bas, A.Y. Effects of two different lipid emulsions on morbidities and oxidant stress statuses in preterm infants: An observational study. J. Matern. Fetal Neonatal Med. 2018, 31, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Edward, R.R.; Innes, J.K.; Marino, L.V.; Calder, P.C. Influence of different intravenous lipid emulsions on growth, development and laboratory and clinical outcomes in hospitalised paediatric patients: A systematic review. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Nghiem-Rao, T.H.; Dahlgren, A.F.; Kalluri, D.; Cao, Y.; Simpson, P.M.; Patel, S.B. Influence of gestational age and birth weight in neonatal cholesterol response to total parenteral nutrition. J. Clin. Lipidol. 2016, 10, 891–897. [Google Scholar] [CrossRef] [PubMed]
- NCEP. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- Gultekin, G.; Sahin, H.; Inanc, N.; Uyanik, F.; Ok, E. Impact of omega-3 and omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak. J. Med. Sci. 2014, 30, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Huschak, G.; Zur Nieden, K.; Hoell, T.; Riemann, D.; Mast, H.; Stuttmann, R. Olive oil based nutrition in multiple trauma patients: A pilot study. Intensive Care Med. 2005, 31, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Puiggròs, C.; Sanchez, J.; Chacon, P.; Sabin, P.; Rosello, J.; Bou, R.; Planas, M. Evolution of lipid profile, liver function, and pattern of plasma fatty acids according to the type of lipid emulsion administered in parenteral nutrition in the early postoperative period after digestive surgery. JPEN J. Parenter. Enter. Nutr. 2009, 33, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Piper, S.N.; Schade, I.; Beschmann, R.B.; Maleck, W.H.; Boldt, J.; Rohm, K.D. Hepatocellular integrity after parenteral nutrition: Comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur. J. Anaesthesiol. 2009, 26, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Pálová, S.; Charvat, J.; Kvapil, M. Comparison of soybean oil- and olive oil-based lipid emulsions on hepatobiliary function and serum triacylglycerols level during realimentation. J. Int. Med. Res. 2008, 36, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Piper, S.; Schollhorn, T.; Schade, I.; Beschmann, R.; Rohm, K. Modulation of lipid utilisation by parenteral administration of a fish-oil-enriched new lipid formula (SMOFlipid) in surgical ICU patients: Comparison with a lipid emulsion based on olive and soybean oil. Crit. Care 2009, 13, S57. [Google Scholar] [CrossRef]
- Kurvinen, A.; Nissinen, M.J.; Gylling, H.; Miettinen, T.A.; Lampela, H.; Koivusalo, A.I.; Rintala, R.J.; Pakarinen, M.P. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, A.A.; van der Crabben, S.N.; Ackermans, M.T.; Endert, E.; Kok, J.H.; Sauerwein, H.P. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: Response depends on fatty acid profile. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E723–E730. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The essentiality of arachidonic acid in infant development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Jill Shaddy, D.; Kerling, E.H.; Gustafson, K.M.; Carlson, S.E. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 2017, 121, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Alshweki, A.; Munuzuri, A.P.; Bana, A.M.; de Castro, M.J.; Andrade, F.; Aldamiz-Echevarria, L.; de Pipaon, M.S.; Fraga, J.M.; Couce, M.L. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Najm, S.; Lofqvist, C.; Hellgren, G.; Engstrom, E.; Lundgren, P.; Hard, A.L.; Lapillonne, A.; Savman, K.; Nilsson, A.K.; Andersson, M.X.; et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hojsak, I.; Colomb, V.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hulst, J.M.; Indrio, F.; et al. ESPGHAN Committee on Nutrition position paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: A systematic review and meta-analysis. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Beath, S.V.; Kelly, D.A. Total parenteral nutrition-induced cholestasis: Prevention and management. Clin. Liver Dis. 2016, 20, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.-J.; Sun, L.-L.; Li, M.-Y.; Ding, C.-L.; Su, Y.-C.; Sun, L.-J.; Xue, S.-H.; Yan, F.; Zhao, C.-H.; Wang, W. Comparison of formulas based on lipid emulsions of olive oil, soybean oil, or several oils for parenteral nutrition: A systematic review and meta-analysis. Adv. Nutr. 2016, 7, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P. Phytosterols, lipid administration, and liver disease during parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2015, 39, 39S–60S. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, C.B.; De Bruin, T.W.A.; Jansen, H.; Erkelens, D.W. Different clearance of intravenously administered olive oil and soybean oil emulsions: Role of hepatic lipase. Am. J. Clin. Nutr. 1993, 57, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. Intestinal failure-associated liver disease: A position paper of the ESPGHAN working group of intestinal failure and intestinal transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Gobel, Y.; Koletzko, B.; Bohles, H.-J.; Engelsberger, I.; Forget, D.; Le Brun, A.; Peters, J.; Zimmermann, A. Parenteral fat emulsions based on olive and soybean oils: A randomized clinical trial in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Grau, T.; Bonet, A.; Rubio, M.; Mateo, D.; Farre, M.; Acosta, J.A.; Blesa, A.; Montejo, J.C.; de Lorenzo, A.G.; Mesejo, A.; et al. Liver dysfunction associated with artificial nutrition in critically ill patients. Crit. Care 2007, 11, R10. [Google Scholar] [CrossRef] [PubMed]
- Hartman, C.; Berkowitz, D.; Elhasid, R.; Ben Harush, M.; Hadad, S.; Shamir, R. Tolerability and safety of short-term olive oil-based intravenous lipid emulsion in pediatric patients undergoing bone marrow transplantation. J. Pediatr. Gastroenterol. Nutr. 2009, 48, E139–E140. [Google Scholar]
- Thomas-Gibson, S.; Jawhari, A.; Atlan, P.; Brun, A.L.A.L.; Farthing, M.; Forbes, A. Safe and efficacious prolonged use of an olive oil-based lipid emulsion (ClinOleic) in chronic intestinal failure. Clin. Nutr. 2004, 23, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, K.; Atlan, P.; Joly, F.; Le Brun, A.; Evard, D.; Perennec, V.; Roux-Haguenau, D.; Bereziat, G.; Messing, B. A 3-month double-blind randomised study comparing an olive oil- with a soyabean oil-based intravenous lipid emulsion in home parenteral nutrition patients. Br. J. Nutr. 2005, 94, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-P.; Yan, X.-L.; Ni, Y.-F.; Guo, K.; Ke, C.-K.; Cheng, Q.-S.; Lu, Q.; Zhang, L.-J.; Li, X.-F. Effects of lipid emulsions in parenteral nutrition of esophageal cancer surgical patients receiving enteral nutrition: A comparative analysis. Nutrients 2013, 6, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Klek, S.; Szczepanek, K.; Scislo, L.; Walewska, E.; Pietka, M.; Pisarska, M.; Pedziwiatr, M. Intravenous lipid emulsions and liver function in adult chronic intestinal failure patients: Results from a randomized clinical trial. Nutrition 2017. [Google Scholar] [CrossRef]
- Johnston, D.E. Special considerations in interpreting liver function tests. Am. Fam. Phys. 1999, 59, 2223–2230. [Google Scholar]
- Chlopicki, S. Perspectives in pharmacology of endothelium: From bench to bedside. Pharmacol. Rep. 2015, 67, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial cell heterogeneity. Crit. Care Med. 2003, 31, S221–S230. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Vestweber, D. How leukocytes trigger opening and sealing of gaps in the endothelial barrier. F1000Res 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New parenteral lipid emulsions for clinical use. JPEN J. Parenter. Enter. Nutr. 2006, 30, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Smiley, D.; Robalino, G.; Peng, L.; Kitabchi, A.E.; Khan, B.; Le, A.; Quyyumi, A.; Brown, V.; Phillips, L.S. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Amissi, S.; Boisrame-Helms, J.; Burban, M.; Rashid, S.K.; Leon-Gonzalez, A.J.; Auger, C.; Toti, F.; Meziani, F.; Schini-Kerth, V.B. Lipid emulsions containing medium chain triacylglycerols blunt bradykinin-induced endothelium-dependent relaxation in porcine coronary artery rings. Lipids 2017, 52, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Harvey, K.A.; Xu, Z.; Pavlina, T.M.; Zaloga, G.P.; Siddiqui, R.A. Modulation of endothelial cell integrity and inflammatory activation by commercial lipid emulsions. Lipid Health Dis. 2015, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Talbott, G.A.; Sharar, S.R.; Harlan, J.M.; Winn, R.K. Leukocyte-endothelial interactions and organ injury: The role of adhesion molecules. New Horiz. 1994, 2, 545–554. [Google Scholar] [PubMed]
- Abbasoglu, O.; Hardy, G.; Manzanares, W.; Pontes-Arruda, A. Fish oil-containing lipid emulsions in adult parenteral nutrition: A review of the evidence. JPEN J. Parenter. Enter. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Adolph, M.; Deutz, N.E.; Grau, T.; Innes, J.K.; Klek, S.; Lev, S.; Mayer, K.; Michael-Titus, A.T.; Pradelli, L.; et al. Lipids in the intensive care unit: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2018, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, C.E.; Brody, R.A.; Parrott, J.S.; Stankorb, S.M.; Heyland, D.K. The effects of different IV fat emulsions on clinical outcomes in critically ill patients. Crit. Care Med. 2014, 42, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, W.; Dhaliwal, R.; Jurewitsch, B.; Stapleton, R.D.; Jeejeebhoy, K.N.; Heyland, D.K. Alternative lipid emulsions in the critically ill: A systematic review of the evidence. Intensive Care Med. 2013, 39, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, D.; Lauterbach, R.; Walczak, M.; Hurkala, J.; Sherman, M.P. Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: A prospective, randomized study. JPEN J. Parenter. Enter. Nutr. 2014, 38, 711–716. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. Globule Size Distribution in Lipid Injectable Emulsions; USP37/NF32; United States Pharmacopeia: Rockville, MD, USA, 2014; Chapter 729; pp. 360–363. [Google Scholar]
- Mirtallo, J.; Canada, T.; Johnson, D.; Kumpf, V.; Petersen, C.; Sacks, G.; Seres, D.; Guenter, P. Safe practices for parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 2004, 28, S39–S70. [Google Scholar] [CrossRef]
- Driscoll, D.F.; Giampietro, K.; Wichelhaus, D.P.; Peterss, H.; Nehne, J.; Niemann, W.; Bistrian, B.R. Physicochemical stability assessments of lipid emulsions of varying oil composition. Clin. Nutr. 2001, 20, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Bhargava, H.N.; Li, L.; Zaim, R.H.; Babayan, V.K.; Bistrian, B.R. Physicochemical stability of total nutrient admixtures. Am. J. Health Syst. Pharm. 1995, 52, 623–634. [Google Scholar] [PubMed]
- Telessy, I.G.; Balogh, J.; Szaba, B.; Csempesz, F.; Zelka, R. Kinetic stability of all-in-one parenteral nutrition admixtures in the presence of high dose Ca2+ additive under clinical application circumstances. Nutr. J. 2012, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.E.; Berlana, D.; Ukleja, A.; Boullata, J. Clinical, ergonomic, and economic outcomes with multichamber bags compared with (Hospital) pharmacy compounded bags and multibottle systems: A systematic literature review. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Wang, S.; Gronlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, C.; Valencia, C.; Partal, P.; Franco, J.M.; Maglio, O.; Abrahamsson, M.; Brito-de la Fuente, E. Droplet-size distribution and stability of commercial injectable lipid emulsions containing fish oil. Am. J. Health Syst. Pharm. 2012, 69, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
| Constituent | Intralipid | ClinOleic | Lipofundin MCT/LCT | Structolipid | Omegaven | Lipoplus/Lipidem | Smoflipid |
|---|---|---|---|---|---|---|---|
| Oil Source | 100% Soybean | 80% Olive 20% Soybean | 50% MCT 50% Soybean | 36% MCT 64% Soybean | 100% Fish | 50% MCT 40% Soybean 10% Fish | 30% MCT 30% Soybean 25% Olive 15% Fish |
| Fatty acid composition, % of total | |||||||
| Medium-chain FA | |||||||
| Caprylic | ND | ND | 27.0 | 14.47 | ND | 24.18–30.1 | 16.0–20.5 |
| Capric | ND | ND | 17.95 | 9.34 | ND | 16.13–19.4 | 9.85–13.0 |
| Long-chain FA | |||||||
| Oleic acid | 20.92 | 59.69 | 11.68 | 16.55 | 10.15 | 7.9–13.44 | 25.2–30.77 |
| α-linolenic | 6.65 | 1.71 | ND | 5.72 | 1.23 | 2.42–3.41 | 2.0–2.75 |
| Eicosapentaenoic | ND | ND | ND | NA | 19.34 | 2.75–3.69 | 2.35–3.03 |
| Docosahexaenoic | 0.11 | 0.06 | 0.06 | 0.19 | 17.67 | 2.3–2.53 | 1.73–2.75 |
| Arachidonic | 0.18 | 0.16 | 0.19 | 0.24 | 1.47 | 0.52–0.66 | 0.27–0.5 |
| Linoleic | 54.68 | 18.56 | 28.89 | 39.18 | 2.98 | 20.88–25.72 | 17.8–21.42 |
| Phytosterols, mg/L | |||||||
| β-sitosterol | 302.6 | 240.6 | 191.6 | 240.0 | ND | NA | 131.6 |
| Campesterol | 55.4 | 13.3 | 30.9 | 44.0 | 1.0 | NA | 20.5 |
| Stigmasterol | 65.1 | 12.2 | 46.0 | 48.8 | 1.4 | NA | 18.5 |
| Tocopherols, µg/mL ± SD | |||||||
| α-tocopherol | 21.0 ± 0.2 | 32.0 ± 0.7 | 132.0 ± 5.6 | 28.4 ± 1.0 | 230.0 ± 0.8 | 177.0 ± 0.7 | 164.5 ± 2.7 |
| β-tocopherol | 3.8 ± 0.7 | 0.6 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.0 | ND | 1.5 ± 0.0 | 1.5 ± 0.1 |
| γ-tocopherol | 108.0 ± 0.9 | 14.0 ± 0.0 | 68.0 ± 1.0 | 68.6 ± 0.7 | 0.2 ± 0.0 | 57.0 ± 0.3 | 29.2 ± 0.6 |
| δ-tocopherol | 33.0 ± 0.2 | 11.0 ± 0.0 | 21.0 ± 0.2 | 27.7 ± 0.1 | 0.0 ± 0.0 | 69.0 ± 0.3 | 10.7 ± 0.1 |
| Study | Population | Intervention and Control (n) (Lipid Dose) | Duration | Outcome in Intervention Group |
|---|---|---|---|---|
| In vitro and in vivo studies | ||||
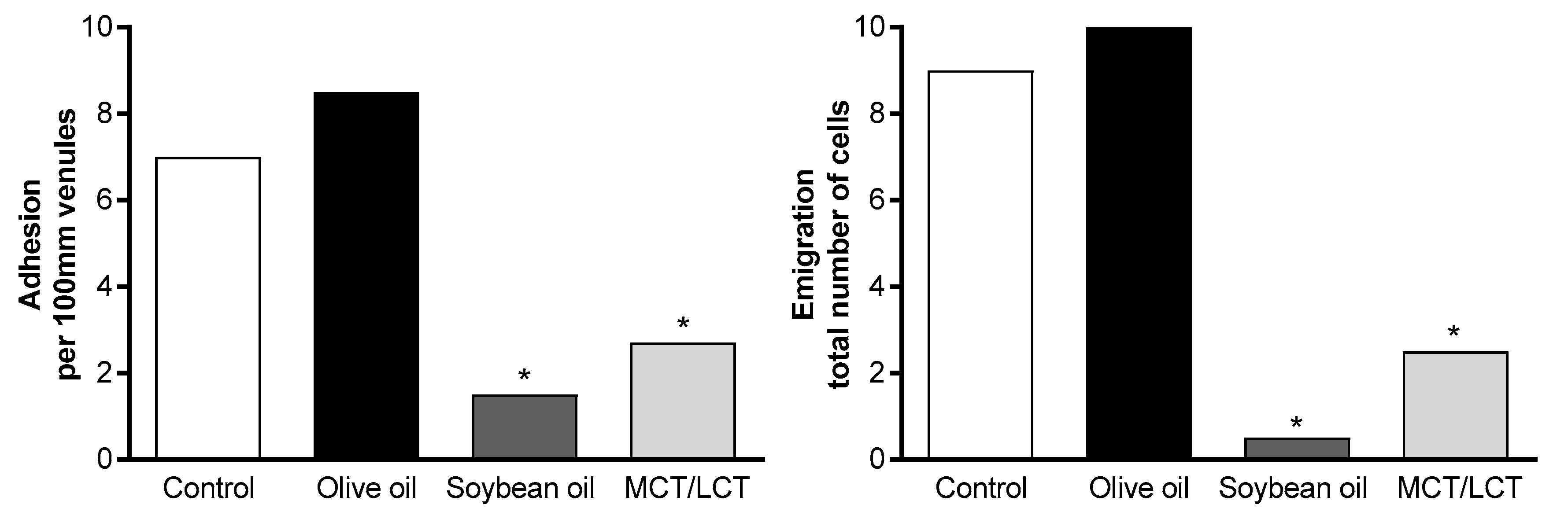
| Buenestado et al., 2006 [7] | In vitro: human neutrophils In vivo: rat leukocytes within mesenteric microcirculation | OO SO MCT/LCT In vitro: lipid-free medium In vivo: saline infusion | In vitro: 1 h to 48 h incubation In vivo: 2 h IV infusion | OO had lower impact on neutrophils (in vitro) and leukocytes (in vivo) compared with other ILEs |
| Buschmann et al., 2015 [27] | In vitro: Murine aortic endothelial cells and bone marrow PMNs In vivo: Mice | OO MCT/LCT SMOF Saline | In vitro: 3 h incubation In vivo: bolus injection (1 to 3 injections) | During systemic inflammation, OO had superior anti-inflammatory properties compared with other ILEs |
| Cury-Boaventura et al., 2008 [22] | In vivo: human lymphocytes and neutrophils | OO (n = 20) Saline (n = 3) | 6 h IV infusion | Decreased lymphocyte proliferation Promoted lymphocyte necrosis (by lipid accumulation) No effect on the proportion of viable neutrophils |
| In vitro: human peripheral white blood cells | OO SO | 48 h incubation | No effect on lymphocyte proliferation | |
| Juttner et al., 2008 [25] | In vitro: human neutrophils and monocytes | OO SO MCT/LCT | Incubations up to 1 h | SO and OO (to lesser extent) induced hydrogen peroxide production in neutrophils and monocytes compared with MCT/LCT, which had no effect |
| Nanhuck et al., 2009 [31] | In vitro: human PMNs and PBMCs | OO SO SMOF FO Saline | 18 h incubation | No difference in the production of lipid bodies from stimulated PMNs or PMBCs between the ILE groups Higher production of eicosanoids and lipid peroxides in FO group |
| Reimund et al., 2004 [32] | In vitro: human PBMCs | OO SO MCT/LCT | 24 h incubation | Basal (non-stimulated) PBMC TNFα production decreased significantly for all ILEs in a dose-dependent manner; however, it was most preserved in the OO group compared with SO (p = 0.0004) and MCT/LCT (p = 0.0483) No effect on IL-6 and IL-8 production was noted for any of the ILEs LPS-stimulated cytokine production was not affected by OO or MCT; however, IL-1 production was significantly inhibited by SO in a dose-dependent manner (p = 0.02) |
| Versleijen et al., 2010 [29] | In vitro: human neutrophils | OO SO MCT/LCT FO SL | 1 h incubation | Basal elimination capacity (pneumococcal elimination mean ± SD: 75% ± 3%) decreased significantly for all ILEs; however, it was most preserved in the OO group (70% ± 6%; p = 0.045) compared with SO (66% ± 10%; p = 0.046), MCT/LCT (47% ± 15%; p = 0.028), FO (67% ± 2%; p = 0.028), and SL (63% ± 9%; p = 0.028) |
| Animal studies | ||||
| Garnacho-Montero et al., 2002 [28] | Rats | OO (n = 15) SO (n = 17) MCT/LCT (n = 12) Chow (n = 15) Glucose (n = 12) | 4 day | OO caused less disruption of bacterial clearing |
| Adult studies | ||||
| Badía-Tahull et al., 2010 [33] | Gastrointestinal surgery (oncology) | OO (n = 14) (0.88 g/kg/day) OO + FO (84.4% + 16.6%) (n = 13) (0.88 g/kg/day) | 5 day | Significantly fewer infections in the OO + FO group compared with the OO group (3 vs. 11; p = 0.007) No differences between groups for C-reactive protein |
| Demirer et al., 2016 [34] | Abdominal surgery (oncology) | OO {100%} (n = 13) (NR) SO + MCT/LCT {75% + 25%} (n = 18) (NR) OO + FO {85% +15%} (NR) (n = 21) | ≥4 day | No significant difference in cytokines (TNFα and IL-6) between groups; however, lower levels were observed for the OO group |
| García-de-Lorenzo et al, 2005 [35] | Patients with severe burns | OO (n = 11) (1.3 g/kg/day) MCT/LCT (n = 11) (1.3 g/kg/day) | 6 day | Significant reduction in TNFα from baseline for OO Non-significant reduction in other cytokines (IL-6 and IL-10) from baseline No difference compared with MCT/LCT ILE |
| Jia et al., 2015 [20] | ICU | OO (n = 226) (0.8 g/kg/day) SO (n = 232) (0.8 g/kg/day) | 5–14 day | Fewer infections in OO-based PN group IL-6 decreased in both groups at Day 5 and was undetectable at Day 14/EOT. The difference between groups at Day 5 was significant (p = 0.0173) C-reactive protein decreased from baseline in both groups with no differences at any time point |
| Mateu-de Antonio et al., 2008 [26] | ICU patients | OO (n = 23) (0.86 g/kg/day) SO (n = 16) (0.91 g/kg/day) | ≥5 day | No effect on infection rate, acute-phase proteins, and major health outcomes Higher leukocyte count at end of PN and higher peak leukocyte count in the OO group |
| Olthof et al., 2013 [36] | Long-term PN | OO (n = 20) (NR) Healthy controls (n = 21) | ≥6 months | No significant difference between groups in C-reactive protein. Values within normal reference range No significant differences between groups in elimination of Streptococcus pneumoniae or expression of membrane surface activation markers |
| Olthof et al., 2016 [37] | Long-term PN | OO (n = 30) (0.97 g/kg/day) Healthy controls (n = 30) | ≥3 months | TNFα production by PBMCs increased 3.6-fold in the OO group compared with controls (p < 0.001), while IL-10, C-reactive protein, and membrane activation markers were not different between groups |
| Onar et al., 2011 [38] | Abdominal surgery (oncology) | OO (n = 10) (0.75 g/kg/day) SO (n = 10) (0.75 g/kg/day) | 7 day | No significant difference in infection rates between OO and SO ILEs |
| Reimund et al., 2005 [39] | Long-term PN | OO (n = 14) (31% of calories) | 3 months | No significant modifications in measured inflammatory (e.g., TNFα and IL-6) and immune parameters concentrations |
| Siqueira et al., 2011 [24] | Healthy subjects | OO (NR) SO (NR) Lipid-free PN Saline | 24 h infusion of each intervention (random order) * | No differences in inflammatory markers (TNFα, IL-6, or C-reactive protein) or immune function parameters (granulocyte or monocyte phagocytosis, and granulocyte or monocyte ROS generation) between groups |
| Umpierrez et al., 2012 [40] | Surgical ICU | OO (n = 51) (22 kcal/kg/day) SO (n = 49) (22 kcal/kg/day) | Maximum 28 day | No difference in plasma inflammatory markers (C-reactive protein, IL-6, and TNFα), or immune cell function (granulocyte or monocyte phagocytosis, granulocyte or monocyte ROS generation), and similar rates of infections between OO and SO ILEs |
| Preterm infant studies | ||||
| Demirel et al., 2012 [41] | ≤32 week | OO (n = 20) (up to 3 g/kg/day) SO (n = 20) (up to 3 g/kg/day) | 14 day | No significant differences in sepsis rates between OO and SO ILEs |
| Gawecka et al., 2008 [42] | <1500 g and <32 week | OO (n = 18) (2.7 g/kg/day) SO (n = 20) (2.7 g/kg/day) | 14 day | Anti-CD3 stimulated IL-6 increased significantly in the SO compared with OO/SO group. No difference in stimulated or unstimulated TNFα and IL-10 between groups |
| Koksal et al., 2011 [43] | ≤34 week | OO (n = 32) (up to 3 g/kg/day) SO (n = 32) (up to 3 g/kg/day) | 7 day | No significant differences in sepsis rates between OO and SO ILEs |
| Savini et al., 2013 [44] | 500–1249 g | OO (n = 29) (up to 3 g/kg/day) SO (n = 30) (up to 3 g/kg/day) MCT/LCT (n = 30) (up to 3 g/kg/day) SO/MCT/FO (n = 27) (up to 3 g/kg/day) MCT/SO/FO (n = 28) (up to 3 g/kg/day) | 21 day | No significant differences in sepsis rates between the 5 tested ILEs |
| Wang et al., 2016 [45] | <2000 g and <37 week | OO (n = 50) (1.45 g/kg/day) SO (n = 50) (1.41 g/kg/day) | >14 day | No significant differences in sepsis rates between OO and SO ILEs |
| Wang et al., 2016 [46] | <2000 g and <37 week | OO (n = 50) (1.42 g/kg/day) SO (n = 50) (1.39 g/kg/day) MCT/LCT (n = 50) (1.30 g/kg/day) | >14 day | No significant differences in sepsis rates between the 5 tested ILEs |
| Study | Population | Intervention and Control (n) [Lipid Dose] | Duration | Outcomes |
|---|---|---|---|---|
| In vitro studies | ||||
| Watkins et al., 1998 [55] | In vitro: HT-29 human colonic adenocarcinoma cells | Oleic acid Linoleic acid Docosahexaenoic acid Eicosapentaenoic acid Arachidonic acid Control | 36 h | ROS production was: oleic acid 6%; linoleic acid 35%, arachidonic acid 94%, eicosapentaenoic acid 40%, and docosahexaenoic acid 429% greater than control |
| Nanhuck et al., 2009 [31] | In vitro: isolated human PBMCs and PMNs | OO SO FO SMOF All ILEs were delivered as 0.01%, 0.02%, or 0.04% | 18 h | In both PMBCs and PMNs, OO and SO consistently showed no effects on LTB4, FO dramatically increased LTB4 in both LPS-stimulated and unstimulated cells Effects on PGE2 were similar, but were not always linear In both PMBCs and PMNs, FO significantly increased lipid peroxide generation, compared with the other ILE and control. SMOF induced a small increase at the highest dose compared with the control, but not the other ILEs |
| Animal studies | ||||
| Fuhrman et al., 2006 [56] | BALB/c mice | Oleic acid Linoleic acid Docosahexaenoic acid OO SO FO Saline | 2 h | Oxidative stress responses increased after intake of all unsaturated fatty acids and oil supplements. However, FO and docosahexaenoic acid induced the greatest increases compared with saline |
| Xu et al., 2016 [54] | Guinea pigs | OO SO FO SMOF | 10 day | MDA levels were increased in the SO, FO, and SMOF groups, with the highest levels seen in the FO group and the lowest seen in the OO group (OO vs. FO; p < 0.05) |
| Adult studies | ||||
| Demirer et al., 2016 [34] | Abdominal surgery (oncology) | OO {100%} (n = 13) (NR) SO + MCT/LCT {75% + 25%} (n = 18) (NR) OO + FO {85% + 15%} (n = 21) (NR) | ≥4 day | TAS decreased slightly in all groups (p = NS) and TBARS increased in all groups, but were lowest in the OO group (p ≤ 0.0015) and remained significant after Bonferroni’s was performed |
| Jia et al., 2015 [20] | ICU | OO (n = 226) (0.8 g/kg/day) SO (n = 232) (0.8 g/kg/day) | 5–14 day | F2-I and MDA were not significantly different from baseline or between groups |
| Onar et al., 2011 [38] | Abdominal surgery (oncology) | OO (n = 10) (0.75 g/kg/day) SO (n = 10) (0.75 g/kg/day) | 7 day | TBARS increased in both groups, no significant difference between groups |
| Olthof et al., 2013 [36] | Long-term PN | OO (n = 20) (NR) Healthy controls (n = 21) | ≥6 months | Total glutathione concentration was not different between groups, oxidized glutathione was higher in PN group (p < 0.001). Lipid peroxidation products, plasma concentrations of vitamin E, and glutathione were not different between groups. Protein carbonyl levels were below detection limits in both groups |
| Reimund et al., 2005 [39] | Long-term PN | OO (n = 14) (31% of calories) | 3 months | Vitamin E and MDA did not change from baseline to 3 months |
| Umpierrez et al., 2012 [40] | ICU | OO (n = 51) (22 kcal/kg/day) SO (n = 49) (22 kcal/kg/day) | 28 day | Markers of oxidative stress were similar between groups at baseline, Day 3, and Day 7 |
| Pediatric studies | ||||
| Goulet et al., 1999 [51] | Long-term PN | OO (n = 9) (1.92 g/kg/day) SO (n = 9) (1.69 g/kg/day) | Mean >30 months | LV-TBARS (p = 0.0027), the ratio of LDL-TBARS to LDL (p = 0.0262), and the ratio of LV-TBARS to LV (p = 0.0146) were significantly increased in the SO group compared with the OO group |
| Hartman et al., 2009 [62] | Bone marrow transplant | OO (n = 15) (1.1 g/kg/day) MCT/LCT (n = 13) (1.1 g/kg/day) | 14 day | TBARS and vitamin E did not change from baseline and there were no differences between groups |
| Preterm neonate studies | ||||
| Deshpande et al., 2014 [64] | <30 week | OO (n = 17) (18.45 g/kg/day) SMOF (n = 17) (18.25 g/kg/day) | 7 day | F2-I did not change from baseline in the OO group and decreased in the FO group. Difference between groups in change from baseline was significant (p = 0.0372) Vitamin E increased significantly in both groups (OO p = 0.0007, FO p = 0.0004), and the change from baseline was significantly higher for FO than for OO (p = 0.0091) |
| Deshpande et al., 2009 [59] | 23–28 week | OO (n = 24) (1.89 g/kg/day) SO (n = 21) (1.89 g/kg/day) | 5 day | F2-I decreased significantly in both groups (OO p = 0.006, SO p = 0.013), but there was no difference between groups in the change from baseline |
| Koksal et al., 2011 [43] | ≤34 week | OO (n = 32) (up to 3 g/kg/day) SO (n = 32) (up to 3 g/kg/day) | 7 day | TAC decreased in both groups from baseline, but there was no difference between groups |
| Pitkanen et al., 2004 [63] | 28–33 week | OO (0.48 g/kg/day) MCT/LCT (0.48 g/kg/day) | 3 h * | Pentane levels significantly increased in both groups during PN infusion, difference between groups was not significant |
| Roggero et al., 2010 [60] | 28–33 week | OO (n = 12) (up to 3 g/kg/day) SO (n = 12) (up to 3 g/kg/day) MCT/LCT (n = 12) (up to 3 g/kg/day) | 7 day | F2-I and TRAP concentrations were not statistically different within and among the 3 groups at any time of the study. No significant interaction effect between the type of lipid emulsion administered and the repeated values of F2-I and TRAP was found. F2-I values showed a trend to decrease throughout the study in all the 3 groups |
| Unal et al., 2017 [65] | 25–32 week | OO (n = 134) (up to 3 g/kg/day) SMOF (n = 93) (up to 3 g/kg/day) | Median 7 day | TAC, TOS, and OSI significantly decreased from baseline to Week 3 in both groups (all p < 0.001) |
| Webb et al., 2008 [61] | 25 week–7 day | OO (n = 39) (23.1 kcal/kg/day) SO (n = 40) (24.3 kcal/kg/day) | 5 day | F2-I levels were not different between groups at baseline or Day 5 |
| Study | Population | Intervention and Control (n) (Lipid Dose) | Duration | Outcomes |
|---|---|---|---|---|
| Animal studies | ||||
| Harvey et al., 2014 [5] | Guinea pigs | OO SO FO SMOF | 6 h infusion 10 d infusion | During 6 h infusion, TG increased significantly in all groups; however, greatest increase in SO group During chronic administration (10 d), TG was significantly lower in SO, OO, and FO lipids compared with SMOF and control diet groups (p < 0.05) |
| Adult studies | ||||
| García-de-Lorenzo et al., 2005 [35] | Severe burns | OO (n = 11) (1.3 g/kg/day) MCT/LCT (n = 11) (1.3 g/kg/day) | 6 day | TG increased from baseline significantly in both groups and TC increased from baseline significantly in OO group. Between-group differences were not significant TC levels remained within normal ranges * for most patients |
| Gultekin et al., 2014 [69] | Sepsis or septic shock | OO (n = 16) (1.3 g/kg/day) OO + FO {90%/10%} (n = 16) (1.3 g/kg/day) | 5 day | No difference from baseline to final measurement for TC, TG, LDL, or VLDL in both groups. HDL significantly decreased from baseline to final measurement in the OO group (p < 0.05) In OO group, TC and TG were within normal ranges * at baseline and final measurement |
| Huschak et al., 2005 [70] | Trauma | OO (n = 18) (0.8 g/kg/day) SO (n = 15) (0.5 g/kg/day) | 14 day | No difference between groups in TG A significant difference in lipid dose delivered was observed between the groups (p < 0.001) |
| Olthof et al., 2013 [36] | Long-term PN | OO (n = 20) (NR) Healthy controls (n = 21) | ≥6 months | TG levels were significantly higher in the PN group; however, TG levels were within normal ranges (as specified in the article) for both groups |
| Onar et al., 2011 [38] | Abdominal surgery (oncology) | OO (n = 10) (0.75 g/kg/day) SO (n = 10) (0.75 g/kg/day) | 7 day | TC, LDL, VLDL, and HDL decreased from baseline in the OO group, no significant difference between groups All values were within normal ranges * |
| Pálová et al., 2008 [73] | Malnourished ≥10% decreased bodyweight | OO (n = 11) (NR) SO (n = 10) (NR) | 14 day | TG deteriorated† in 1/11 patients in OO group vs. 7/10 in SO group (p < 0.01) |
| Piper et al., 2009 [74] | Abdominal or major maxillofacial surgery (oncology) | OO (n = 22) (NR) SMOF (n = 22) (NR) | 5 day | TG increased from baseline in both groups, and the increase was greater in the OO group. TG levels remained mostly within normal range * Significant between-group differences at Day 2 (p < 0.03) and Day 5 (p < 0.01) |
| Puiggròs et al., 2009 [71] | Abdominal surgery | OO (n = 7) (1.1–1.2 g/kg/day) SO (n = 7) (1.1–1.2 g/kg/day) MCT/LCT {50%/50%} (n = 7) (1.1–1.2 g/kg/day) MCT/LCT {36%/64%} (n = 7) (1.1–1.2 g/kg/day) | 5 day | No change from baseline in TC, HDL, LDL, or TG in OO group, all values within normal ranges. No difference between groups for any of these measures |
| Reimund et al., 2005 [39] | Long-term PN | OO (n = 14) (31% of calories) | 3 months | No change from baseline in TC, HDL, LDL, or TG in OO group No difference between groups for any of these measures Baseline and 3-month values within normal ranges for all measures |
| Siqueira et al., 2011 [24] | Healthy volunteers | OO (NR) SO (NR) Lipid-free PN Saline | 24-h infusion of each intervention (random order) ‡ | TG significantly increased from baseline in OO and SO groups compared with saline No difference between OO and SO groups in TC, HDL, or LDL between groups All values were within normal range * |
| Pediatric studies | ||||
| Goulet et al., 1999 [51] | Long-term PN | OO (n = 9) (1.92 g/kg/day) SO (n = 9) (1.69 g/kg/day) | Mean >30 months | TC, HDL, LDL, and TG decreased in OO group and increased in SO group Differences between groups not significant except for TC and LDL |
| Hartman et al., 2009 [62] | Bone marrow transplant | OO (n = 15) (1.1 g/kg/day) MCT/LCT (n = 13) (1.1 g/kg/day) | 14 day | TC decreased from baseline in both groups; however, the decrease was significantly greater in the OO group (p = 0.017). TG decreased from baseline in both groups, but the difference between groups was not significant TC remained within normal ranges in both groups, TG was above normal range * at baseline and decreased to within normal range in the OO group. TG remained within normal ranges in MCT/LCT group |
| Kurvinen et al., 2011 [75] | Intestinal failure | OO (n = 11) (0.9 g/kg/day) Normal controls (n = 20) | 3 months | TC was significantly lower in the OO group; however, TC remained within normal range * in both groups |
| Preterm neonate studies | ||||
| Demirel et al., 2012 [41] | ≤32 week | OO (n = 20) (up to 3 g/kg/day) SO (n = 20) (up to 3 g/kg/day) | 14 day | TC and TG within normal ranges * in both groups. No significant differences between groups except for VLDL, which was significantly higher in the OO group (p < 0.05), baseline levels NR |
| Koksal et al., 2011 [43] | ≤34 week | OO (n = 32) (up to 3 g/kg/day) SO (n = 32) (up to 3 g/kg/day) | 7 day | TC, VLDL, and TG increased, no significant difference between groups. All measures were within normal ranges * |
| Pitkanen et al., 2004 [63] | 28–33 week | OO (0.48 g/kg/day) MCT/LCT (0.48 g/kg/day) | 3 h § | TG increased significantly (p < 0.001) in both groups |
| Wang et al., 2016 [46] | <2000 g and <37 week | OO (n = 50) (1.42 g/kg/day) MCT/LCT (n = 50) (1.30 g/kg/day) SO (n = 50) (1.39 g/kg/day) | >14 day | No significant differences were observed in TC, TG, apolipoprotein A-I, apolipoprotein B, Lp(a), and apolipoprotein A-I/B among the groups. However, on Day 7, HDL level in the MCT/LCT group (0.89 ± 0.31 mmol/L) was significantly lower than in the OO (1.06 ± 0.40 mmol/L) or SO (1.05 ± 0.33 mmol/L) groups (p < 0.05). On Day 7, LDL levels were significantly higher in OO (1.77 ± 0.44 mmol/L) than in MCT/LCT (1.58 ± 0.44 mmol/L) or SO (1.54 ± 0.38 mmol/L) groups (p < 0.05). TC, TG, HDL, and LDL levels were within normal ranges * |
| Study | Population | Intervention and Control (n) (Lipid Dose) | Duration | Outcomes |
|---|---|---|---|---|
| Adult studies | ||||
| Badía-Tahull et al., 2010 [33] | Gastrointestinal surgery | OO (n = 14) (0.88 g/kg/day) OO + FO {84% + 17%} (n = 13) (0.88 g/kg/day) | 5 day | No significant differences between groups in LFTs (ALT, ALP, and GGT) at Day 6 |
| García-de-Lorenzo et al., 2005 [35] | Severe burns | OO (n = 11) (1.3 g/kg/day) MCT/LCT (n = 11) (1.3 g/kg/day) | 6 day | At Day 6, of 11 MCT/LCT and 9 OO patients, more in the MCT/LCT group had abnormal LFTs: ALT 8 vs. 4, AST 6 vs. 5, ALP 7 vs. 3, GGT 9 vs. 6, and bilirubin (total or conjugated) 4 vs. 2 Markers of cholestasis * in a significantly greater proportion of MCT/LCT vs. OO group (9/11 vs. 3/9, p = 0.04, Suissa-Shuster test) Markers of cytolysis† associated with cholestasis in 3 MCT/LCT and 2 OO patients |
| Grau et al., 2007 [88] | ICU | OO or MCT/LCT (n = 303 initial TPN group) (NR) EN (n = 422) (NR) | Not pre-specified | Multivariate analysis showed TPN is significantly associated with LD (p < 0.001) LD in 91/303 (30%) TPN patients LD in 75/233 (32%) TPN patients receiving MCT/LCT Multivariate model found no relationship between ILE used and liver dysfunction Median duration of TPN was 5 d for patients with LD vs. 0 d for those without LD (p = 0.001) Cholestasis occurred in 31/303 (10%) TPN patients |
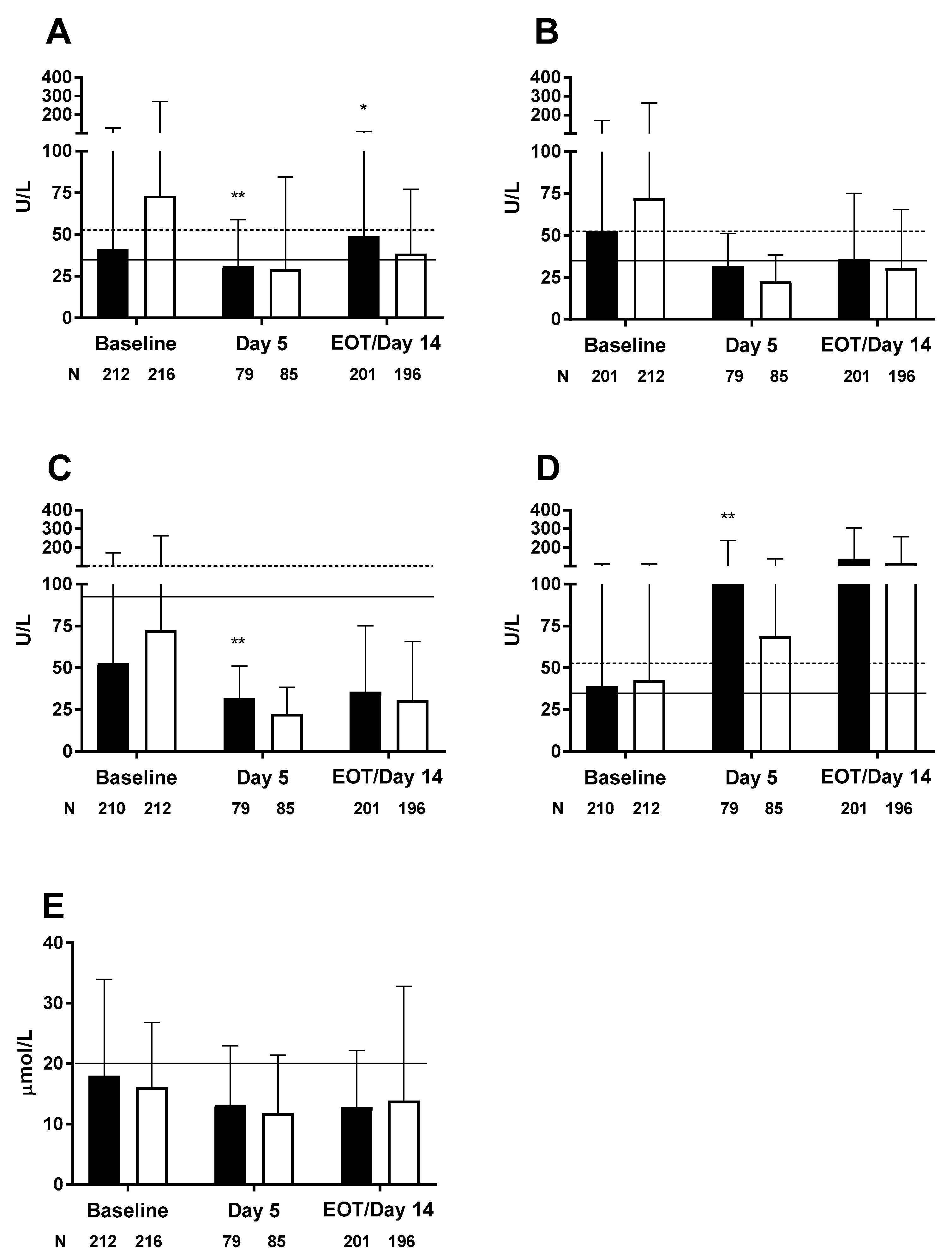
| Jia et al., 2015 [20] | ICU | OO (n = 226) (0.8 g/kg/day) SO (n = 232) (0.8 g/kg/day) | 5–14 day | LFTs generally within normal limits ALT change from BL significantly greater in OO vs. SO group at Day 5 (p = 0.002) and EOT/Day 14 (p = 0.006) ALP and GGT changes from BL significantly greater in OO vs. SO group at Day 5 (p = 0.001 and p = 0.004, respectively), but not at EOT/Day 14. Increases in both enzymes suggest OO and SO associated with mild cholestasis No significant differences between groups for AST and total bilirubin Short-term PN with OO or SO did not appear to negatively impact liver function |
| Klek et al., 2017 [93] | Long-term PN | OO (n = 17) (0.6 g/kg/day) SO (n = 14) (0.7 g/kg/day) MCT/LCT {50% + 50%} (n = 18) (0.7 g/kg/day) SMOF (n = 16) (0.7 g/kg/day) | 12 months | No significant change from BL for ALT, AST, AP, or GGT for SO, MCT, or SMOF. Bilirubin and GGT significantly decreased from BL (p = 0.0023 and p = 0.0079) in OO group; ALT, AST, and AP remained unchanged |
| Onar et al., 2011 [38] | Abdominal surgery (oncologic) | OO (n = 10) (0.75 g/kg/day) SO (n = 10) (0.75 g/kg/day) | 7 day | ALP and GGT significantly increased from BL (p < 0.05) in both groups at Day 7 Total bilirubin significantly decreased from BL (p < 0.05) in OO group at Day 7 No significant change from BL for ALT and AST in both groups nor for total bilirubin in SO group at Day 7 No significant differences between groups for LFTs Increases in LFTs (AST, ALT, ALP, and bilirubin) occurred in 10% of patients; abnormalities resolved post PN |
| Pálová et al., 2008 [73] | Malnourished ≥10% decreased body weight | OO (n = 11) (NR) SO (n = 10) (NR) | 14 day | No significant difference between groups in number of patients with deterioration ‡ in cytosolic enzymes (1 SO [ALT] vs. 1 OO [AST]) Significantly more patients with deterioration ‡ in cholestatic enzymes in SO vs. OO group (5 vs. 1, p < 0.05): conjugated bilirubin 3 vs. 0, ALP 3 vs. 1, and GGT 6 vs. 1 |
| Piper et al., 2009 [74] | Abdominal surgery or major maxillofacial surgery | OO (n = 22) (NR) SMOF (n = 22) (NR) | 5 day | Mean AST significantly lower in SMOF vs. OO group at Day 2 (27 vs. 47 U/L, p < 0.02) and Day 5 (31 vs. 56 U/L, p < 0.02) Mean ALT significantly lower in SMOF vs. OO group at Day 2 (20 vs. 42 U/L, p < 0.03) and Day 5 (26 vs. 49 U/L, p < 0.03) Mean α-GST significantly lower in SMOF vs. OO group at Day 2 (5 vs. 17 µg/L, p < 0.03) and Day 5 (6 vs. 24 µg/L, p < 0.01) |
| Puiggròs et al., 2009 [71] | Abdominal surgery | OO (n = 7) (1.1–1.2 g/kg/day) SO (n = 7) (1.1–1.2 g/kg/day) MCT/LCT {50%/50%}) (n = 7) (1.1–1.2 g/kg/day) MCT/LCT {37%/63%} (n = 7) (1.1–1.2 g/kg/day) | 5 day | No significant differences between groups in changes from BL to Day 6 for LFTs (ALT, AST, ALP, GGT, and total bilirubin) A tendency (NS) to increase GGT in the SO and MCT/LCT structured groups and AST in the MCT/LCT mixture group at Day 6 was observed; however, values remained within normal limits |
| Reimund et al., 2005 [39] | Long-term PN | OO (n = 14) (31% of calories) | 3 months | No significant changes from BL in bilirubin (total and conjugated), AST, ALT, ALP, and GGT at Month 3 |
| Thomas-Gibson et al., 2004 [90] | Long-term PN | OO (n = 13) (up to 1 g/kg/day) | 6 months SO followed by 6 months OO followed by 6 months SO § | In 12 patients with >2 mo OO PN, bilirubin was within normal limits and AST was ≤15% outside normal range at BL. LFTs increased transiently in 4 patients and were persistently high in 1 severely septic patient who also had abnormal levels at baseline 1 new case of cholelithiasis was identified No biliary outflow abnormality at BL or endpoint of OO PN despite 6 patients having BL biliary disease In 11 patients with >2 mo SO PN after OO PN, no significant changes in LFTs occurred in 6 mo post OO PN |
| Vahedi et al., 2005 [91] | Long-term PN | MCT/LCT {50%/50%)}run-in, followed by OO (n = 6) (0.7 g/kg/day) SO (n = 7) (0.7 g/kg/day) | 3 months | No differences between groups in changes in LFTs from BL to Day 90 1 case of cholestasis (SO) and 1 case of cytolysis (OO) existing at BL had resolved at Day 90 Hepatic ultrasound on Day 90 detected no hepatobiliary changes compared with BL |
| Wang et al., 2013 [92] | Resectable esophageal cancer | EN + OO PN (n = 46) (~0.83 g/kg/day) EN + MCT/LCT PN (n = 48) (~0.83 g/kg/day) | PN 7 day, EN added after Day 7 | Liver function was measured at regular intervals; results not reported |
| Pediatric studies | ||||
| Goulet et al., 1999 [51] | Long-term PN | OO (n = 9) (1.92 g/kg/day) SO (n = 9) (1.69 g/kg/day) | Mean >30 months | No significant differences between groups in changes from BL to Day 60 in bilirubin (total and conjugated), LFTs (AST, ALT, ALP, and GGT) and biliary acids Total bilirubin increased from BL in both groups ALT increased from BL in both groups AST increased from BL in OO group and decreased in SO group ALP increased from BL in OO group and decreased in SO group GGT essentially unchanged in OO group and increased from BL in SO group Biliary acids increased from BL in OO group and essentially unchanged in SO group |
| Hartman et al., 2009 [62] | Bone marrow transplant | OO (n = 15) (1.1 g/kg/day) MCT/LCT (n = 13) (1.1 g/kg/day) | 14 day | No significant differences for LFTs between groups |
| Kurvinen et al., 2011 [75] | Long-term PN | OO (n = 11) (0.9 g/kg/day) | >3 months | ALT, AST, GGT, and bilirubin remained close to normal or within the normal range during follow-up # GGT correlated with serum PS (r = 0.61–0.62, p < 0.05). Liver biopsies showed fibrosis in 5/8 (63%) patients and cholestasis in 3/8 (38%) patients Liver fibrosis in 5 patients reflected increased serum PS (r = 0.55–0.60, p = 0.16–0.12) |
| Preterm neonate studies | ||||
| Demirel et al., 2012 [41] | <32 wk | OO (n = 20) (up to 3 g/kg/day) SO (n = 20) (up to 3 g/kg/day) | 14 day | LFTs normal and similar in both groups at 14th day of life |
| Deshpande et al., 2014 [64] | <30 wk | OO (n = 17) (18.45 g/kg/day) SMOF (n = 17) (18.25 g/kg/day) | 7 day | No significant difference between groups in bilirubin (total and conjugated) or LFTs (ALT and GGT) on Day 8; values within normal limits in both groups |
| Gobel et al., 2003 [87] | NICU patients, gestational age 28–36 wk | OO (n = 18) (up to 2 g/kg/day) SO (n = 15) (up to 2 g/kg/day) | 7 day | No significant differences between groups for changes from BL to Day 8 for LFTs (bilirubin [total and conjugated], AST, ALT, ALP, and GGT) AST significantly lower at Day 8 vs. BL in both groups (OO: 14.2 vs. 27.2 IU/L, p = 0.0001; SO: 13.9 vs. 25.4 IU/L, p = 0.0007) ALT lower at Day 8 vs. BL (NS) in both groups ALP significantly higher at Day 8 vs. BL in both groups (OO: 286 vs. 222 IU/L, p = 0.0039; SO: 269 vs. 207 IU/L, p = 0.0028) GGT significantly lower at Day 8 vs. BL in both groups (OO: 64.0 vs. 75.0 IU/L, p = 0.0139; SO: 63.8 vs. 83.3 IU/L, p = 0.0073) Total bilirubin lower at Day 8 vs. BL (NS) in both groups Conjugated bilirubin higher at Day 8 vs. BL in OO group (NS) and lower in SO group (NS) |
| Koksal et al., 2011 [43] | ≤34 wk | OO (n = 32) (up to 3 g/kg/day) SO (n = 32) (up to 3 g/kg/day) | 7 day | AST, ALT, and bilirubin (total and indirect) decreased from BL to Day 7 while ALP and GGT increased in both groups (NS) No significant differences between groups in LFTs |
| Savini et al., 2013 [44] | 500–1249 g | OO (n = 29) (up to 3 g/kg/day) SO (n = 30) (up to 3 g/kg/day) MCT/LCT (n = 30) (up to 3 g/kg/day) MSF (n = 27) (up to 3 g/kg/day) SMOF (n = 28) (up to 3 g/kg/day) | 21 day | No significant differences between groups in mean AST, ALT, ALP, GGT, or bilirubin (total and conjugated) at 6 weeks of age Cholestasis (conjugated bilirubin >2.0 mg/dL) in 3 (2.1%) patients (1 MCT/LCT, 1 MSF, 1 SMOF) at 6 weeks of age, when all infants were receiving minimal enteral feeding No significant correlations between phytosterol intake, conjugated bilirubin, and LFTs at 6 weeks of age |
| Wang et al., 2016 [45] | <2000 g and <37 wk | OO (n = 50) (1.45 g/kg/day) SO (n = 50) (1.41 g/kg/day) | >14 day | Mean total bilirubin elevated at BL in both groups (OO 2.75 mg/dL, SO 38.80 mg/dL). At Day 7, mean values significantly increased in OO group (8.35 mg/dL) and significantly decreased in SO group (9.00 mg/dL) (p < 0.05). At Day 14, mean values significantly decreased from Day 7 in both groups (OO 4.13 mg/dL, SO 3.83 mg/dL) (p < 0.05) Direct bilirubin elevated at BL in both groups (OO 0.55 mg/dL, SO 0.59 mg/dL) and significantly increased at Day 7 (1.01 mg/dL, p < 0.05) and Day 14 (0.67 mg/dL) in SO group; however, increases not significant in OO group (0.75 and 0.73 mg/dL) Direct bilirubin significantly different between groups (p = 0.039) ALT not significantly different from BL at Days 7 and 14 in both groups; mean values remained within normal limits At Days 7 and 14, AST significantly decreased into normal range from high values at BL (p < 0.05) in both groups. ALP similarly elevated at BL and similarly increased significantly at Days 7 and 14 in both groups (p < 0.05) Mean GGT elevated at BL in both groups (OO 98 IU/L, SO 215 IU/L). At Days 7 and 14, mean GGT significantly increased from BL in OO group (100 and 139 IU/L) and significantly decreased in SO group (112 and 89 IU/L) (p < 0.05) No significant differences between groups for ALT, AST, ALP, GGT, and total bilirubin |
| Wang et al., 2016 [46] | <2000 g and <37 wks | OO (n = 50) (1.42 g/kg/day) MCT/LCT (n = 50) (1.30 g/kg/day) SO (n = 50) (1.39 g/kg/day) | >14 day | Total and direct bilirubin highest at Day 7 in all groups AST decreased from high values at BL to within normal limits in all groups at Days 7 and 14 ALT remained within normal limits in all groups ALP elevated at BL and increased at Days 7 and 14 in all groups GGT elevated at BL and decreased but remained elevated at Days 7 and 14 in all groups No significant differences in LFTs among groups at BL and Days 7 and 14 |
| Webb et al., 2008 [61] | ≥25 wk | OO (n = 39) (23.1 kcal/kg/day) SO (n = 39) (24.3 kcal/kg/day) | 5 day | LFTs were similar in both groups at BL and Day 5 No abnormalities or differences between groups in ALP, GGT, or conjugated bilirubin at BL or Day 5 Bile acids increased at Day 5 in both groups; no difference between groups |
| Systematic literature reviews and meta-analyses | ||||
| Dai et al., 2016 [83] | SLR and meta-analysis of RCTs: Neonates, infants, children, and adults | OO 8 studies SMOF 7 studies SO (control in each study) | Various | No differences for any analyses of total bilirubin. ALP significantly higher in OO vs. SO group (infants plus children, p < 0.00001) AST and ALP significantly lower in SMOF vs. SO group (all ages combined, p = 0.004 and p = 0.02) ALT, AST, and ALP significantly lower in SMOF vs. SO group (only adults, p = 0.004, p = 0.006, and p = 0.03) GGT lower in SMOF vs. SO group (all ages combined and only adults, p = 0.07 and p = 0.08) |
| Edward et al., 2017 [66] | SLR of 17 RCTs in hospitalized pediatric patients | OO 7 studies (control in 1 study control) SMOF 8 studies SO (control 15 studies) MCT/LCT 2 studies (control in 1 study) FO 2 studies | Various | The evidence does not point toward a particular ILE being superior in terms of effect on liver enzymes or total bilirubin. The majority of studies did not find significant differences between use of different ILEs and liver enzymes |
| Hojsak et al., 2016 [81] | SLR and meta-analysis of 23 RCTs: Preterm neonates, infants, and children | OO 2 studies SMOF 4 studies SMF 1 study MCT 1 study SO (control) | Various | Meta-analysis showed no differences in the rate of cholestasis or bilirubin levels associated with short-term use of different ILEs in preterm infants, neonates, and children Some evidence that use of multicomponent FO-containing ILE may contribute to a decrease in total bilirubin levels in children with intestinal failure on long-term PN |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Calder, P.C.; Cury-Boaventura, M.F.; De Waele, E.; Jakubowski, J.; Zaloga, G. Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review. Nutrients 2018, 10, 776. https://doi.org/10.3390/nu10060776
Cai W, Calder PC, Cury-Boaventura MF, De Waele E, Jakubowski J, Zaloga G. Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review. Nutrients. 2018; 10(6):776. https://doi.org/10.3390/nu10060776
Chicago/Turabian StyleCai, Wei, Phillip C. Calder, Maria F. Cury-Boaventura, Elisabeth De Waele, Julie Jakubowski, and Gary Zaloga. 2018. "Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review" Nutrients 10, no. 6: 776. https://doi.org/10.3390/nu10060776
APA StyleCai, W., Calder, P. C., Cury-Boaventura, M. F., De Waele, E., Jakubowski, J., & Zaloga, G. (2018). Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion—A Review. Nutrients, 10(6), 776. https://doi.org/10.3390/nu10060776





