α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167)
Abstract
1. Introduction
2. Materials and Methods
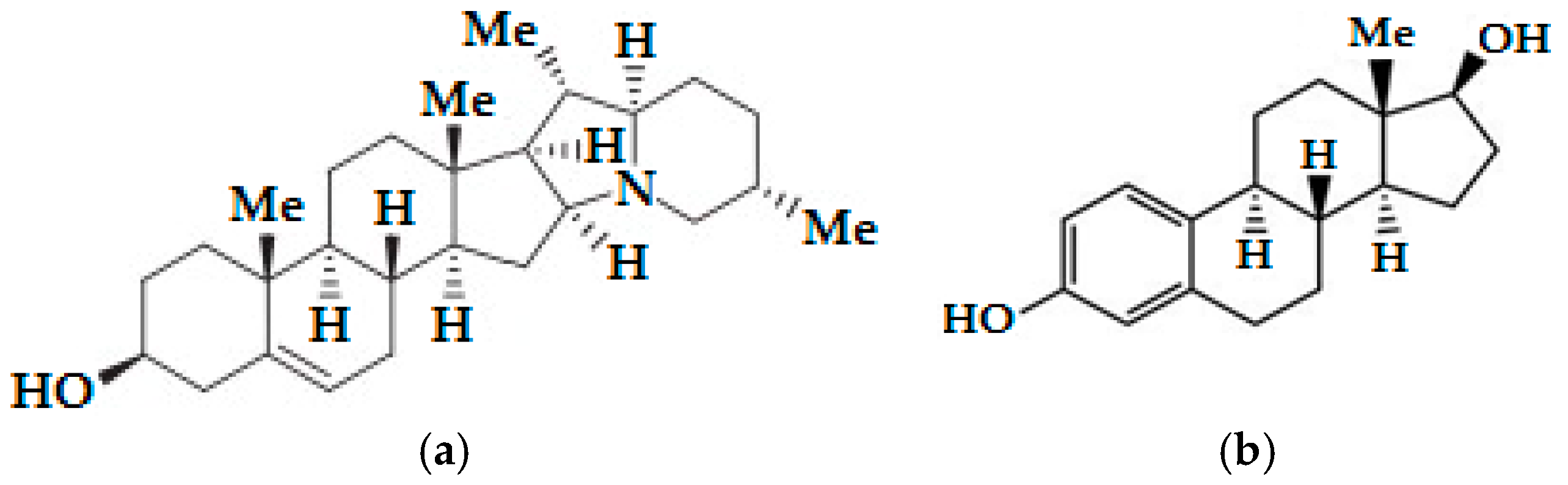
2.1. Materials
2.2. Cancer Cell Line and Culture
2.3. Cell Growth and Proliferation Assay Using xCELLigence Real-Time Cell Analysis (RTCA)
2.4. Cytotoxicity Assay Using xCELLigence RTCA
2.5. Determination of Cell Viability via Sulphorhodamine B Assay
2.6. Protein Extraction and Western Blot Analysis
2.7. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.8. Statistical Analysis
3. Results
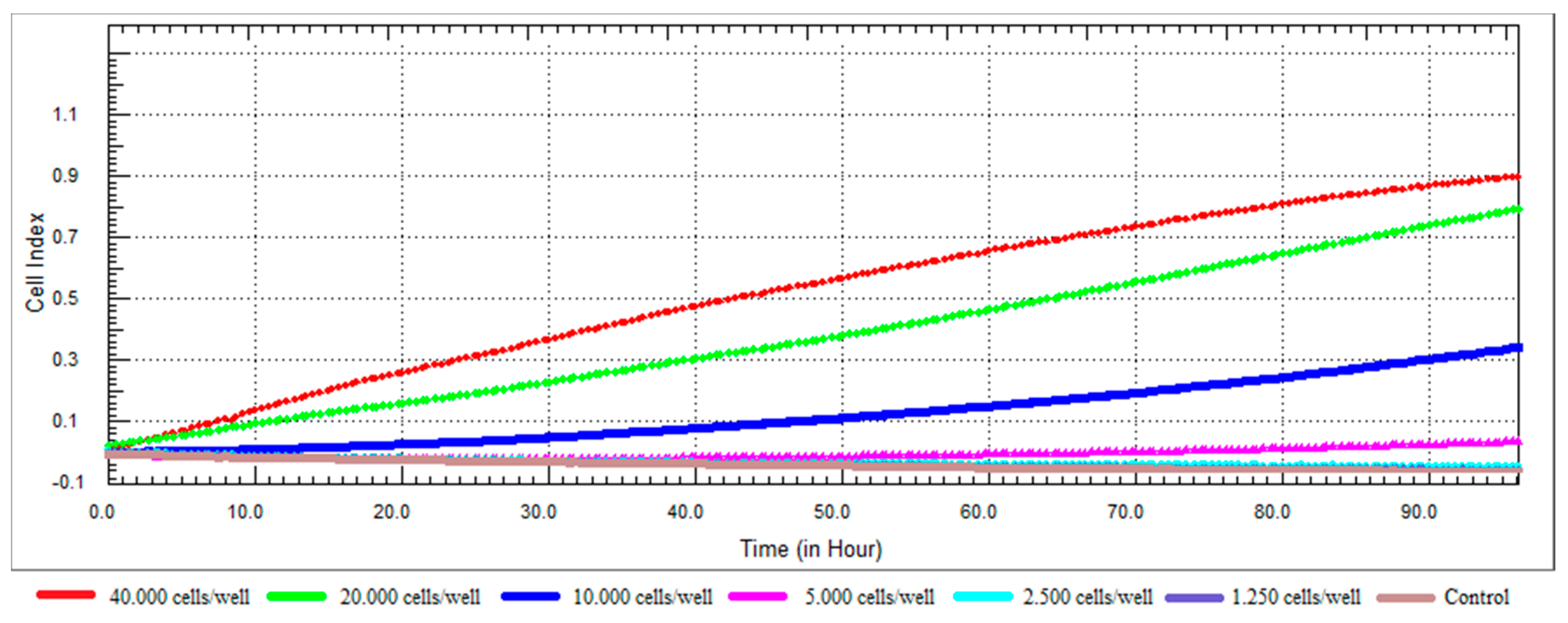
3.1. Real Time Cell Growth Profile Curve
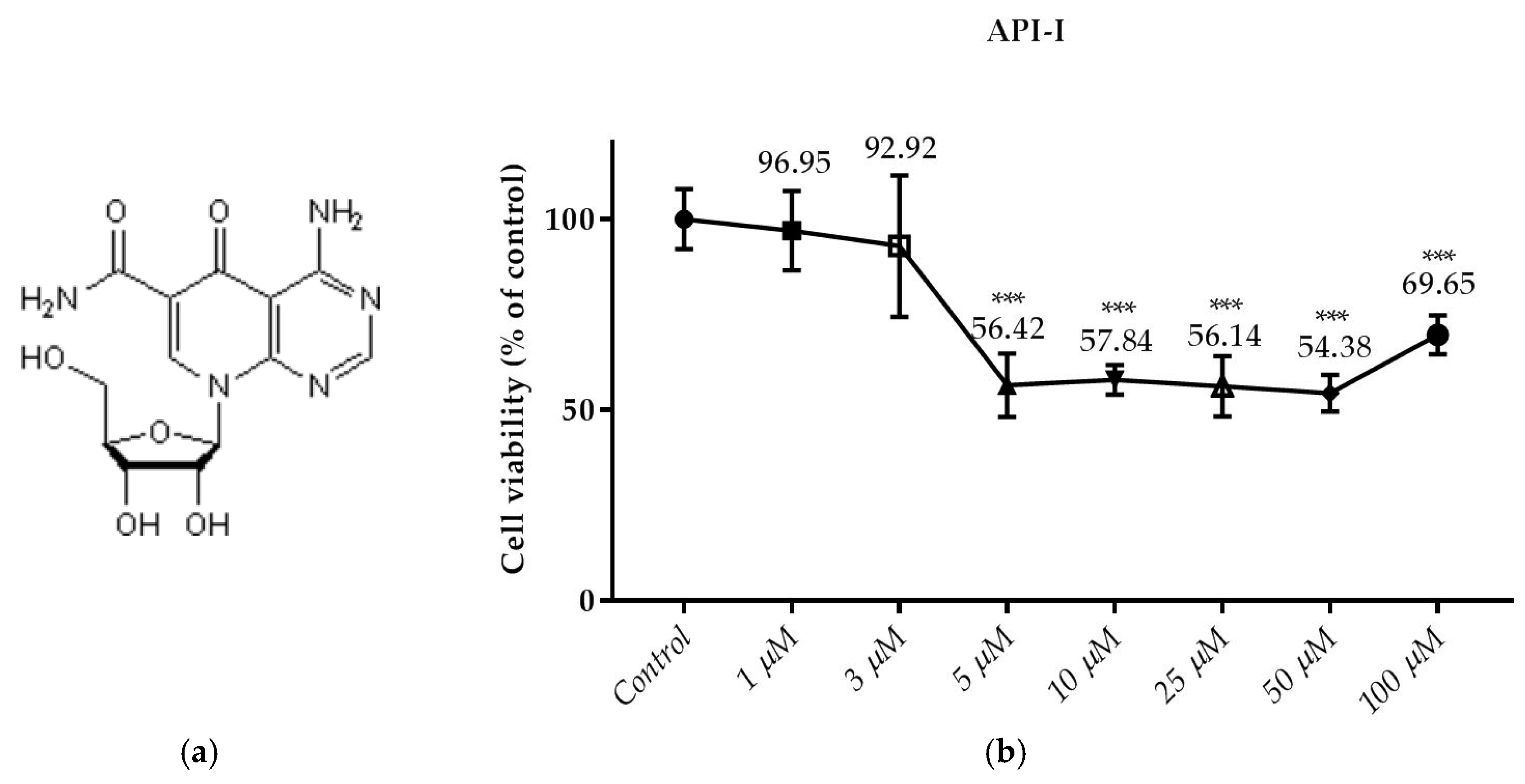
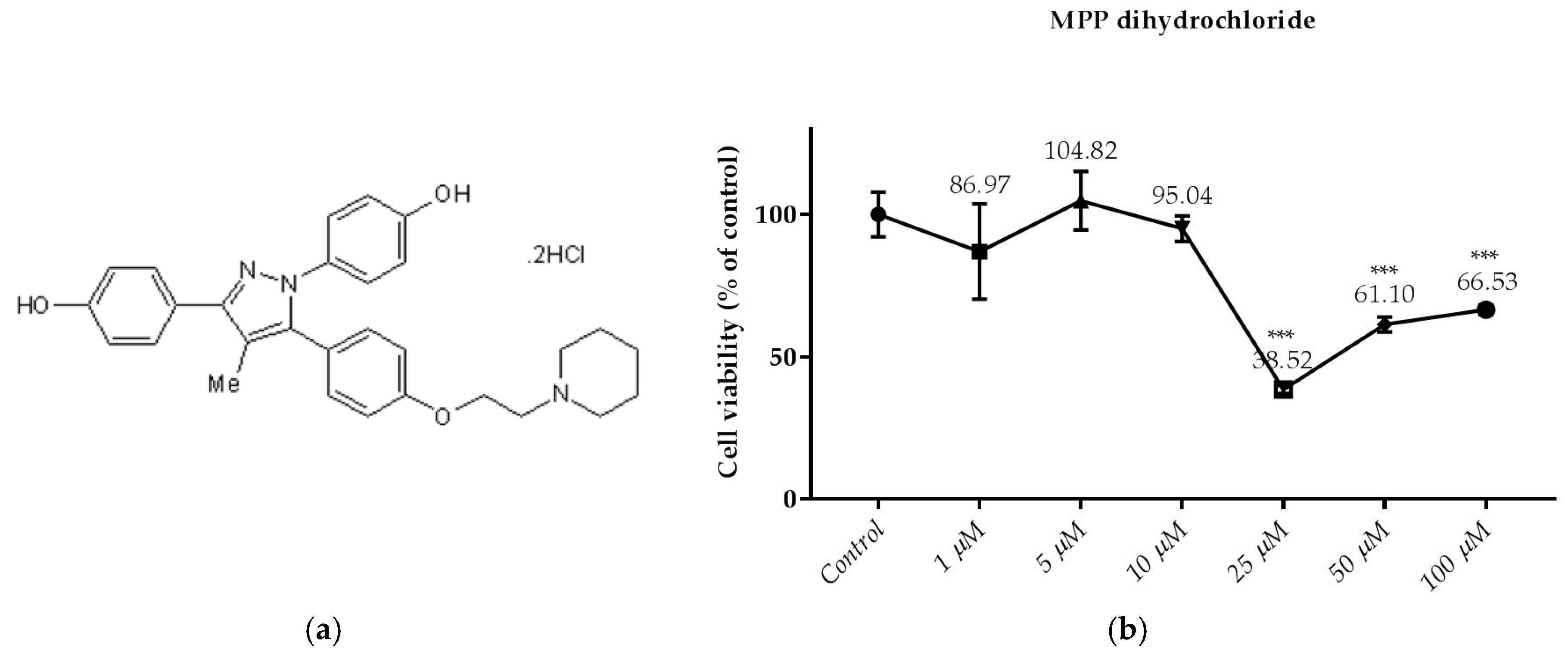
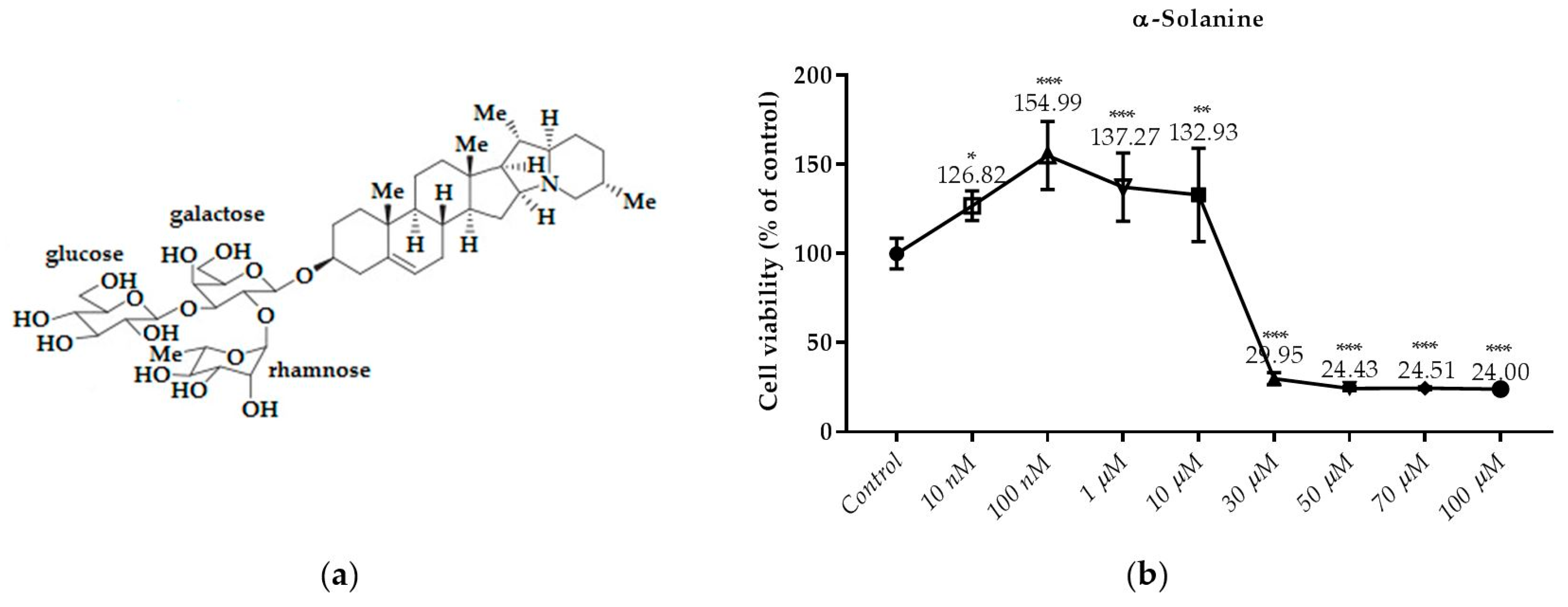
3.2. Effects of the Compounds on the Cell Viability
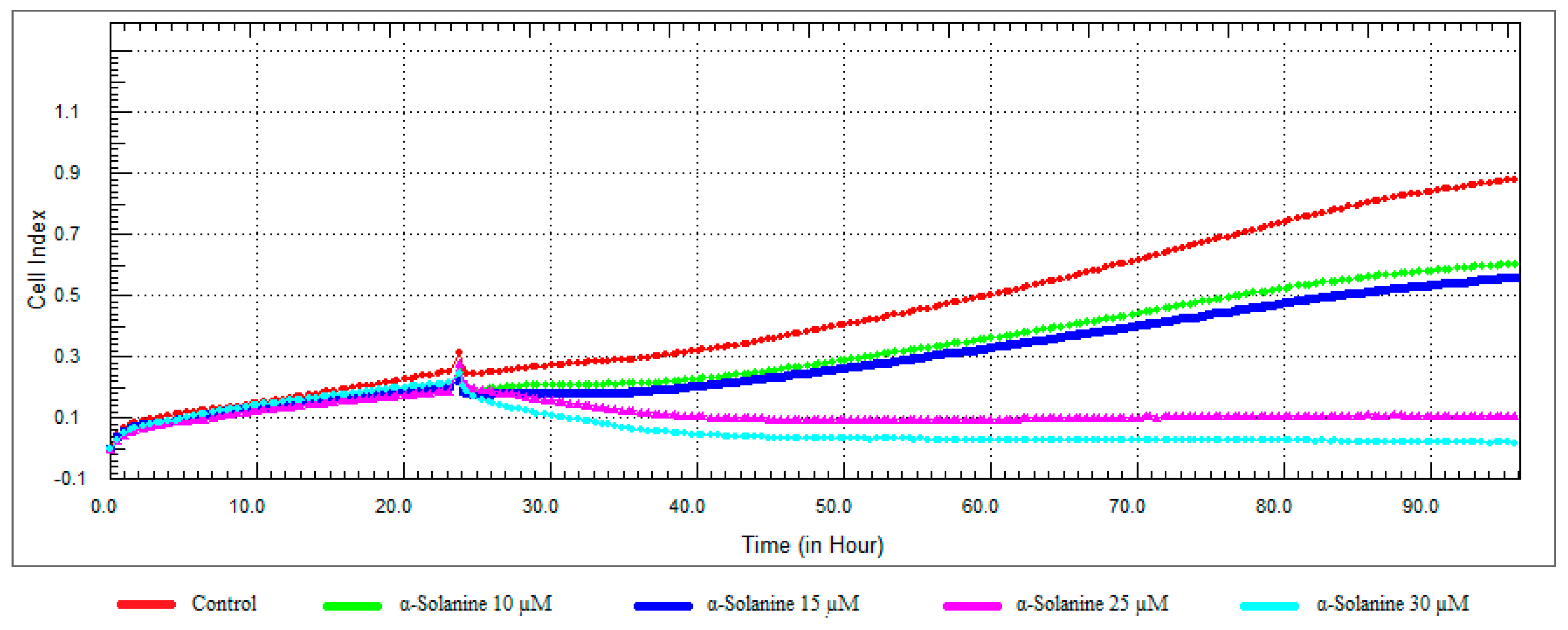
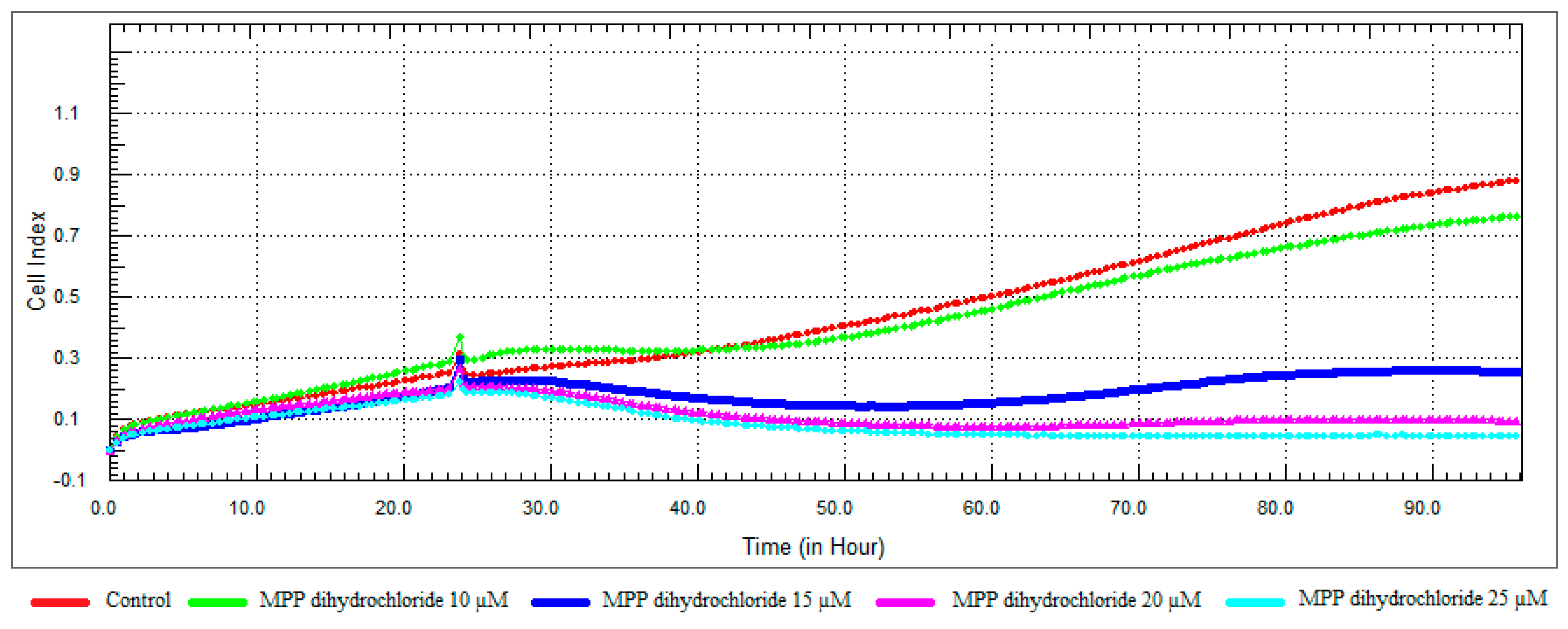
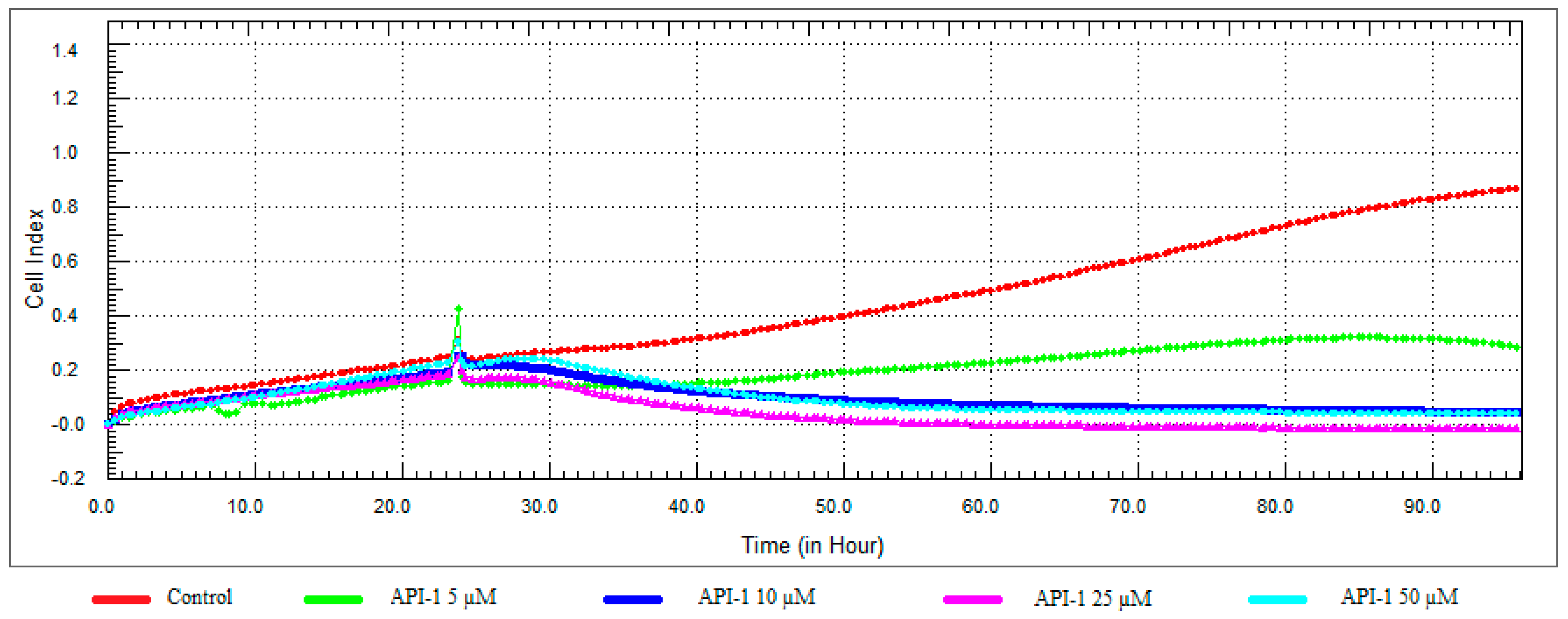
3.3. Monitoring of Cytotoxicity in Real-Time Using xCELLigence System
3.4. α-Chaconine, α-Solanine Inhibits Phosphorylation of Akt (Ser473) and ERα
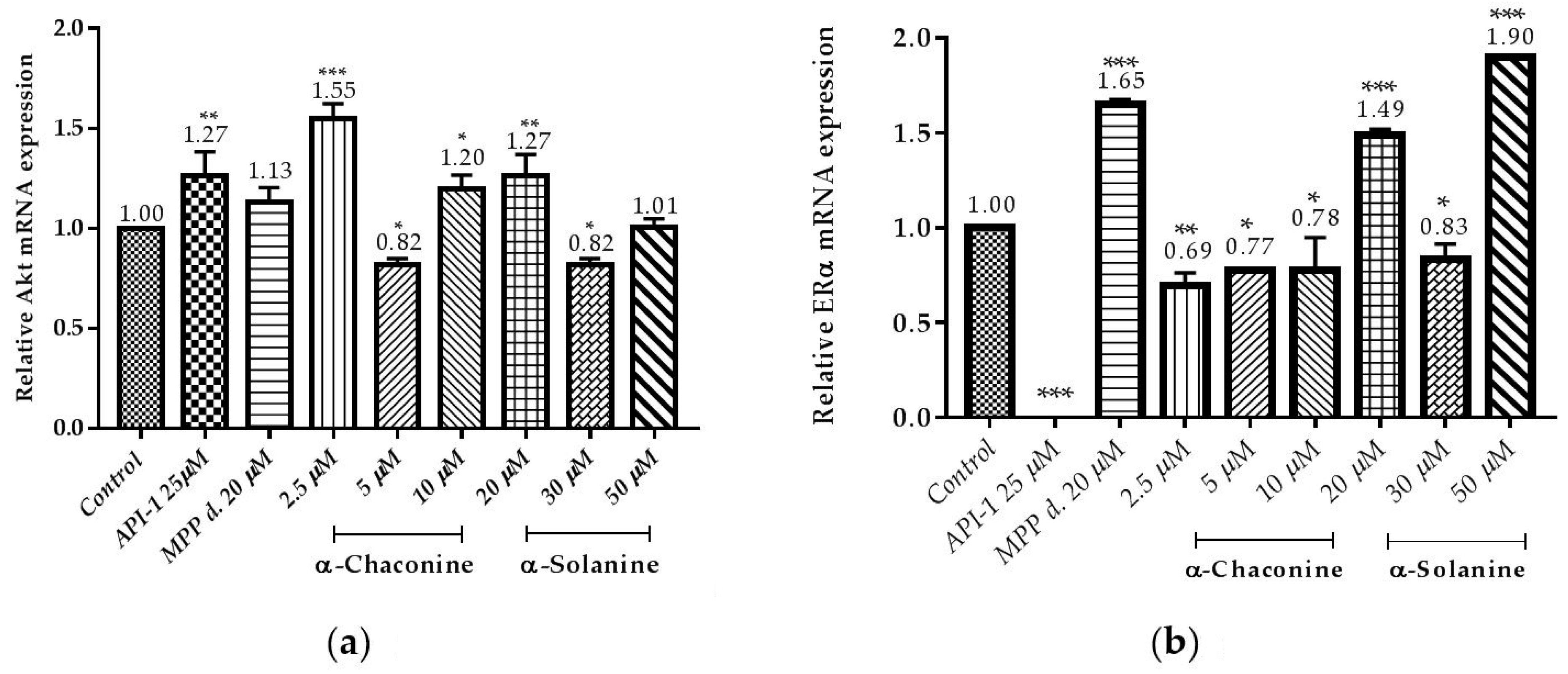
3.5. Changes in the Expression of Akt and ERα Genes
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Milner, S.E.; Brunton, N.P.; Jones, P.W.; O’Brien, N.M.; Collins, S.G.; Maguire, A.R. Bioactivities of glycoalkaloids and their aglycones from solanum species. J. Agric. Food Chem. 2011, 59, 3454–3484. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.H.; Liao, A.C.; Hung, J.H.; Lee, W.J.; Hu, K.C.; Lin, P.T.; Liao, R.F.; Chen, P.S. Alpha-solanine inhibits invasion of human prostate cancer cell by suppressing epithelial-mesenchymal transition and mmps expression. Molecules 2014, 19, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.; Sonawane, P.; Heinig, U.; Bocobza, S.; Burdman, S.; Aharoni, A. The bitter side of the nightshades: Genomics drives discovery in solanaceae steroidal alkaloid metabolism. Phytochemistry 2015, 113, 24–32. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Maiello, M.R.; D’Alessio, A.; Pergameno, M.; Normanno, N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: Role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets 2012, 16, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.W.; Chen, M.W.; Cheng, K.J.; Yu, P.Z.; Wei, X.; Shi, Z. Therapeutic potential of steroidal alkaloids in cancer and other diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.F.; Liu, S.P.; Pan, B.; Tang, Z.F.; Zhong, J.G.; Zhou, F.J. Solanine inhibits prostate cancer Du145 xenograft growth in nude mice by inducing cell cycle arrest in G1/S phase. J. South. Med. Univ. 2016, 36, 665–670. [Google Scholar]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Antifungal activity of secondary plant metabolites from potatoes (Solanum tuberosum L.): Glycoalkaloids and phenolic acids show synergistic effects. J. Appl. Microbiol. 2016, 120, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Lee, S.G.; Lee, H.H.; Lee, H.J.; Shin, J.S.; Kim, N.J.; An, H.J.; Nam, J.H.; Jang, D.S.; Lee, K.T. Alpha-chaconine isolated from a Solanum tuberosum L. cv Jayoung suppresses lipopolysaccharide-induced pro-inflammatory mediators via AP-1 inactivation in RAW 264.7 macrophages and protects mice from endotoxin shock. Chem. Biol. Interact. 2015, 235, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G. Teratogenic effect of potato glycoalkaloids. Zhonghua Fu Chan Ke Za Zhi 1993, 28, 73–75. [Google Scholar] [PubMed]
- Thorne, H.V.; Clarke, G.F.; Skuce, R. The inactivation of herpes simplex virus by some solanaceae glycoalkaloids. Antivir. Res. 1985, 5, 335–343. [Google Scholar] [CrossRef]
- Mitchell, G.; Lafrance, M.; Boulanger, S.; Séguin, D.L.; Guay, I.; Gattuso, M.; Marsault, É.; Bouarab, K.; Malouin, F. Tomatidine acts in synergy with aminoglycoside antibiotics against multiresistant staphylococcus aureus and prevents virulence gene expression. J. Antimicrob. Chemother. 2011, 67, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.C.; Bhat, K.P.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Novel bioactive steroidal alkaloids from pachysandra procumbens. Tetrahedron 2000, 56, 3133–3138. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhat, K.P.; Pisha, E.; Kennelly, E.J.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Activity-guided isolation of steroidal alkaloid antiestrogen-binding site inhibitors from pachysandra procumbens. J. Nat. Prod. 1998, 61, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dixit, V. Effects of short term treatment of solasodine on cauda epididymis in dogs. Indian J. Exp. Biol. 2002, 40, 169–173. [Google Scholar] [PubMed]
- Zha, X.M.; Zhang, F.R.; Shan, J.Q.; Zhang, Y.H.; Liu, J.O.; Sun, H.B. Synthesis and evaluation of in vitro anticancer activity of novel solasodine derivatives. Chin. Chem. Lett. 2010, 21, 1087–1090. [Google Scholar] [CrossRef]
- Lu, M.-K.; Chen, P.-H.; Shih, Y.-W.; Chang, Y.-T.; Huang, E.-T.; Liu, C.-R.; Chen, P.-S. Alpha-chaconine inhibits angiogenesis in vitro by reducing matrix metalloproteinase-2. Biol. Pharm. Bull. 2010, 33, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Vanamala, J.; Safe, S.H.; Miller, J.C., Jr. The bioactive compounds α-chaconine and gallic acid in potato extracts decrease survival and induce apoptosis in LNCaP and PC3 prostate cancer cells. Nutr. Cancer 2010, 62, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Lee, K.R.; Kim, H.J.; Lee, I.S.; Kozukue, N. Anticarcinogenic effects of glycoalkaloids from potatoes against human cervical, liver, lymphoma, and stomach cancer cells. J. Agric. Food Chem. 2005, 53, 6162–6169. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Komori, C.; Lee, Y.-Y.; Hashimoto, F.; Yahara, S.; Nohara, T.; Ejima, A. Cytotoxic activities of solanum steroidal glycosides. Biol. Pharm. Bull. 1996, 19, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-K.; Shih, Y.-W.; Chang Chien, T.-T.; Fang, L.-H.; Huang, H.-C.; Chen, P.-S. Alpha-solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Kong, H.; Dong, G.; Liu, L.; Tong, K.; Sun, H.; Chen, B.; Zhang, C.; Zhou, M. Antitumor efficacy of α-solanine against pancreatic cancer in vitro and in vivo. PLoS ONE 2014, 9, e87868. [Google Scholar] [CrossRef] [PubMed]
- Mohsenikia, M.; Alizadeh, A.M.; Khodayari, S.; Khodayari, H.; Karimi, A.; Zamani, M.; Azizian, S.; Mohagheghi, M.A. The protective and therapeutic effects of alpha-solanine on mice breast cancer. Eur. J. Pharmacol. 2013, 718, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; Brunton, N.P.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Cytotoxic and apoptotic potential of potato glycoalkaloids in a number of cancer cell lines. J. Agric. Sci. Appl. 2013, 2, 184–192. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Bansal, N.; Yendluri, V.; Wenham, R.M. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control J. Moffitt Cancer Cent. 2009, 16, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Cancer 2017. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 20 August 2017).
- Burke, W.M.; Orr, J.; Leitao, M.; Salom, E.; Gehrig, P.; Olawaiye, A.B.; Brewer, M.; Boruta, D.; Herzog, T.J.; Shahin, F.A. Endometrial cancer: A review and current management strategies: Part II. Gynecol. Oncol. 2014, 134, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, T.; Ofengeim, D.; Noh, K.-M.; Latuszek-Barrantes, A.; Hemmings, B.A.; Follenzi, A.; Zukin, R.S. The endogenous inhibitor of AKT, CTMP, is critical to ischemia-induced neuronal death. Nat. Neurosci. 2009, 12, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Q.; Lindsley, C.W.; Cheng, G.Z.; Yang, H.; Nicosia, S.V. The AKT/PKB pathway: Molecular target for cancer drug discovery. Oncogene 2005, 24, 7482–7492. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.B.; Honig, A.; Schönhals, T.; Weidler, C.; Häusler, S.; Krockenberger, M.; Grunewald, T.G.; Dombrowski, Y.; Rieger, L.; Dietl, J.; et al. Perifosine inhibits growth of human experimental endometrial cancers by blockade of AKT phosphorylation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Saglam, A.S.; Alp, E.; Elmazoglu, Z.; Menevse, E.S. Effect of API-1 and FR180204 on cell proliferation and apoptosis in human DLD-1 and LoVo colorectal cancer cells. Oncol. Lett. 2016, 12, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Sun, M.; He, L.; Zhou, Q.H.; Chen, J.; Sun, X.M.; Bepler, G.; Sebti, S.M.; Cheng, J.Q. A small molecule inhibits AKT through direct binding to AKT and preventing AKT membrane translocation. J. Biol. Chem. 2010, 285, 8383–8394. [Google Scholar] [CrossRef] [PubMed]
- Zivadinovic, D.; Watson, C.S. Membrane estrogen receptor-α levels predict estrogen-induced ERK1/2 activation in MCF-7 cells. Breast Cancer Res. 2004, 7, R130. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Acconcia, F.; Ascenzi, P. Estrogen receptor signalling: Bases for drug actions. Curr. Drug Targets Immune Endocr. Metab. Disord. 2005, 5, 305–314. [Google Scholar] [CrossRef]
- Vasquez, Y.M. Estrogen-regulated transcription: Mammary gland and uterus. Steroids 2018, 133, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.R.; Sheng, S.; Barnett, D.H.; Petz, L.N.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol. Cell. Endocrinol. 2003, 206, 13–22. [Google Scholar] [CrossRef]
- RTCA SP Instrument Operator’s Manual. Available online: http://sydney.edu.au/medicine/bosch/facilities/molecular-biology/live-cell/RTCA%20SP%20Instrument%20Operator%20Manual%20v4.pdf (accessed on 20 May 2017).
- RTCA Software Manual Software Version 1.2. Available online: http://sydney.edu.au/medicine/bosch/facilities/molecular-biology/live-cell/RTCA%20SP%20Instrument%20Operator%20Manual%20v4.pdf (accessed on 20 May 2017).
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.Z.; Zhu, L.; Jackson, J.A.; Gabos, S.; Sun, X.J.; Wang, X.B.; Xu, X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated jurkat and raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Roddick, J.G. The acetylcholinesterase-inhibitory activity of steroidal glycoalkaloids and their aglycones. Phytochemistry 1989, 28, 2631–2634. [Google Scholar] [CrossRef]
- Gubarev, M.I.; Enioutina, E.Y.; Taylor, J.L.; Visic, D.M.; Daynes, R.A. Plant-derived glycoalkaloids protect mice against lethal infection with salmonella typhimurium. Phytother. Res. 1998, 12, 79–88. [Google Scholar] [CrossRef]
- Fewell, A.M.; Roddick, J.G. Interactive antifungal activity of the glycoalkaloids α-solanine and α-chaconine. Phytochemistry 1993, 33, 323–328. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Sun, F.; Han, H.; Zhang, X.; Fan, Y.; Tai, G.; Zhou, Y. In vivo antimalarial activities of glycoalkaloids isolated from solanaceae plants. Pharm. Biol. 2010, 48, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis of biologically active compounds in potatoes (Solanum tuberosum), tomatoes (Lycopersicon esculentum), and jimson weed (Datura stramonium) seeds. J. Chromatogr. A 2004, 1054, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Keukens, E.A.; de Vrije, T.; van den Boom, C.; de Waard, P.; Plasman, H.H.; Thiel, F.; Chupin, V.; Jongen, W.M.; de Kruijff, B. Molecular basis of glycoalkaloid induced membrane disruption. Biochim. Biophys. Acta 1995, 1240, 216–228. [Google Scholar] [CrossRef]
- Yang, S.A.; Paek, S.H.; Kozukue, N.; Lee, K.R.; Kim, J.A. Alpha-chaconine, a potato glycoalkaloid, induces apoptosis of HT-29 human colon cancer cells through caspase-3 activation and inhibition of ERK 1/2 phosphorylation. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.W.; Chen, P.S.; Wu, C.H.; Jeng, Y.F.; Wang, C.J. Alpha-chaconine-reduced metastasis involves a PI3K/AKT signaling pathway with downregulation of NF-kappaB in human lung adenocarcinoma A549 cells. J. Agric. Food Chem. 2007, 55, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B. Continuously monitoring the cytotoxicity of api-1, α-chaconine and α-solanine on human lung carcinoma A549. Multidiscip. Digit. Publ. Inst. Proc. 2017, 1, 998. [Google Scholar] [CrossRef]
- Lee, K.R.; Kozukue, N.; Han, J.S.; Park, J.H.; Chang, E.Y.; Baek, E.J.; Chang, J.S.; Friedman, M. Glycoalkaloids and metabolites inhibit the growth of human colon (HT29) and liver (HepG2) cancer cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, G. Inhibitory effect of solanine on prostate cancer cell line PC-3 in vitro. Natl. J. Androl. 2011, 17, 284–287. [Google Scholar]
- Moxley, K.M.; McMeekin, D.S. Endometrial carcinoma: A review of chemotherapy, drug resistance, and the search for new agents. Oncologist 2010, 15, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-B.; Gao, S.-Y. Antihepatocarcinoma effect of solanine and its mechanisms. Chin. Herb. Med. 2012, 4, 126–135. [Google Scholar]
- Ji, Y.; Gao, S.; Ji, C.; Zou, X. Induction of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J. Ethnopharmacol. 2008, 115, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Henika, P.R.; Mackey, B.E. Effect of feeding solanidine, solasodine and tomatidine to non-pregnant and pregnant mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2003, 41, 61–71. [Google Scholar] [CrossRef]
- Keepers, Y.P.; Pizao, P.E.; Peters, G.J.; van Ark-Otte, J.; Winograd, B.; Pinedo, H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur. J. Cancer Clin. Oncol. 1991, 27, 897–900. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, R.; Zhang, G.; Cheng, R.; Bai, Y.; Zhao, H.; Lu, X.; Li, H.; Chen, S.; Li, J. Anticancer function of α-solanine in lung adenocarcinoma cells by inducing microrna-138 expression. Tumor Biol. 2016, 37, 6437–6446. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-Y.; Wang, Q.-J.; Ji, Y.-B. Effect of solanine on the membrane potential of mitochondria in HepG2 cells and [Ca2+] i in the cells. World J. Gastroenterol. 2006, 12, 3359. [Google Scholar] [CrossRef] [PubMed]
- Lilja, J.F.; Wu, D.; Reynolds, R.K.; Lin, J. Growth suppression activity of the PTEN tumor suppressor gene in human endometrial cancer cells. Anticancer Res. 2001, 21, 1969–1974. [Google Scholar] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Langkilde, S.; Mandimika, T.; Schrøder, M.; Meyer, O.; Slob, W.; Peijnenburg, A.; Poulsen, M. A 28-day repeat dose toxicity study of steroidal glycoalkaloids, α-solanine and α-chaconine in the Syrian Golden hamster. Food Chem. Toxicol. 2009, 47, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Rayburn, J.R.; Friedman, M.; Bantle, J.A. Synergistic interaction of glycoalkaloids α-chaconine and α-solanine on developmental toxicity in Xenopus embryos. Food Chem. Toxicol. 1995, 33, 1013–1019. [Google Scholar] [CrossRef]
- Groen, K.; Pereboom-De Fauw, D.P.K.H.; Besamusca, P.; Beekhof, P.K.; Speijers, G.J.A.; Derks, H.J.G.M. Bioavailability and disposition of ′H-solanine in rat and hamster. Xenobiotica 1993, 23, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Alozie, S.O.; Sharma, R.P.; Salunkhe, D.K. Physiological Disposition, Subcellular Distribution and Tissue Binding of A-Chaconine (3h). J. Food Saf. 1978, 1, 257–273. [Google Scholar] [CrossRef]




| Compound | 24 h |
|---|---|
| α-Chaconine | 4.72 µM |
| α-Solanine | 26.27 µM |
| API-1 | 56.67 µM |
| MPP dihydrochloride | 20.01 µM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaboğa Arslan, A.K.; Yerer, M.B. α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167). Nutrients 2018, 10, 672. https://doi.org/10.3390/nu10060672
Karaboğa Arslan AK, Yerer MB. α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167). Nutrients. 2018; 10(6):672. https://doi.org/10.3390/nu10060672
Chicago/Turabian StyleKaraboğa Arslan, Ayşe Kübra, and Mükerrem Betül Yerer. 2018. "α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167)" Nutrients 10, no. 6: 672. https://doi.org/10.3390/nu10060672
APA StyleKaraboğa Arslan, A. K., & Yerer, M. B. (2018). α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167). Nutrients, 10(6), 672. https://doi.org/10.3390/nu10060672






