Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
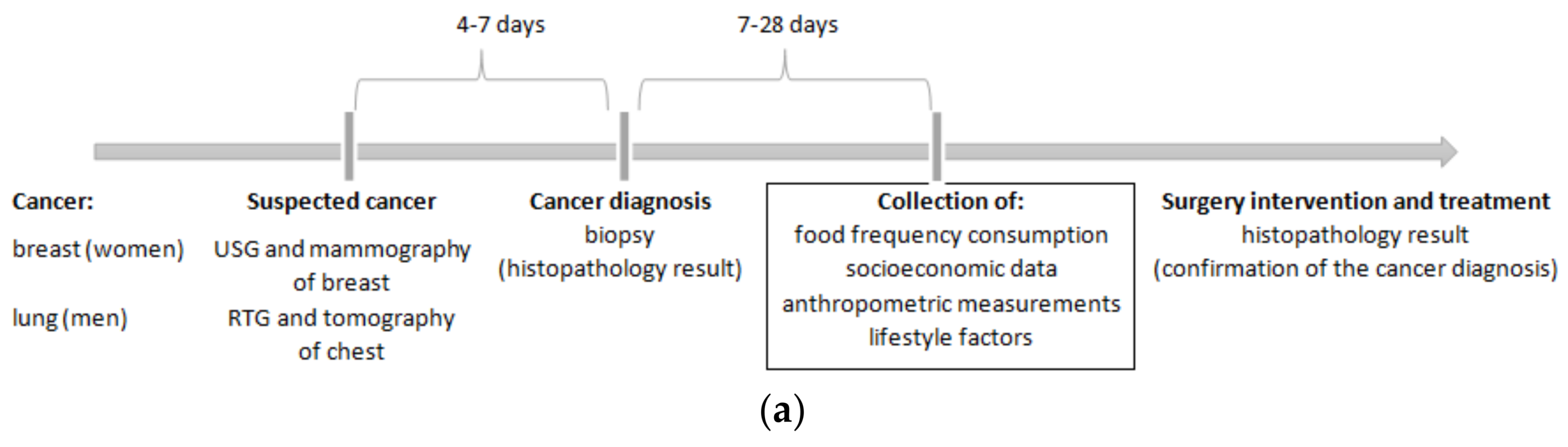
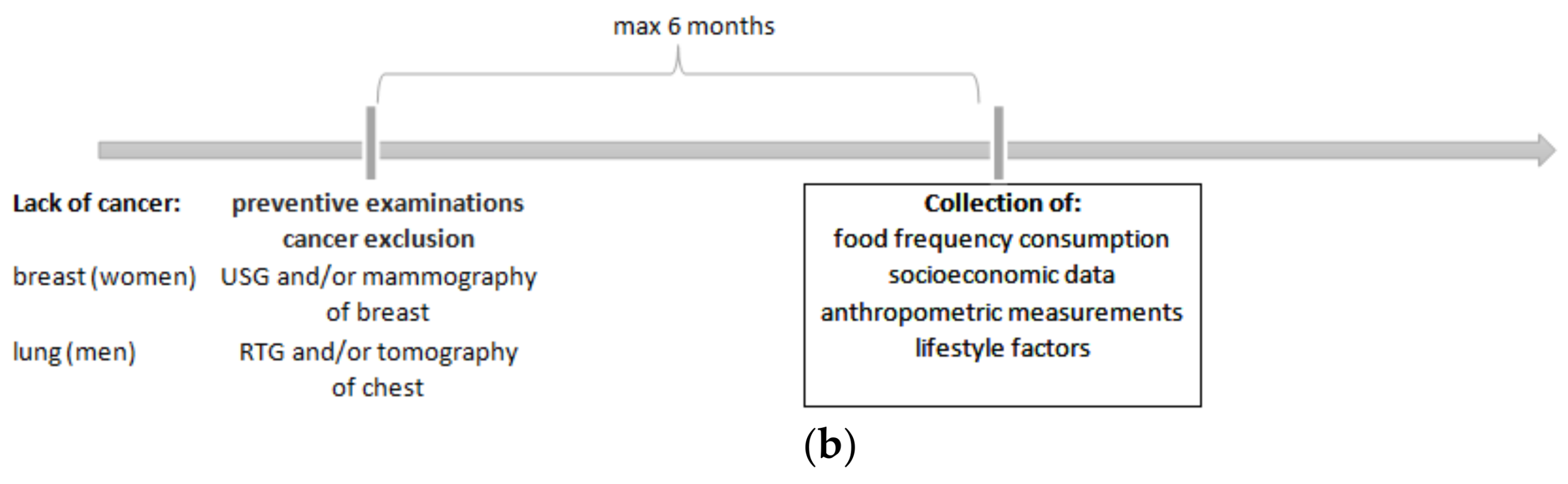
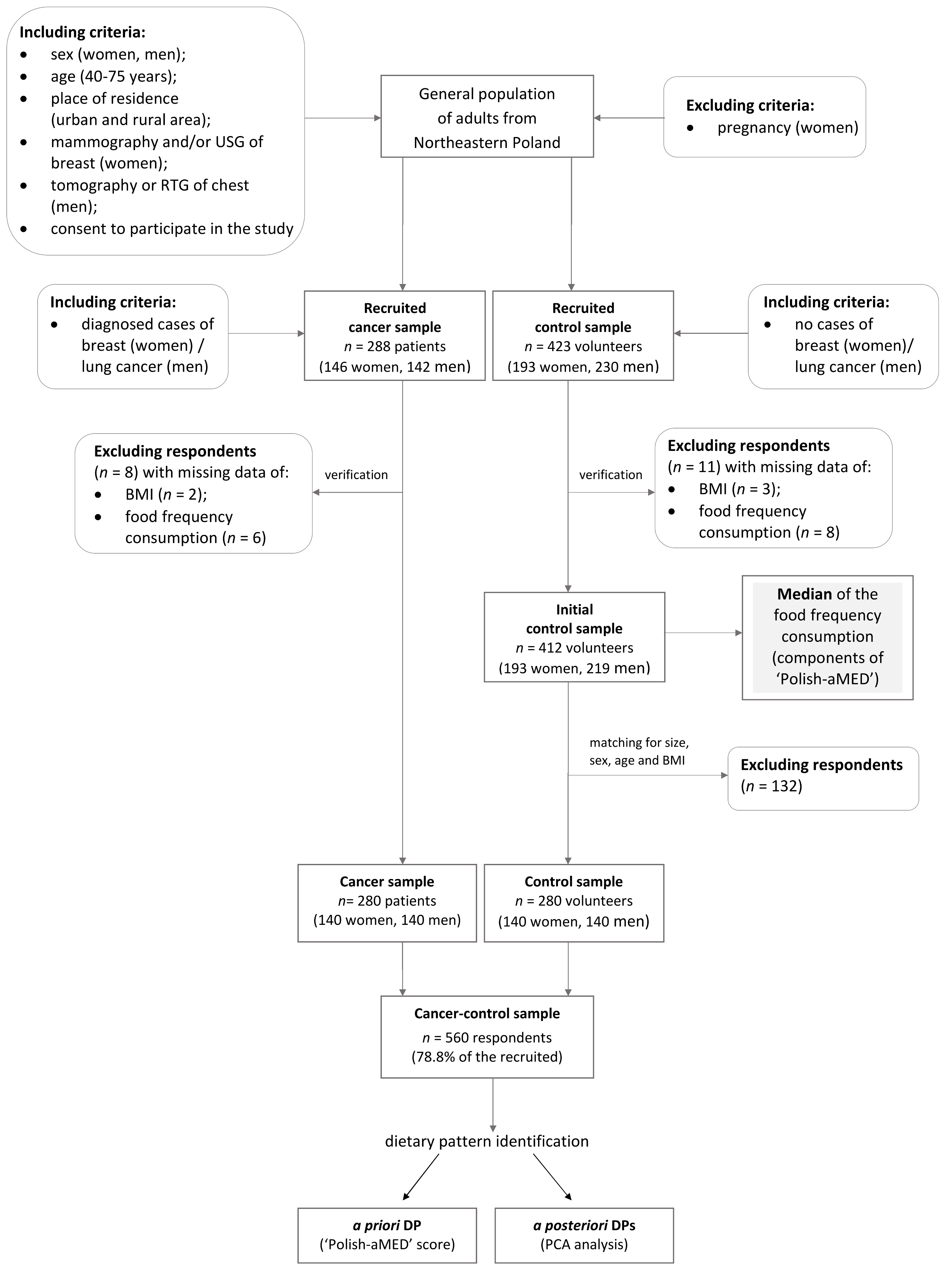
2.2. Study Design and Sample Characteristics
2.3. Food Frequency Consumption
2.4. Polish-Adapted Mediterranean Diet Score
2.5. Confounders
2.6. Statistical Analysis
3. Results
3.1. Food Frequency Consumption and Dietary Patterns
3.2. Dietary Patterns and Breast or Lung Cancer Risk
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; IARC: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 15 August 2017).
- World Cancer Report 2014; World Health Organization, International Agency for Research on Cancer, WHO Press: Geneva, Switzerland, 2015; Available online: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014 (accessed on 15 August 2017).
- Krajowy Rejestr Nowotworów, Centrum Onkologii—Instytut im. Marii Skłodowskiej—Curie (Polish National Cancer Registry, Oncology Center. Institute of M. Sklodowska-Curie). Available online: http://onkologia.org.pl/k/epidemiologia/ (accessed on 20 August 2017). (In Polish).
- World Health Organization—Cancer Country Profiles. 2014. Available online: http://www.who.int/cancer/country-profiles/pol_en.pdf?ua=1 (accessed on 20 August 2017).
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer; Continuous Update Project. Keeping the Science Current. Breast Cancer 2010 Report; American Institute for Cancer Research: Washington, DC, USA, 2010. [Google Scholar]
- Xu, S.; Wang, P.; You, Z.; Meng, H.; Mu, G.; Bai, X.; Zhang, G.; Zhang, J.; Pang, D. The long non-coding RNA EPB41L4A-AS2 inhibits tumor proliferation and is associated with favorable prognoses in breast cancer and other solid tumors. Oncotarget 2016, 7, 20704–20717. [Google Scholar] [CrossRef] [PubMed]
- Wirfält, E.; Drake, I.; Wallström, P. What do review papers conclude about food and dietary patterns? Food Nutr. Res. 2013, 57, 20523. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.; Vrieling, A.; Flesch-Janys, D.; Chang-Claude, J. Dietary patterns and the risk of postmenopausal breast cancer in a German case-control study. Cancer Causes Control 2011, 22, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Link, L.B.; Canchola, A.J.; Bernstein, L.; Clarke, C.A.; Stram, D.O.; Ursin, G.; Horn-Ross, P.L. Dietary patterns and breast cancer risk in the California Teachers Study cohort. Am. J. Clin. Nutr. 2013, 98, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Balder, H.F.; Goldbohm, R.A.; van den Brandt, P.A. Dietary Patterns Associated with Male Lung Cancer Risk in the Netherlands Cohort Study. Cancer Epidemiol. Biomark. Prev. 2005, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Boffetta, P.; Ronco, A.L.; Deneo-Pellegrini, H.; Acosta, G.; Gutierrez, L.P.; Mendilaharsua, M. Nutrient patterns and risk of lung cancer: NA factor analysis in Uruguayan men. Lung Cancer 2008, 61, 283–291. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.L.; Deneo-Pellegrini, H.; Correa, P.; Boffetta, P.; Acosta, G.; Mendilaharsu, M. Dietary patterns and risk of adenocarcinoma of the lung in males: A factor analysis in Uruguay. Nutr. Cancer 2011, 63, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Bessaoud, F.; Tretarre, B.; Daures, J.P.; Gerber, M. Identification of dietary patterns using two statistical approaches and their association with breast cancer risk: A case-control study in southern France. Ann. Epidemiol. 2012, 22, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Polla, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Can, J.M.; Lope, V.; Antoli, S.; Ramos, M.; Mun, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Pot, G.K.; Stephen, A.M.; Dahm, C.C.; Key, T.J.; Cairns, B.J.; Burley, V.J.; Cade, J.E.; Greenwood, D.C.; Keogh, R.H.; Bhaniani, A.; et al. Dietary patterns derived with multiple methods from food diaries and breast cancer risk in the UK Dietary Cohort Consortium. Eur. J. Clin. Nutr. 2014, 68, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Cottet, V.; Touvier, M.; Fournier, A.; Touillaud, M.S.; Lafay, L.; Clavel-Chapelon, F.; Boutron-Ruaulty, M. Postmenopausal Breast Cancer Risk and Dietary Patterns in the E3N-EPIC Prospective Cohort Study. Am. J. Epidemiol. 2009, 170, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Baglietto, L.; Krishnan, K.; Severi, G.; Hodge, A.; Brinkman, M.; English, D.R.; McLean, C.; Hopper, J.L.; Giles, G.G. Dietary patterns and risk of breast cancer. Br. J. Cancer 2011, 104, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, C.A.; Hadjisavvas, A.; Loizidou, M.A.; Loucaides, G.; Neophytou, I.; Sieri, S.; Kakouri, E.; Middleton, N.; Vineis, P.; Kyriacou, K. The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: A case control study. BMC Cancer 2012, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Sandin, S.; LÖf, M.; Ursin, G.; Adami, H.O.; Weiderpass, E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE 2013, 8, e55374. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Cottet, V.; Gonzalez, C.A.; Lujan-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the Mediterranean diet and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Gnagnarella, P.; Maisonneuve, P.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann. Oncol. 2013, 24, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Voevodina, O.; Billich, C.; Arand, B.; Nagel, G. Association of Mediterranean diet, dietary supplements and alcohol consumption with breast density among women in South Germany: A cross-sectional study. BMC Public Health 2013, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.M.; Bassett, J.K.; Shivappa, N.; He’bert, J.R.; English, D.R.; Giles, G.G.; Severi, G. Dietary inflammatory index, Mediterranean diet score, and lung cancer: A prospective study. Cancer Causes Control 2016, 27, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Matalas, A.L. Disparities Within Traditional Mediterranean Food Patterns: An Historical Approach of the Greek Diet. Int. J. Food Sci. Nutr. 2006, 57, 529–536. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Representative List of the Intangible Cultural Heritage of Humanity. 2010. Available online: www.unesco.org/culture/ich/index.php?lg=en&pg=00011&RL=00394 (accessed on 20 October 2017).
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Lidia Wadolowska Website. Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/ (accessed on 25 March 2017).
- Krupa-Kozak, U.; Drabińska, N.; Jarocka-Cyrta, E. The effect of oligofructose-enriched insulin supplementation on gut microbiota, nutritional status and gastrointestinal symptoms in paediatric coeliac disease patients on a gluten-free diet: Study protocol for a pilot randomized controlled trial. Nutr. J. 2017, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodrigo, C.; Escauriaza, B.A.; Bartrina, J.A.; Polanco Allúe, I. Dietary assessment in children and adolescents: Issues and recommendations. Nutr. Hosp. 2015, 31 (Suppl. 3), 76–83. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Ryterska, K.; Maciejewska, D.; Banaszczak, M.; Milkiewicz, P.; Milkiewicz, M.; Gutowska, I.; Ossowski, P.; Kaczorowska, M.; Jamioł-Milc, D.; et al. Nutritional Strategies for the individualized treatment of Non-Alcoholic Fatty Liver Disease (NAFLD) based on the Nutrient-Induced Insulin Output Ratio (NIOR). Int. J. Mol. Sci. 2016, 17, 1192. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Krusinska, B.; Hawrysz, I.; Slowinska, M.A.; Wadolowska, L.; Biernacki, M.; Czerwinska, A.; Golota, J.J. Dietary patterns and breast or lung cancer risk: A pooled analysis of two case-control studies in northern-eastern Poland. Adv. Clin. Exp. Med. 2017, 9, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Berry, G.; Matthews, J.N.S. Statistical Methods in Medical Research, 4th ed.; Blackwell Science: Oxford, UK, 2001. [Google Scholar]
- Previdelli, Á.N.; de Andrade, S.C.; Fisberg, R.M.; Marchioni, D.M. Using two different approaches to assess dietary patterns: Hypothesis-driven and data-driven analysis. Nutrients 2016, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Taraszewska, A. Nadwaga i otyłość oraz wybrane elementy stylu życia jako czynniki ryzyka GERD. (Overweight and obesity and selected lifestyle elements as risk factors for GERD). Postępy Nauk Medycznych 2011, 9, 749–759. (In Polish) [Google Scholar]
- Wadolowska, L.; Krusinska, B. The manual for developing nutritional data from the KomPAN questionnaire. In Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data; Gawecki, J., Ed.; The Committee of Human Nutrition, Polish Academy of Sciences: Warsaw, Poland, 2014; pp. 34–51. ISBN 978-83-63305-19-2. Available online: http://www.knozc.pan.pl/ (accessed on 2 February 2018).
- Anic, G.M.; Park, Y.; Subar, A.F.; Schap, T.E.; Reedy, J. Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study. Eur. J. Clin. Nutr. 2016, 70, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Shivappa, N.; Hébert, R.; Bellomi, M.; Rampinelli, C.; Bertolotti, R.; Spaggiari, L.; Palli, D.; Veronesi, G.; Gnagnarella, P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur. J. Nutr. 2016, 55, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.D.; Park, K.; Shin, A.; Ro, J.; Kim, J. Glycemic index and glycaemic load dietary patterns and the associated risk of breast cancer: A case-control study. Asian Pac. J. Cancer Prev. 2013, 14, 5193–5198. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P. Helping women to good health: Breast cancer, omega-3/omega-6 lipids, and related lifestyle factors. BMC Med. 2014, 12, 54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirko, K.A.; Willett, W.C.; Hankinson, S.E.; Rosner, B.A.; Beck, A.H.; Tamimi, R.M.; Eliassen, A.H. Healthy dietary patterns and risk of breast cancer by molecular subtype. Breast Cancer Res. Treat. 2016, 155, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E.; Taylor, E.F.; Burley, V.J.; Greenwood, D.C. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur. J. Clin. Nutr. 2011, 65, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.Y.; Weng, S.F.; Hernandez, L.; Spitz, M.R.; Forman, M.R. Dietary patterns affect lung cancer risk in never smokers. Nutr. Cancer 2011, 63, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Sweeney, C.; Giuliano, R.; Herrick, J.S.; Hines, L.; Byers, T.; Baumgartner, K.B.; Slattery, M.L. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: The Four-Corners Breast Cancer Study. Am. J. Clin. Nutr. 2008, 87, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational Studies: Cohort and Case-Control Studies. Plast. Reconstr. Surg. 2010, 6, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
| Food Groups | PCA-Derived Dietary Patterns | ‘Polish-aMED’ Score | ||
|---|---|---|---|---|
| ‘Prudent’ | ‘Non-Healthy’ | ‘Dressings and Sweetened-Low-Fat Dairy’ | ||
| Vegetables | 0.55 | 0.00 | 0.04 | 0.49 * |
| Fruits | 0.55 | 0.02 | −0.04 | 0.46 * |
| Milk, fermented milk drinks, curd cheese | 0.54 | 0.00 | 0.28 | 0.28 * |
| Whole grain products | 0.53 | −0.42 | −0.03 | 0.47 * |
| Fish | 0.51 | −0.05 | 0.05 | 0.34 * |
| Legumes | 0.48 | −0.01 | −0.14 | 0.33 * |
| Nuts and seeds | 0.46 | −0.28 | −0.16 | 0.44 * |
| Vegetable oils (including olive oil) | 0.44 | 0.27 | −0.04 | 0.30 * |
| Eggs | 0.43 | 0.24 | −0.01 | 0.18 * |
| Fruit, vegetable, vegetable-fruit juices | 0.39 | 0.19 | 0.06 | 0.12 * |
| Cereals | 0.35 | −0.07 | 0.19 | 0.18 * |
| Cheese | 0.31 | 0.30 | 0.08 | 0.11 * |
| White meat | 0.30 | 0.28 | 0.22 | 0.11 * |
| Refined grain products | −0.22 | 0.71 | 0.12 | −0.32 * |
| Sugar, honey and sweets | −0.02 | 0.60 | 0.09 | −0.10 * |
| Red and processed meats | 0.11 | 0.56 | 0.04 | −0.18 * |
| Potatoes | 0.03 | 0.52 | 0.04 | −0.10 * |
| Animal fats | 0.12 | 0.47 | −0.65 | −0.16 * |
| Sweetened beverages, energy drinks | 0.03 | 0.35 | −0.13 | −0.02 |
| Other edible fats (margarine, mayonnaise, dressings) | −0.06 | 0.17 | 0.81 | −0.01 |
| Sweetened milk drinks and flavoured homogenized cheese | 0.28 | 0.22 | 0.39 | 0.05 |
| Ratio of vegetable oils to animal fat | NA | NA | NA | 0.37 |
| Share in explaining the variance (%) | 14 | 12 | 7 | NA |
| Food Group/Dietary Items | Criteria for 1 Point |
|---|---|
| Vegetables: raw or cooked: cabbage, brussels sprouts, cauliflower, broccoli, kale, carrot, pepper, spinach, endive, lettuce, leek, celery, parsley, tomato, cucumber, cabbage, zucchini, pumpkin, eggplant, beets, parsnips, onion, garlic, radish, turnip, artichoke, asparagus, salads with mixed vegetables | Greater than median intake (times/day) * |
| Fruit: apricots, cherries, nectarines, peaches, plums, grapes, kiwis, oranges, mandarins, grapefruit, lemons, pomelos, pineapple, watermelon, melon, fresh dactyls, fresh figs, strawberries, raspberries, blackberries, blueberries, currants, bananas, apples, pears, avocado | Greater than median intake (times/day) * |
| Whole grains: whole-grain bread, whole-grain groats, brown rice, wholemeal pasta | Greater than median intake (times/day) * |
| Fish: freshwater fish (perch, panga, trout, carp, eel,) and marine fish (cod, salmon, sardines, hake, herring, tuna, mackerel, halibut) | Greater than median intake (times/day) * |
| Legumes: fresh or canned: corn, green beans, dry seeds of legumes in dishes: beans, soybeans, peas, chickpeas, hummus | Greater than median intake (times/day) * |
| Nuts and seeds: peanuts, hazelnuts, walnuts, almonds, pistachios, cashews, coconut, chestnuts,pumpkin seeds, sesame seeds, sunflower seeds, wheat germ | Greater than median intake (times/day) * |
| Ratio of vegetable oils (rapeseed oil, sunflower oil, linseed oil, olives) to animal fat (butter, cream, lard) instead of ratio of monounsaturated to saturated fat | Greater than median intake (times/day) * |
| Red and processed meat: red meat (pork, beef, veal), venison, sausages, ham, liver, entrails, bacon, pate | Lower than median intake (times/day) * |
| Characteristics | Initial Control Sample | |
|---|---|---|
| % or Mean (95% CI) | Median * | |
| Sample size | 412 | |
| Sex | ||
| Men | 53.2 | |
| Women | 46.8 | |
| Age (years) | 58.5 (57.8; 59.2) | |
| BMI (kg/m2) | 28.2 (27.8; 28.7) | |
| Frequency of consumption of food groups # (times/day) | ||
| Vegetables | 1.064 (1.010; 1.117) | 1.000 |
| Fruit | 0.917 (0.867; 0.967) | 1.000 |
| Whole grains | 0.767 (0.703; 0.832) | 0.671 |
| Fish | 0.268 (0.238; 0.297) | 0.200 |
| Legumes | 0.208 (0.181; 0.235) | 0.125 |
| Nuts and seeds | 0.281 (0.239; 0.323) | 0.100 |
| Ratio of vegetable oils to animal fat | 1.745 (1.231; 2.258) | 0.500 |
| Red and processed meat | 1.519 (1.431; 1.607) | 1.342 |
| Variable | Cancer-Control Sample | Cancer Sample | Control Sample | p-Value |
|---|---|---|---|---|
| Sample Size | 560 | 280 | 280 | |
| Sex | ||||
| Men | 50.0 | 50.0 | 50.0 | |
| Women | 50.0 | 50.0 | 50.0 | |
| Age (years *) | 60.9 (7.2) | 61.1 (8.0) | 60.7 (6.3) | 0.4483 |
| BMI (kg/m2 *) a | 27.3 (4.6) | 27.0 (5.1) | 27.5 (4.1) | 0.2006 |
| Place of residence | ||||
| village | 28.8 | 32.9 a | 24.6 a | |
| town (<20,000 inhabitants) | 22.3 | 23.9 | 20.7 | |
| town (20–100,000 inhabitants) | 20.0 | 19.3 | 20.7 | 0.0304 |
| city (>100,000 inhabitants) | 28.9 | 23.9 b | 33.9 b | |
| Education level | ||||
| primary | 18.6 | 27.5 a | 9.6 a | |
| secondary | 59.3 | 59.6 | 58.9 | <0.0001 |
| higher | 22.1 | 12.9 b | 31.4 b | |
| Economic situation | ||||
| below the average | 19.5 | 22.9 a | 16.1 a | |
| average | 67.5 | 66.1 | 68.9 | 0.0766 |
| above average | 13.0 | 11.1 | 15.0 | |
| Socioeconomic status b | ||||
| low | 32.5 | 41.1 a | 23.9 a | |
| average | 16.8 | 15.4 | 18.2 | <0.0001 |
| high | 50.7 | 43.6 b | 57.9 b | |
| Physical activity at work c | ||||
| low | 51.3 | 60.4 a | 42.1 a | |
| moderate | 33.6 | 25.7 b | 41.4 b | <0.0001 |
| high | 15.2 | 13.9 | 16.4 | |
| Physical activity in leisure time d | ||||
| low | 28.0 | 30.7 | 25.4 | |
| moderate | 58.9 | 60.0 | 57.9 | 0.0226 |
| high | 13.0 | 9.3 a | 16.8 a | |
| Overall physical activity e | ||||
| low | 51.8 | 61.4 a | 42.1 a | |
| moderate | 43.6 | 34.3 b | 52.9 b | <0.0001 |
| high | 4.6 | 4.3 | 5.0 | |
| Smokers f | 73.4 | 80.0 | 66.8 | 0.0004 |
| Current smokers | 30.0 | 32.5 | 27.5 | 0.1967 |
| Former smokers | 71.6 | 79.3 | 63.9 | <0.0001 |
| Abuse of alcohol g | 20.5 | 22.9 | 18.2 | 0.1739 |
| ‘Polish-aMED’ score (points) * | 4.3 (1.9) | 4.0 (1.9) | 4.6 (1.8) | 0.0002 |
| low (0–2) | 20.2 | 26.8 a | 13.6 a | |
| average (3–5) | 49.6 | 48.6 | 50.7 | 0.0001 |
| high (6–8) | 30.2 | 24.6 b | 35.7 b |
| Dietary Patterns | Tertiles/Levels | Sample Size | % | p-Value | Control | Breast or Lung Cancer | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | ORcrude | 95% CI | p-Value | ORadj | 95% CI | p-Value | |||||
| ‘Polish-aMED’ | low (0–2 points; ref.) | 113 | 66.4 | Ref. | Ref. | Ref. | |||||
| average (3–5 points) | 278 | 48.9 | <0.001 | 1.00 | 0.49 | 0.31; 0.77 | <0.01 | 0.49 | 0.30; 0.80 | <0.01 | |
| high (6–8 points) | 169 | 40.8 | 1.00 | 0.35 | 0.21; 0.58 | <0.0001 | 0.37 | 0.21; 0.64 | <0.001 | ||
| ‘Prudent’ | bottom (ref.) | 186 | 58.6 | Ref. | Ref. | Ref. | |||||
| middle | 188 | 44.7 | <0.05 | 1.00 | 0.57 | 0.38; 0.86 | <0.01 | 0.67 | 0.43; 1.05 | ns | |
| upper | 186 | 46.8 | 1.00 | 0.62 | 0.41; 0.94 | <0.05 | 0.73 | 0.45; 1.67 | ns | ||
| ‘Non-healthy’ | bottom (ref.) | 187 | 43.3 | Ref. | Ref. | Ref. | |||||
| middle | 187 | 47.6 | <0.01 | 1.00 | 1.19 | 0.79; 1.79 | ns | 0.98 | 0.64; 1.52 | ns | |
| upper | 186 | 59.1 | 1.00 | 1.89 | 1.25; 2.86 | <0.01 | 1.65 | 1.05; 2.59 | <0.05 | ||
| ‘Dressings and sweetened-low-fat dairy’ | bottom (ref.) | 186 | 46.2 | Ref. | Ref. | Ref. | |||||
| middle | 187 | 56.1 | ns | 1.00 | 1.49 | 0.99; 2.24 | ns | 1.50 | 0.98; 2.31 | ns | |
| upper | 187 | 47.6 | 1.00 | 1.06 | 0.70; 1.60 | ns | 1.04 | 0.68; 1.60 | ns | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krusinska, B.; Hawrysz, I.; Wadolowska, L.; Slowinska, M.A.; Biernacki, M.; Czerwinska, A.; Golota, J.J. Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients 2018, 10, 470. https://doi.org/10.3390/nu10040470
Krusinska B, Hawrysz I, Wadolowska L, Slowinska MA, Biernacki M, Czerwinska A, Golota JJ. Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients. 2018; 10(4):470. https://doi.org/10.3390/nu10040470
Chicago/Turabian StyleKrusinska, Beata, Iwona Hawrysz, Lidia Wadolowska, Malgorzata Anna Slowinska, Maciej Biernacki, Anna Czerwinska, and Janusz Jacek Golota. 2018. "Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study" Nutrients 10, no. 4: 470. https://doi.org/10.3390/nu10040470
APA StyleKrusinska, B., Hawrysz, I., Wadolowska, L., Slowinska, M. A., Biernacki, M., Czerwinska, A., & Golota, J. J. (2018). Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients, 10(4), 470. https://doi.org/10.3390/nu10040470







