Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design
Abstract
1. Introduction
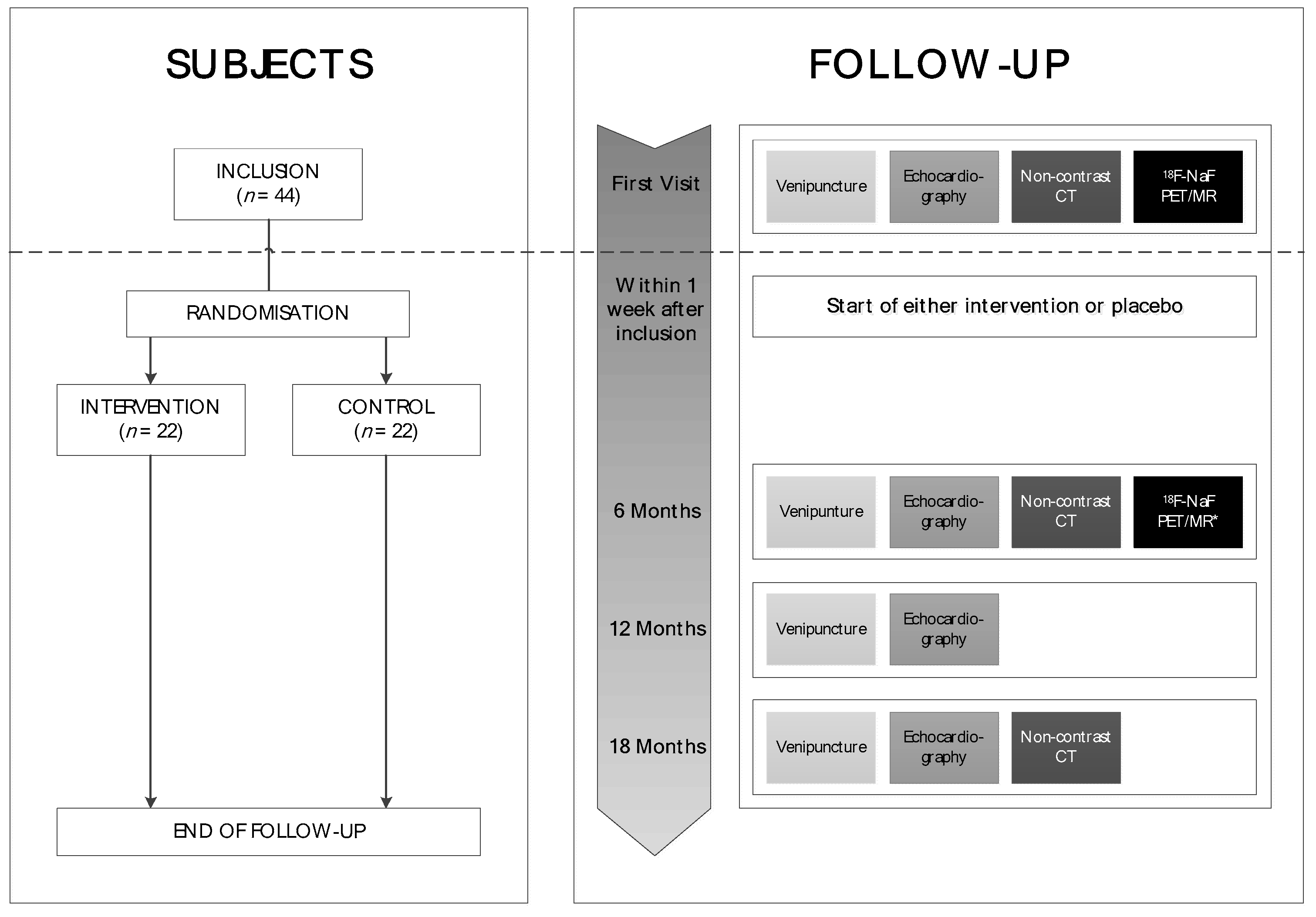
2. Trial Design
3. Inclusion and Exclusion Criteria
4. Study Objectives and Statistical Analyses Plan
4.1. Primary End Point and Sample Size Calculation
4.2. Secondary Endpoints
4.3. Additional Analyses
5. Study Procedures
5.1. PET and MR Imaging
5.2. Computed Tomography (CT) Imaging
5.3. Echocardiography
5.4. Laboratory Assessments
5.5. Randomization and Study Intervention
5.6. Study Intervention
6. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Intervention | Trial | Year or Clinicaltrials.gov Number | No. of Patients | Main Inclusion Criteria | Primary Endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Simvastatin + ezetimibe vs. placebo | SEAS (Simvastatin and Ezetimibe Aortic Stenosis) [46] | 2008 | 1873 | Patients (45–85 years) with asymptomatic mild to moderate aortic valve stenosis (Vmax 2.5–4.0 m/s) | Major cardiovascular events | No difference in occurrence of major cardiovascular events |
| Rosuvastatin vs. placebo | ASTRONOMER (Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin) [47] | 2010 | 269 | Patients (18–82 years) with asymptomatic mild to moderate aortic valve stenosis (Vmax 2.5–4.0 m/s) | Peak gradient and AVA progression in rosuvastatin arm vs. placebo (using echocardiography at baseline and annual measurements) | Rosuvastatin had no effect on the progression of aortic valve stenosis based on peak gradient and AVA |
| Rosuvastatin vs. placebo | PROCAS (Progression of Stenosis in Adult Patients With Congenital Aortic Stenosis) [48] | 2011 | 63 | Patients (18–45 years) with asymptomatic congenital aortic valve stenosis (Vmax ≥ 2.5 m/s) | Aortic valve stenosis progression based on Vmax in rosuvastatin arm vs. placebo (using echocardiography at baseline and annual measurements) | Rosuvastatin had no effect on the progression of congenital aortic valve stenosis (based on Vmax, mean gradient and AVA) |
| Fluvastatin vs. placebo | AORTICA 1 (Randomized Study to Evaluate the Efficacy of Fluvastatin on Inflammatory Markers in Patients With Aortic Stenosis) | NCT00404287 | 164 | Patients (>18 years) with asymptomatic aortic valve stenosis (Vmax > 2 m/s) | Changes in CRP (mg/dL) concentrations at 12 months | Not available |
| Fluvastatin vs. placebo | Statin Therapy in Asymptomatic Aortic Stenosis | NCT00176410 | 100 | Patients (21–80 years) with asymptomatic mild to moderate aortic valve stenosis (Vmax > 2.5 m/s, 0.8 <AVA <1.5 cm2) | Progression of aortic valve stenosis and hemodynamic parameters (using TTE and catheterization at 24 months) | Not available |
| Ramipril vs. placebo | RIAS (a prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor ramipril in aortic stenosis) [49] | 2015 | 100 | Patients (>18 years) with asymptomatic moderate to severe aortic valve stenosis (valve area < 1.5 cm2 or Vmax > 3.0 m/s) without indications for valve replacement surgery | Change in LVM in the ramipril arm vs. the placebo arm (using CMR at baseline at 6 months and 1 year) | Modest (but significant) difference in LVM between the two groups after 1 year (regression of LVM in the ramipril arm vs. increased LVM in the placebo arm) |
| Captopril and trandolapril vs. placebo | ACCESS (Acute Haemodynamic Effects of Treatment With Angiotensin Converting Enzyme (ACE)-inhibitors in Patients With Symptomatic Aortic Stenosis | NCT00252317 | 64 | Patients (>18 years) with asymptomatic and symptomatic severe aortic valve stenosis (AVA < 1.0 cm2) | Improvement of haemodynamic parameters after 8 weeks of treatment with ACE-inhibitor vs. placebo | Not available |
| Eplerenone vs. placebo | ZEST (A randomized trial of the aldosterone-receptor antagonist, eplerenone, in asymptomatic moderate–severe aortic stenosis) [50] | 2008 | 65 | Patients with asymptomatic moderate to severe aortic valve stenosis (Vmax > 3.0 m/s) with ejection fraction > 50%, without indications for valve replacement surgery | Delay of onset of LV systolic dysfunction or reduction of progression of LV hypertrophy in the eplerenone arm vs. placebo (using CMR) | Eplerenone did not show a clear effect on primary endpoints. |
| Candesartan vs. placebo | Is blockade of the renin–angiotensin system able to reverse the structural and functional remodeling of the left ventricle in severe aortic stenosis? [51] | 2015 | 51 | Patients (>18 years) with severe aortic valve stenosis referred for valve replacement surgery | Changes in LV structure and function and improvement of exercise capacity in the eplerenone arm vs. placebo (at 5 months) | Candesartan did not have favorable effects on the left ventricle or exercise tolerance. |
| Candesartan vs. placebo | ROCK-AS (The Potential of Candesartan to Retard the Progression of Aortic Stenosis) | NCT00699452 | 120 | Patients (>18 years) with clinically symptomatic severe aortic valve stenosis, not treated with ACE-inhibitors or AT1R antagonists | Inflammation in the valves at 3–5 months | Not available |
| Fimasartan vs. placebo | ALFA (A Randomized Trial of Angiotensin Receptor bLocker, Fimasarta, in Aortic Stenosis) | NCT01589380 | 100 | Patients (20–75 years), with moderate to severe (asymptomatic) aortic valve stenosis (Vmax 3.0–4.5 m/s, mean gradient 25–49 mmHg or AVA 0.76–1.5 cm2), able to undergo cardiopulmonary exercise testing | Change in VO2max during cardiopulmonary exercise testing at 1 year | Not available |
| Tadalafil vs. placebo | ASPEN (Aortic stenosis and phosphodiesterase type 5 inhibition: a pilot study) | NCT01275339 | 56 | Patients (>18 years) with moderate to severe aortic valve stenosis (AVA < 1.5 cm2), without indications for valve replacement surgery | Change in LVM (using CMR at 6 months), change in diastolic function (using tissue Doppler e’ at 12 weeks and 6 months) and change in LV longitudinal peak systolic strain (using echocardiography at 12 weeks and 6 months) | Not available. |
References
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Aikawa, E.; Merryman, W.D. Potential drug targets for calcific aortic valve disease. Nat. Rev. Cardiol. 2014, 11, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Ko, J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chandra, S.; Sucosky, P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS ONE 2012, 7, e48843. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.; Aikawa, E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ. Res. 2011, 108, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Pawade, T.A.; Newby, D.E.; Dweck, M.R. Calcification in Aortic Stenosis: The Skeleton Key. J. Am. Coll. Cardiol. 2015, 66, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; O’Brien, K.D.; et al. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Thomas, G.R.; Pardini, A.W.; Figueira, W.F.; Caputo, J.M.; Williamson, M.K. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J. Biol. Chem. 2002, 277, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Venardos, N.; Bennett, D.; Weyant, M.J.; Reece, T.B.; Meng, X.; Fullerton, D.A. Matrix Gla protein regulates calcification of the aortic valve. J. Surg. Res. 2015, 199, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. NMCD 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.; van der Meer, I.M.; Hofman, A.; Witteman, J.C. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyak, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Jenkins, W.S.; Vesey, A.T.; Pringle, M.A.; Chin, C.W.; Malley, T.S.; Cowie, W.J.; Tsampasian, V.; Richardson, H.; Fletcher, A.; et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ. Cardiovasc. Imaging 2014, 7, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, W.S.; Vesey, A.T.; Shah, A.S.; Pawade, T.A.; Chin, C.W.; White, A.C.; Fletcher, A.; Cartlidge, T.R.; Mitchell, A.J.; Pringle, M.A.; et al. Valvular (18)F-Fluoride and (18)F-Fluorodeoxyglucose Uptake Predict Disease Progression and Clinical Outcome in Patients With Aortic Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1200–1201. [Google Scholar] [CrossRef] [PubMed]
- LaForest, R.; Woodard, P.K.; Gropler, R.J. Cardiovascular PET/MRI: Challenges and Opportunities. Cardiol. Clin. 2016, 34, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ratib, O.; Nkoulou, R. Potential Applications of PET/MR Imaging in Cardiology. J. Nucl. Med. 2014, 55 (Suppl. 2), 40S–46S. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease: The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [PubMed]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A.; Scottish Aortic, S.; Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Dichtl, W.; Alber, H.F.; Feuchtner, G.M.; Hintringer, F.; Reinthaler, M.; Bartel, T.; Sussenbacher, A.; Grander, W.; Ulmer, H.; Pachinger, O.; et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am. J. Cardiol. 2008, 102, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, V.M.; Reinartz, S.; Kaesler, N.; Kruger, T.; Dirrichs, T.; Kramann, R.; Peeters, F.; Floege, J.; Keszei, A.; Marx, N.; et al. Slower Progress of Aortic Valve Calcification With Vitamin K Supplementation: Results From a Prospective Interventional Proof-of-Concept Study. Circulation 2017, 135, 2081–2083. [Google Scholar] [CrossRef] [PubMed]
- Sievers, H.H.; Schmidtke, C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 2007, 133, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Messika-Zeitoun, D.; Bielak, L.F.; Peyser, P.A.; Sheedy, P.F.; Turner, S.T.; Nkomo, V.T.; Breen, J.F.; Maalouf, J.; Scott, C.; Tajik, A.J.; et al. Aortic valve calcification: Determinants and progression in the population. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Noordzij, M.; Tripepi, G.; Dekker, F.W.; Zoccali, C.; Tanck, M.W.; Jager, K.J. Sample size calculations: Basic principles and common pitfalls. Nephrol. Dial. Transplant. 2010, 25, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Jones, C.; Joshi, N.V.; Fletcher, A.M.; Richardson, H.; White, A.; Marsden, M.; Pessotto, R.; Clark, J.C.; Wallace, W.A.; et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012, 125, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Kai, H.; Ishibashi, M.; Nakaura, H.; Kaida, H.; Baba, K.; Hayabuchi, N.; Imaizumi, T. Simvastatin attenuates plaque inflammation: Evaluation by fluorodeoxyglucose positron emission tomography. J. Am. Coll. Cardiol. 2006, 48, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Kao, H.L.; Huang, C.L.; Chen, M.F.; Lin, L.Y.; Wang, Y.C.; Lin, Y.H.; Lin, H.J.; Tzen, K.Y.; Yen, R.F.; et al. The effects of 3-month atorvastatin therapy on arterial inflammation, calcification, abdominal adipose tissue and circulating biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Eveborn, G.W.; Schirmer, H.; Heggelund, G.; Lunde, P.; Rasmussen, K. The evolving epidemiology of valvular aortic stenosis. The Tromso study. Heart 2013, 99, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quinones, M.; American Society of Echocardiography. European Association of Echocardiography. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Baron-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L.; European Association of Echocardiography. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I.; et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [PubMed]
- MenaQ7. Available online: http://menaq7.com/why-menaq7/varieties/ (accessed on 2 March 2018).
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Westenfeld, R.; Krueger, T.; Schlieper, G.; Cranenburg, E.C.; Magdeleyns, E.J.; Heidenreich, S.; Holzmann, S.; Vermeer, C.; Jahnen-Dechent, W.; Ketteler, M.; et al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: A randomized Trial. Am. J. Kidney Dis. 2012, 59, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Teunissen, K.J.; Spronk, H.M.; Hamulyak, K.; Ten Cate, H.; Shearer, M.J.; Vermeer, C.; Schurgers, L.J. Effect of low-dose supplements of menaquinone-7 (vitamin K2) on the stability of oral anticoagulant treatment: Dose-response relationship in healthy volunteers. J. Thromb. Haemost. JTH 2013, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Rossebo, A.B.; Pedersen, T.R.; Boman, K.; Brudi, P.; Chambers, J.B.; Egstrup, K.; Gerdts, E.; Gohlke-Barwolf, C.; Holme, I.; Kesaniemi, Y.A.; et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 2008, 359, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Teo, K.; Dumesnil, J.G.; Ni, A.; Tam, J.; Investigators, A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010, 121, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, D.; Yap, S.C.; van Dijk, A.P.; Budts, W.; Pieper, P.G.; van der Burgh, P.H.; Mulder, B.J.; Witsenburg, M.; Cuypers, J.A.; Lindemans, J.; et al. Effects of rosuvastatin on progression of stenosis in adult patients with congenital aortic stenosis (PROCAS Trial). Am. J. Cardiol. 2011, 108, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.; Loudon, M.; Francis, J.M.; Joseph, J.; Gerry, S.; Karamitsos, T.D.; Prendergast, B.D.; Banning, A.P.; Neubauer, S.; Myerson, S.G. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial). Eur. Heart J. Cardiovasc. Imaging 2015, 16, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.A.; Kerr, A.J.; Cowan, B.R.; Young, A.A.; Occleshaw, C.; Richards, A.M.; Edwards, C.; Whalley, G.A.; Freidlander, D.; Williams, M.; et al. A randomized trial of the aldosterone-receptor antagonist eplerenone in asymptomatic moderate-severe aortic stenosis. Am. Heart J. 2008, 156, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Helske-Suihko, S.; Laine, M.; Lommi, J.; Kaartinen, M.; Werkkala, K.; Kovanen, P.T.; Kupari, M. Is blockade of the Renin-Angiotensin system able to reverse the structural and functional remodeling of the left ventricle in severe aortic stenosis? J. Cardiovasc. Pharmacol. 2015, 65, 233–240. [Google Scholar] [CrossRef] [PubMed]
| Intervention | Trial | Year or Clinicaltrials.gov Number | No. of Patients | Main Inclusion Criteria | Primary Endpoint | Conclusion |
|---|---|---|---|---|---|---|
| Atorvastatin vs. placebo | SALTIRE (Scottish Aortic Stenosis and Lipid Lowering Trial: Impact on Regression) [23] | 2005 | 155 | Patients (>18 years) with aortic valve stenosis (Vmax ≥ 2.5 m/s) and aortic valve calcifications, without indications for AVR | Calcium score and Vmax progression in atorvastatin, arm vs. placebo (using echocardiography and cardiac CT at baseline, 12 and 24 months) | Atorvastatin had no effect on the rate of change in Vmax or valvular calcification |
| Atorvastatin vs. placebo | TASS (Tyrolean Aortic Stenosis Study) [24] | 2008 | 47 | Patients (>18 years) with aortic valve stenosis (mean gradient ≥15 mmHg, Vmax ≥ 2.0 m/s) and aortic valve calcifications, without indications for AVR | Calcium score and mean pressure gradient progression in atorvastatin arm vs. placebo (using echocardiography and cardiac CT at baseline, 12 and 24 months) | Atorvastatin did not reduce progression of CAVS based on mean pressure gradient and aortic valve calcification |
| Vitamin K1 | Slower progress of aortic valve calcification with vitamin K supplementation. Results from a prospective interventional proof-of-concept study [25] | 2017 | 99 | Patients with asymptomatic or mildly symptomatic aortic valve calcification (Vmax > 2.0 m/s), without indications for aortic valve replacement | Difference in progression of aortic valve calcification between the vitamin K arm and the placebo arm (using cardiac CT at 1 year) | Vitamin K might decelerate the progression of aortic valve calcification, measured by cardiac CT when compared to placebo. |
| PCSK9 inhibitor vs. placebo | PCSK9 inhibitors in the progression of aortic stenosis | NCT03051360 | 140 | Patients (>18 years) with mild to moderate aortic valve stenosis | Calcium score progression in the PCSK9 treated arm vs. placebo arm (using cardiac CT and NaF PET at 2 years) | Not available |
| Niacin vs. placebo | EAVaLL (Early Aortic Valve Lipoprotein(a) lowering trial) | NCT02109614 | 238 | Patients (51–84 years) with presence of aortic sclerosis or mild aortic stenosis (AVA > 1.5 cm2, mean gradient 25 mmHg) and high Lp(a) (>50 mg/dL) | Calcium score progression in the niacin arm compared to the placebo arm (using cardiac CT at 2 years) | Not available |
| Alendronic acid vs. placebo; Denosumab vs. placebo | SALTIRE II and RANKL inhibition in aortic stenosis (Study investigating the effect of drugs used to treat osteoporosis on the progression of calcific aortic stenosis) | NCT02132026 | 150 | Patients (>50 years) with aortic valve stenosis based on echocardiography (Vmax > 2.5 m/s and grade 2–4 calcification), without indications for valve replacement surgery | Change in aortic valve calcium score (using CT at baseline, 6 months and 2 years) | Not available |
| Ataciguat vs. placebo | A Study Evaluating the Effects of Ataciguat (HMR1766) on Aortic Valve Calcification (CAVS) | NCT02481258 | 35 | Patients (>50 years) with mild to moderate aortic valve stenosis/calcification (1.0 < AVA < 2.0 cm2, calcium level > 300 AU and LVEF > 50%) | Change in aortic valve calcium between the HMR1766 arm vs. the placebo arm (using CT at 6 and 12 months) | Not available |
| Phytine | CALCIFICA (Value of oral phytate in the prevention of progression of cardiovascular calcifications) | NCT01000233 | 250 | Patients (>18 years) with calcium in the aortic valve (characterized by Rosenhek score grade 2/3 on echocardiography) | Calcium in the aortic valve and in the coronary arteries in the phytine arm vs. the placebo arm (using CT at 24 months) | Not available |
| Inclusion criteria |
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Anatomy and function AoV [34,35,36] |
| Diameter LVOT, aortic sinus, STJ, ascending aorta |
| Systolic LV function and dimension [37,38] |
| Filling pressure and LV diastolic function [39] |
| RV function [40] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peeters, F.E.C.M.; Van Mourik, M.J.W.; Meex, S.J.R.; Bucerius, J.; Schalla, S.M.; Gerretsen, S.C.; Mihl, C.; Dweck, M.R.; Schurgers, L.J.; Wildberger, J.E.; et al. Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design. Nutrients 2018, 10, 386. https://doi.org/10.3390/nu10040386
Peeters FECM, Van Mourik MJW, Meex SJR, Bucerius J, Schalla SM, Gerretsen SC, Mihl C, Dweck MR, Schurgers LJ, Wildberger JE, et al. Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design. Nutrients. 2018; 10(4):386. https://doi.org/10.3390/nu10040386
Chicago/Turabian StylePeeters, Frederique E. C. M., Manouk J. W. Van Mourik, Steven J. R. Meex, Jan Bucerius, Simon M. Schalla, Suzanne C. Gerretsen, Casper Mihl, Marc R. Dweck, Leon J. Schurgers, Joachim E. Wildberger, and et al. 2018. "Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design" Nutrients 10, no. 4: 386. https://doi.org/10.3390/nu10040386
APA StylePeeters, F. E. C. M., Van Mourik, M. J. W., Meex, S. J. R., Bucerius, J., Schalla, S. M., Gerretsen, S. C., Mihl, C., Dweck, M. R., Schurgers, L. J., Wildberger, J. E., Crijns, H. J. G. M., & Kietselaer, B. L. J. H. (2018). Bicuspid Aortic Valve Stenosis and the Effect of Vitamin K2 on Calcification Using 18F-Sodium Fluoride Positron Emission Tomography/Magnetic Resonance: The BASIK2 Rationale and Trial Design. Nutrients, 10(4), 386. https://doi.org/10.3390/nu10040386






