Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Standardized Extracts (KBH-JP-040)
2.2. Quantitative Analysis of Liriodendrin, Betulin, and Formononetin by HPLC
2.3. Isolation and Culture of Chondrocytes
2.4. Cytotoxicity Measurement and Treatment Procedures
2.5. Evaluation of Protein Level by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Measurement of Protein Expression by Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
2.7. Reactive Oxygen Species (ROS) Measurement
2.8. Western Blot Analysis
2.9. Animal Experimental Design
2.10. Induction of Arthritis
2.11. Magnetic Resonance Imaging (MRI) Examination
2.12. Sample Collection
2.13. Histopathological Examination
2.14. Serum Substance-P and Cytokine Measurement
2.15. Statistical Analysis
3. Results
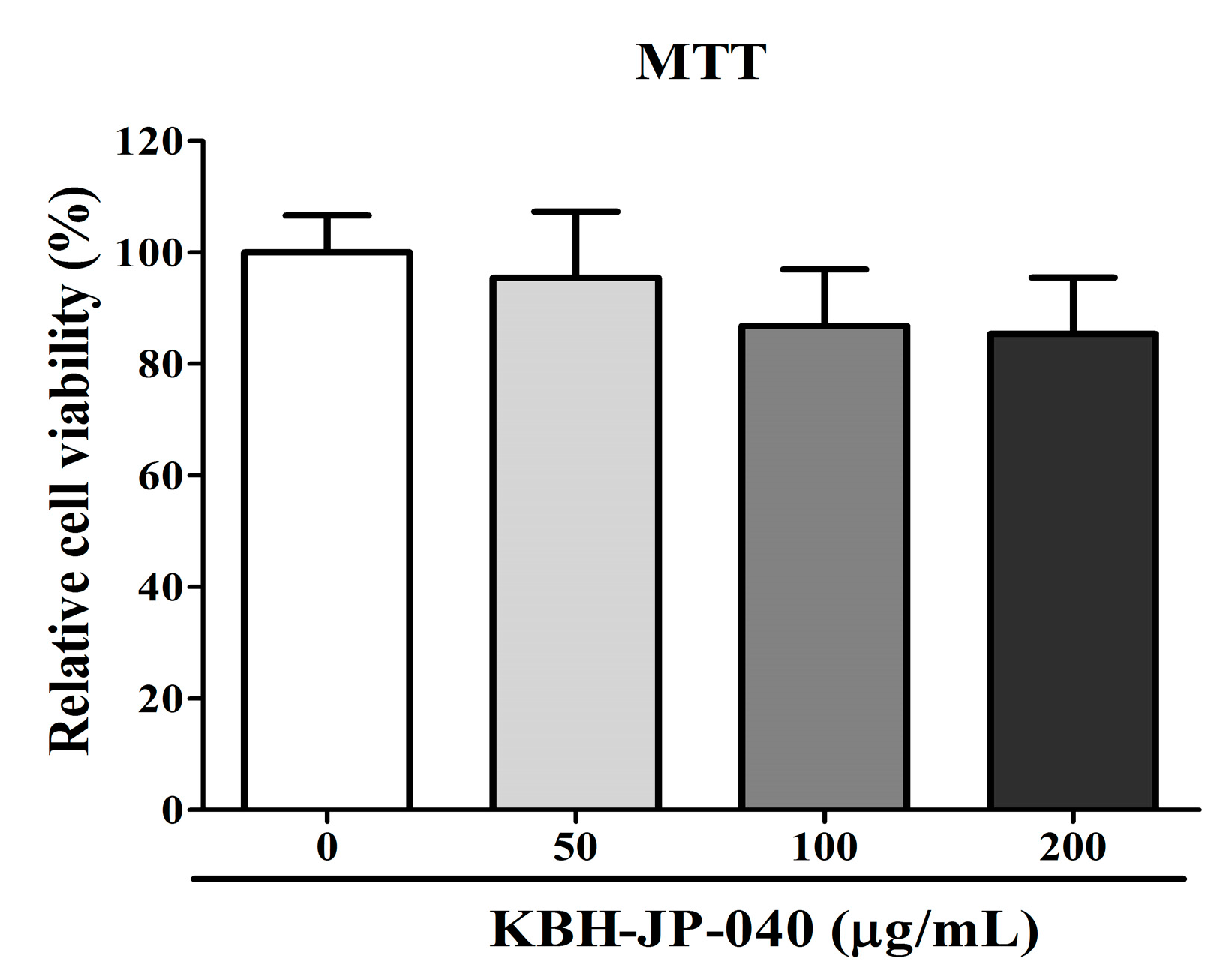
3.1. Cell Viability
3.2. Effects of KBH-JP-040 on Aggrecan and Collagen Type-II, PGE2, and ROS
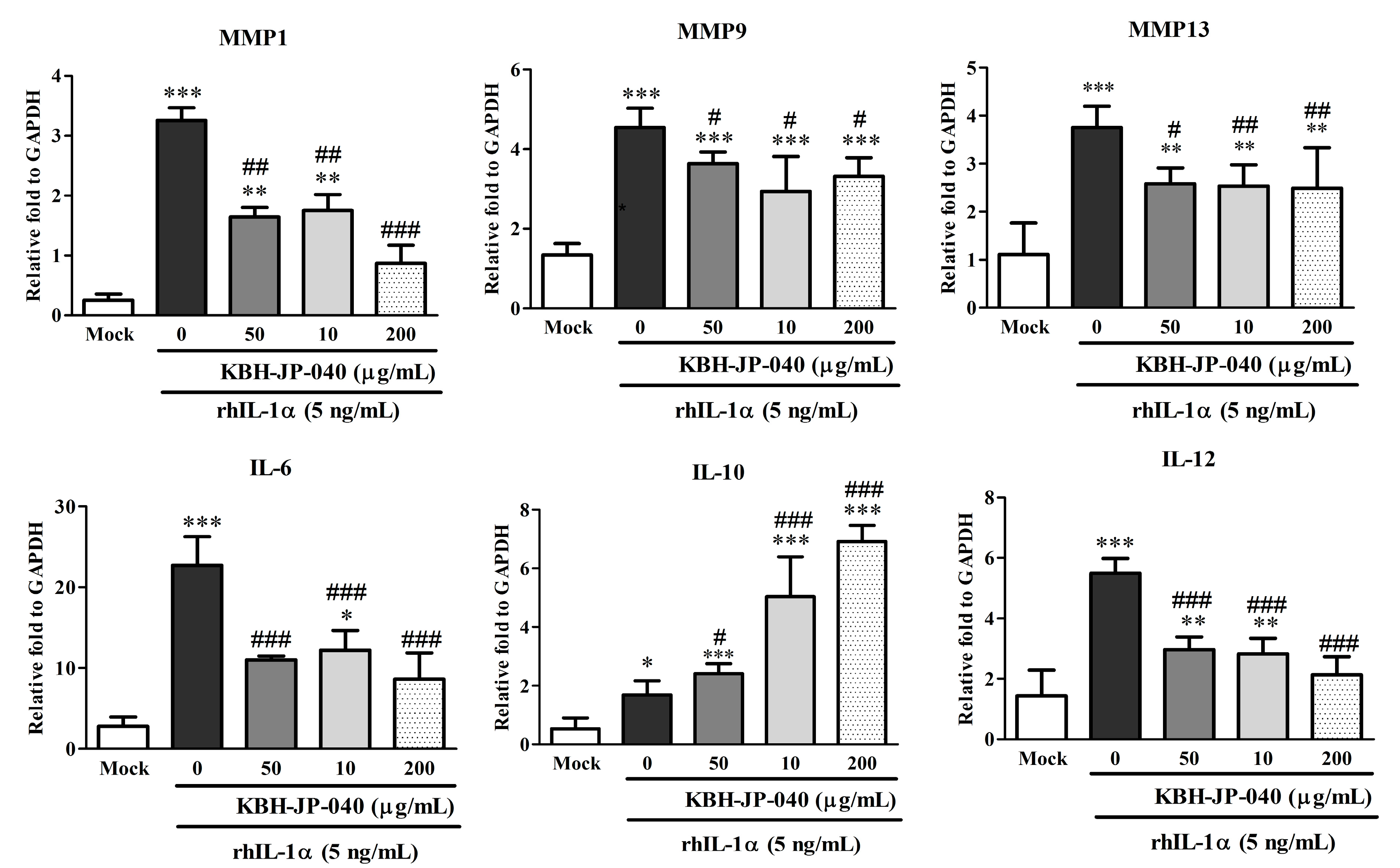
3.3. Effects of KBH-JP-040 on Matrix Metalloproteinase (MMP) and Interleukin (IL) Protein Levels
3.4. Effects of KBH-JP-040 on NF-κB, p38, JNK Activation, and IκBα Degradation
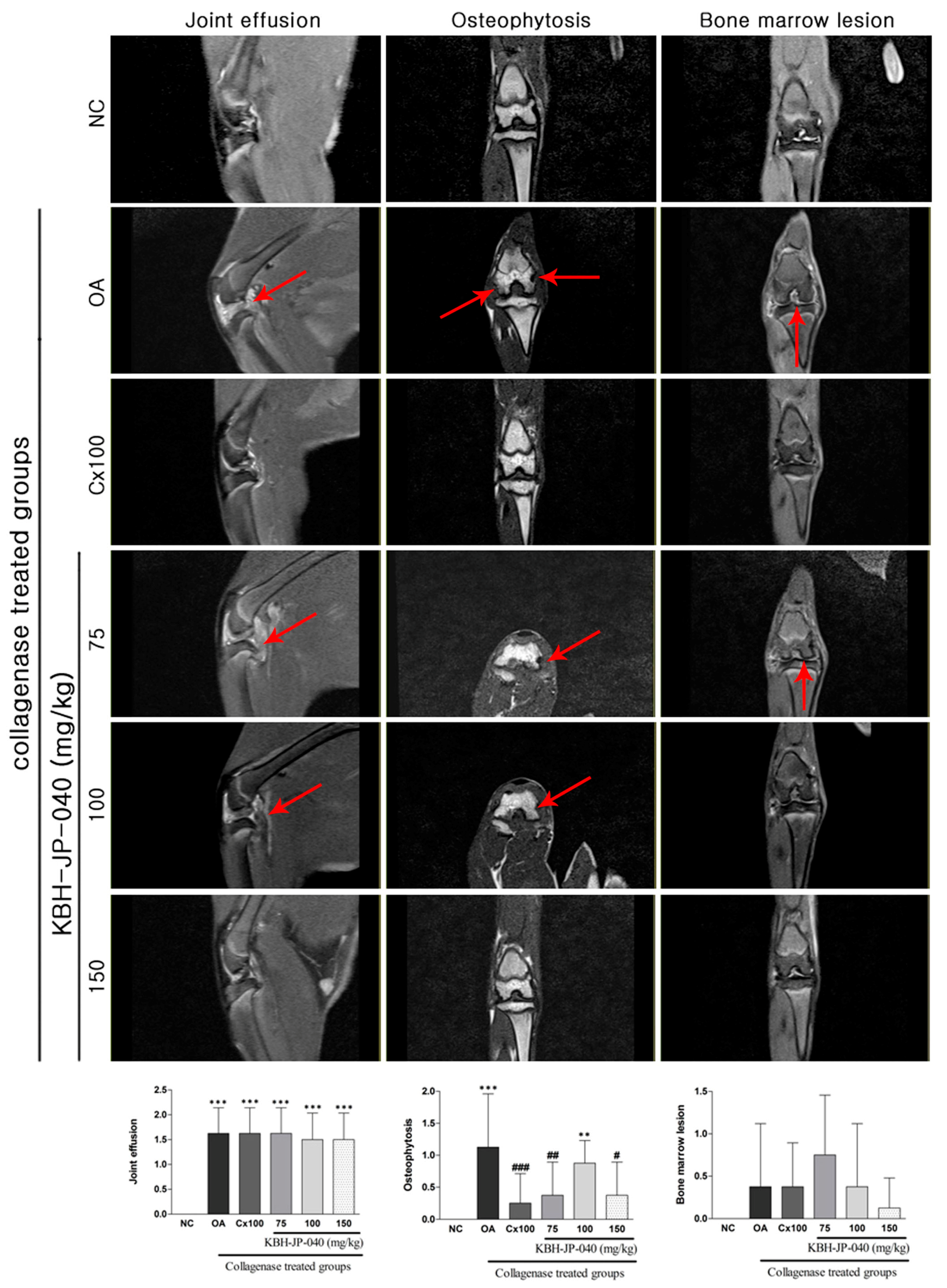
3.5. MRI Analysis
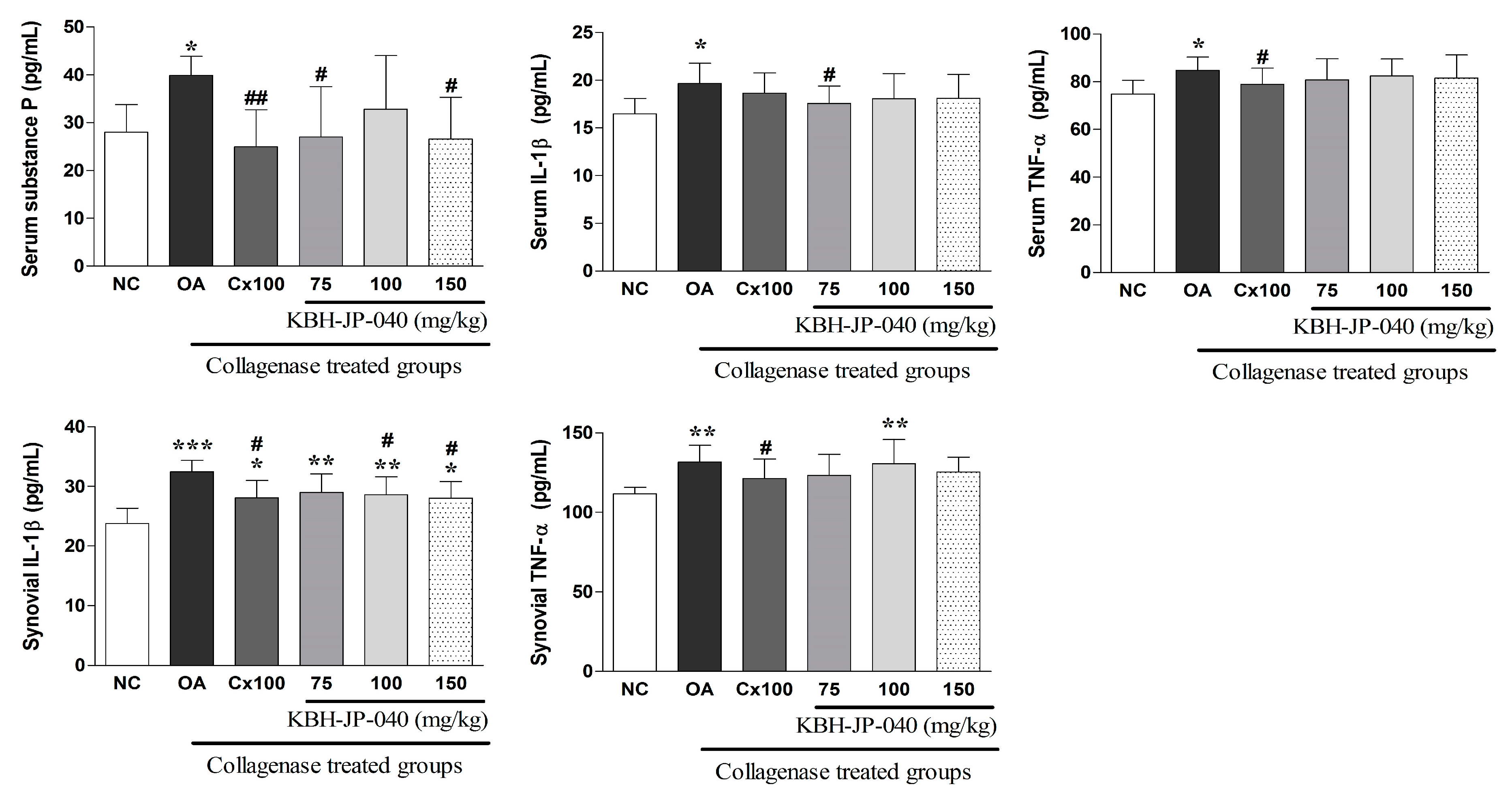
3.6. Inflammatory Cytokines and Serum Substance-P Level
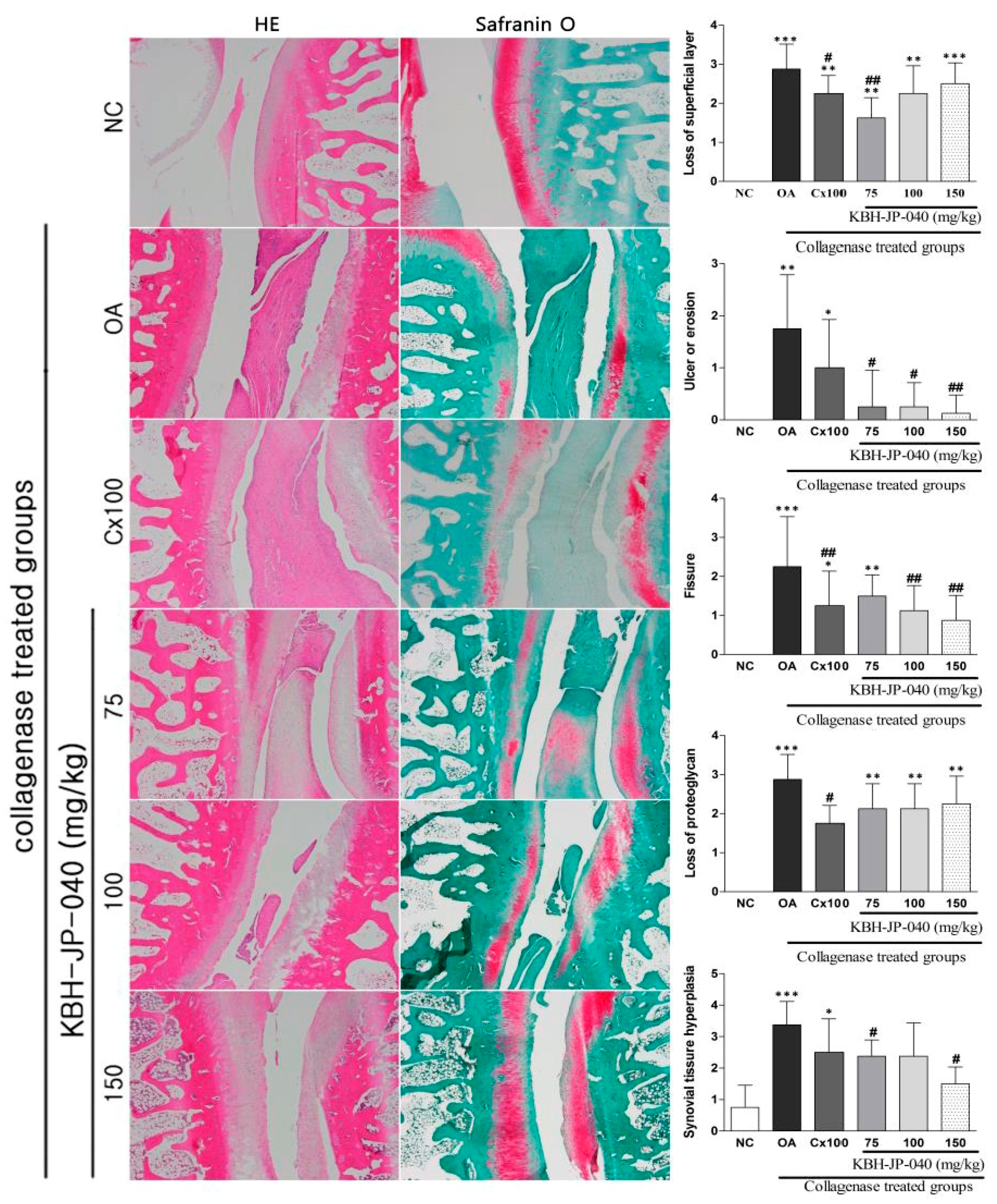
3.7. Histopathological Examination
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haq, I.; Murphy, E.; Dacre, J. Osteoarthritis. J. Postgrad. Med. 2003, 79, 377–383. [Google Scholar] [CrossRef]
- Park, D.S.; Huh, J.E.; Baek, Y.H. Therapeutic effect of Aralia cordata extracts on cartilage protection in collagenase-induced inflammatory arthritis rabbit model. J. Ethnopharmacol. 2009, 125, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, P.; Koppikar, S.; Bhondave, P.; Narkhede, A.; Nagarkar, B.; Kulkarni, V.; Wagh, N.; Kulkarni, O.; Harsulkar, A.; Jagtap, S. Influence of six medicinal herbs on collagenase-induced osteoarthritis in rats. Am. J. Chin. Med. 2013, 41, 1407–1425. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Cimmino, M.A.; Scarpa, R.; Caporali, R.; Parazzini, F.; Zaninelli, A.; Atzeni, F.; Canesi, B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin. Arthritis Rheum. 2005, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Morey, V.; Kang, J.Y.; Kim, K.W.; Kim, T.K. Prevalence and risk factors of spine, shoulder, hand, hip, and knee osteoarthritis in community-dwelling Koreans older than age 65 years. Clin. Orthop. Relat. Res. 2015, 473, 3307–3314. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.E.; Murphy, L.B.; Helmick, C.G.; Hootman, J.M.; Renner, J.B.; Jordan, J.M. Bone mineral density and the risk of hip and knee osteoarthritis: The Johnston county osteoarthritis project. Arthritis Care Res. 2017, 69, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Egloff, C.; Hugle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Baek, Y.H.; Lee, J.D.; Choi, D.Y.; Park, D.S. Therapeutic effect of siegesbeckia pubescens on cartilage protection in a rabbit collagenase-induced model of osteoarthritis. J. Pharmacol. Sci. 2008, 107, 317–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Vecchis, R.; Baldi, C.; Di Biase, G.; Ariano, C.; Cioppa, C.; Giasi, A.; Valente, L.; Cantatrione, S. Cardiovascular risk associated with celecoxib or etoricoxib: A meta-analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiol. 2014, 62, 437–448. [Google Scholar] [PubMed]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Huh, K.; Kim, S.H.; Lee, K.T.; Lee, H.K.; Park, H.J. Kalopanaxsaponin a from Kalopanax pictus, a potent antioxidant in the rheumatoidal rat treated with freund’s complete adjuvant reagent. J. Ethnopharmacol. 2002, 79, 113–118. [Google Scholar] [CrossRef]
- Kim, I.T.; Park, Y.M.; Shin, K.M.; Ha, J.; Choi, J.; Jung, H.J.; Park, H.J.; Lee, K.T. Anti-inflammatory and anti-nociceptive effects of the extract from Kalopanax pictus, pueraria thunbergiana and rhus verniciflua. J. Ethnopharmacol. 2004, 94, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Yang, H.L.; Pan, J.H.; Korivi, M.; Pan, J.Y.; Hsieh, M.C.; Chao, P.M.; Huang, P.J.; Tsai, C.T.; Hseu, Y.C. Hericium erinaceus inhibits tnf-alpha-induced angiogenesis and ros generation through suppression of mmp-9/nf-kappab signaling and activation of nrf2-mediated antioxidant genes in human ea.Hy926 endothelial cells. Oxid. Med. Cell. Longev. 2016, 8257238. [Google Scholar] [CrossRef]
- Sohn, Y.A.; Hwang, S.A.; Lee, S.Y.; Hwang, I.Y.; Kim, S.W.; Kim, S.Y.; Moon, A.; Lee, Y.S.; Kim, Y.H.; Kang, K.J.; et al. Protective effect of liriodendrin isolated from Kalopanax pictus against gastric injury. Biomol. Ther. 2015, 23, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.T.; Kim, J.S. The pharmacology and clinical properties of Kalopanax pictus. J. Med. Plants Res. 2009, 3, 613–620. [Google Scholar]
- Jeong, Y.H.; Hyun, J.W.; Kim Van Le, T.; Kim, D.H.; Kim, H.S. Kalopanaxsaponin an exerts anti-inflammatory effects in lipopolysaccharide-stimulated microglia via inhibition of jnk and nf-kappab/ap-1 pathways. Biomol. Ther. 2013, 21, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (huangqi). Phytother. Res. PTR 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Ma, N.; Wang, W.; Fu, W.; Zhang, H.; Zhao, M.; Gao, X.; Hao, X.; Zhang, Z. Formononetin, an active compound of Astragalus membranaceus (fisch) bunge, inhibits hypoxia-induced retinal neovascularization via the hif-1alpha/vegf signaling pathway. Drug Des. Dev. Ther. 2016, 10, 3071–3081. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (lion’s mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate ngf-mediated neurite outgrowth on pc12 cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessey, K.; Matuska, A.; Hoeppner, J.; Farr, J.; Klaassen, M.; Kaeding, C.; Lattermann, C.; King, W.; Woodell-May, J. Autologous protein solution prepared from the blood of osteoarthritic patients contains an enhanced profile of anti-inflammatory cytokines and anabolic growth factors. J. Orthop. Res. 2014, 32, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Adcocks, C.; Collin, P.; Buttle, D.J. Catechins from green tea (camellia sinensis) inhibit bovine and human cartilage proteoglycan and typeⅡ collagen degradation in vitro. J. Nutr. 2002, 132, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bouchgua, M.; Alexander, K.; D’Anjou, M.A.; Girard, C.A.; Carmel, E.N.; Beauchamp, G.; Richard, H.; Laverty, S. Use of routine clinical multimodality imaging in a rabbit model of osteoarthritis—Part I. Osteoarthr. Cartil. 2009, 17, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Butler, M.; O’Byrne, E.; Hickman, L.; Swartzendruber, D.; Selwyn, M.; Steinetz, B. A new model of osteoarthritis in rabbits. I. Development of knee joint pathology following lateral meniscectomy and section of the fibular collateral and sesamoid ligaments. Arthritis Rheum. 1983, 26, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Liang, Y.Q.; Tang, L.J.; Ding, Y.; Wang, X.H. Antioxidant and anti-inflammatory effects of astragalus polysaccharide on ea.Hy926 cells. Exp. Ther. Med. 2013, 6, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, D.; Zhuo, Y.; Zhang, S.; Wang, X.; Gao, H. Protective role of liriodendrin in sepsis-induced acute lung injury. Inflammation 2016, 39, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Haavikko, R.; Hamalainen, M.; Leppanen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin derivatives effectively suppress inflammation in vitro and in vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Zhou, J.; Lv, H.; Yu, Q.; Peng, L.; Hua, S. Betulin exhibits anti-inflammatory activity in lps-stimulated macrophages and endotoxin-shocked mice through an ampk/akt/nrf2-dependent mechanism. Cell Death Dis. 2017, 8, e2798. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ji, W.; Fu, Q.; Ma, S. Formononetin inhibited the inflammation of lps-induced acute lung injury in mice associated with induction of ppar gamma expression. Inflammation 2013, 36, 1560–1566. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; An, J. Formononetin ameliorates mast cell-mediated allergic inflammation via inhibition of histamine release and production of pro-inflammatory cytokines. Exp. Ther. Med. 2017, 14, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Tang, J.L.; Bao, J.P.; Hu, P.F.; Shi, Z.L.; Wu, L.D. Anti-arthritic effects of chlorogenic acid in interleukin-1beta-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. Immunopharmacol. 2011, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.K.; Chan, J.Y.; Wu, S.B.; Cheng, L.; Ho, G.K.; Lau, C.P.; Kennelly, E.J.; Leung, P.C.; Fung, K.P.; Lau, C.B. Anti-inflammatory activities of an active fraction isolated from the root of Astragalus membranaceus in raw 264.7 macrophages. Phytother. Res. 2014, 28, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Lee, S.W.; Oh, C.H.; Rhee, Y.H. Hericium erinaceus suppresses lps-induced pro-inflammation gene activation in raw264.7 macrophages. Immunopharmacol. Immunotoxicol. 2012, 34, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, R.G.; Park, S.J.; Ha, J.H.; Choi, J.W.; Park, H.J.; Lee, K.T. In vitro antiinflammatory activity of kalopanaxsaponin an isolated from Kalopanax pictus in murine macrophage raw 264.7 cells. Biol. Pharm. Bull. 2002, 25, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Huh, J.E.; Baek, Y.H.; Lee, J.D.; Choi, D.Y.; Park, D.S. Effect of phellodendron amurense in protecting human osteoarthritic cartilage and chondrocytes. J. Ethnopharmacol. 2011, 134, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Zhong, H.; Qi, Y.; Cheng, Y.; Li, W.; Yan, S.; Wang, X. Anti-arthritic effects of crocin in interleukin-1beta-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm. Res. 2013, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Ngan, N.T.; Minh, C.V.; Kiem, P.V.; Nhiem, N.X.; Tai, B.H.; Thao, N.P.; Tung, N.H.; Song, S.B.; Kim, Y.H. Anti-inflammatory triterpenoid saponins from the stem bark of Kalopanax pictus. J. Nat. Prod. 2011, 74, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Heo, S.I.; Wang, M.H. Antioxidant and anti-inflammatory activity of Kalopanax pictus leaf. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 360–366. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, X.; Li, C.; Fu, P. Systematic review of the renal protective effect of Astragalus membranaceus (root) on diabetic nephropathy in animal models. J. Ethnopharmacol. 2009, 126, 189–196. [Google Scholar] [CrossRef] [PubMed]
- D’Anjou, M.A.; Moreau, M.; Troncy, E.; Martel-Pelletier, J.; Abram, F.; Raynauld, J.P.; Pelletier, J.P. Osteophytosis, subchondral bone sclerosis, joint effusion and soft tissue thickening in canine experimental stifle osteoarthritis: Comparison between 1.5 t magnetic resonance imaging and computed radiography. Vet. Surg. 2008, 37, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Chen, J.; Wang, Y.; Liu, Y.; Zhang, Y.; Chen, W. Magnetic resonance imaging of osteophytic, chondral, and subchondral structures in a surgically-induced osteoarthritis rabbit model. PLoS ONE 2014, 9, e113707. [Google Scholar] [CrossRef] [PubMed]

| Forward | Reverse | |
|---|---|---|
| Collagen type 2 | 5′ CCG ATC CCC TGC AGT ACA TG 3′ | 5′ TGC TCT CGA TCT GGT TGT TCA 3′ |
| SOX9 | 5′ CTG AAG GGC TAC GAC TGG AC 3′ | 5′ TAC TGG TCT GCC AGC TTC CT 3′ |
| MMP1 | 5′-CTC CCT TGG ACT CAC TCA TTC TA-3 | 5′-AGA ACA TCA CCT CTC CCC TAA AC-3′ |
| MMP2 | 5′-TGG GGG AGA TTC TCA CTT TG 3′ | 5′CCA TCA GCG TTC CCA TAC TT 3′ |
| MMP3 | 5′ TGG GAA GCC AGT GGA AAT G 3′ | 5′CCA TGC AAT GGG TAG GAT GAG 3′ |
| MMP9 | 5′ TGC TCC TGG CTC TAG GCT AC 3′ | 5′ TTG GAG GTT TTC AGG TCT CG 3′ |
| MMP13 | 5′ CTG ACC TGG GAT TTC CAA AA 3′ | 5′ ACA CGT GGT TCC CTG AGA AG 3′ |
| IL-6 | 5′ TGATGGATGCTTCCAAACTG 3′ | 5′GAGCATTGGAAGTTGGGGTA 3′ |
| IL-10 | 5′ GAGAGAAGCTGAAGACCCTCTG 3′ | 5′TCATTCATGGCCTTGTAGACA C 3′ |
| IL-12 | 5′-AGG CCC AGC AGC AGA ATA AAT A-3′ | 5′-GTG CTC CAG GAG TCA GGG TAC T-3′ |
| GAPDH | 5′ GG GTG TGA ACC ACG AGA AAT 3′ | 5′ ACT GTG GTC ATG AGC CCT TC 3′ |
| NC | RhIL-1 | RhIL-1+KBH-JP-040 50 | RhIL-1+KBH-JP-040100 | RhIL-1+KBH-JP-040200 | |
|---|---|---|---|---|---|
| Aggrecan (ng/mL) | 12.88 ± 0.62 | 10.90 ± 0.60 ** | 11.54 ± 1.30 * | 11.57 ± 1.05 **,## | 11.74 ± 1.06 **,# |
| Collagen type II (ng/mL) | 12.85 ± 1.15 | 9.51 ± 0.48 ** | 11.80 ± 0.54 **,# | 12.93 ± 3.81 *,## | 13.32 ± 1.22 *,## |
| IL-6 (pg/mL) | 35.20 ± 8.24 | 60.30 ± 0.76 | 51.66 ± 2.44 | 51.24 ± 1.60 | 50.63 ± 5.32 |
| IL-10 (pg/mL) | 3.34 ± 2.06 | 3.53 ± 1.66 *** | 4.00 ± 1.43 ** | 4.79 ± 2.95 *,# | 4.65 ± 3.35 * |
| IL-12 (pg/mL) | 1.32 ± 0.67 | 2.30 ± 0.64 ** | 1.51 ± 0.36 *,## | 1.39 ± 0.24 ### | 1.01 ± 0.52 ### |
| MMP1 (pg/mL) | 52.98 ± 10.69 | 93.06 ± 34.37 *** | 46.19 ± 30.69 ***,# | 38.31 ± 15.35 **,### | 41.37 ± 12.95 **,### |
| MMP9 (pg/mL) | 34.82 ± 12.80 | 54.05 ± 10.05 *** | 39.24 ± 4.94 ***,# | 38.43 ± 5.88 **,### | 33.21 ± 3.45 **,### |
| MMP13 (pg/mL) | 189.35 ± 23.33 | 269.50 ± 37.08 ** | 211.45 ± 20.37 * | 208.25 ± 38.84 # | 192.15 ± 38.73 |
| PGE2 (pg/mL) | 58.30 ± 1.33 | 81.78 ± 1.19 ** | 54.32 ± 0.81 * | 41.20 ± 0.69 # | 47.22 ± 2.50 |
| ROS (%) | 545.20 ± 17.25 | 645.40 ± 36.73 *** | 563.20 ± 6.45 **,# | 567.60 ± 11.01 *,## | 555.60 ± 13.43 **,# |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Kim, H.-K.; Kim, S.-E.; Kim, M.-J.; Kim, D.-H.; Lee, H.S. Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients 2018, 10, 356. https://doi.org/10.3390/nu10030356
Rahman MM, Kim H-K, Kim S-E, Kim M-J, Kim D-H, Lee HS. Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients. 2018; 10(3):356. https://doi.org/10.3390/nu10030356
Chicago/Turabian StyleRahman, Md. Mahbubur, Hyun-Kyu Kim, Seong-Eun Kim, Myung-Jin Kim, Do-Hyung Kim, and Hak Sung Lee. 2018. "Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models" Nutrients 10, no. 3: 356. https://doi.org/10.3390/nu10030356
APA StyleRahman, M. M., Kim, H.-K., Kim, S.-E., Kim, M.-J., Kim, D.-H., & Lee, H. S. (2018). Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients, 10(3), 356. https://doi.org/10.3390/nu10030356






