Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Proanthocyanidin Extract
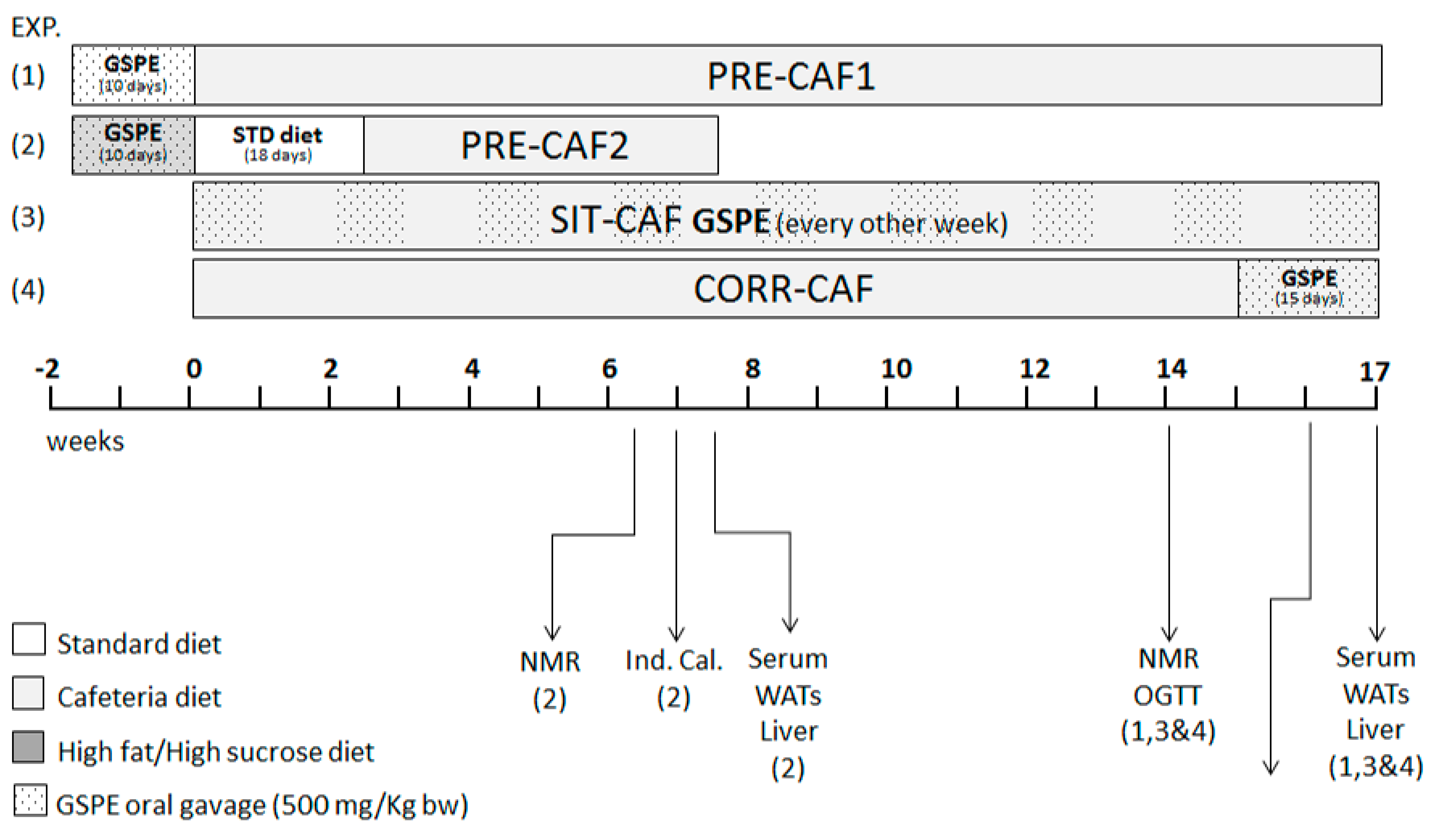
2.2. Animal Models
2.3. Blood and Tissue Collection
2.4. Morphometric Variables
2.5. Biochemical Variables
2.6. Indirect Calorimetry
2.7. Statistical Analysis
3. Results
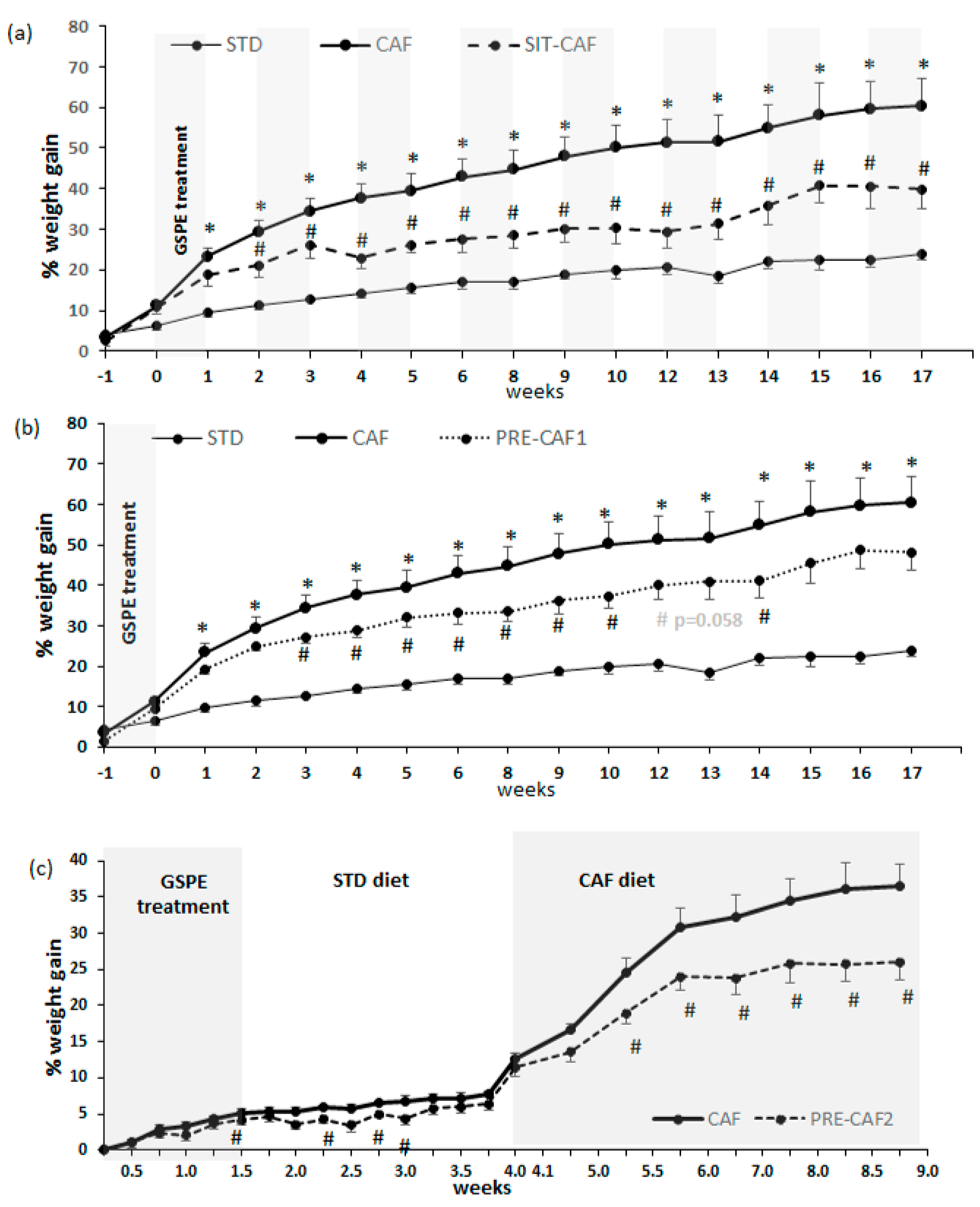
3.1. GSPE Limits Body Weight Gain under Obesogenic Diets When Administered as a Preventive Agent
3.2. Adipose Tissue is an Important Target of GSPE Preventive Effects against Obesogenic Diets
3.3. All GSPE Treatments Reduced RQ but Did Not Modify Oxygen Expenditure
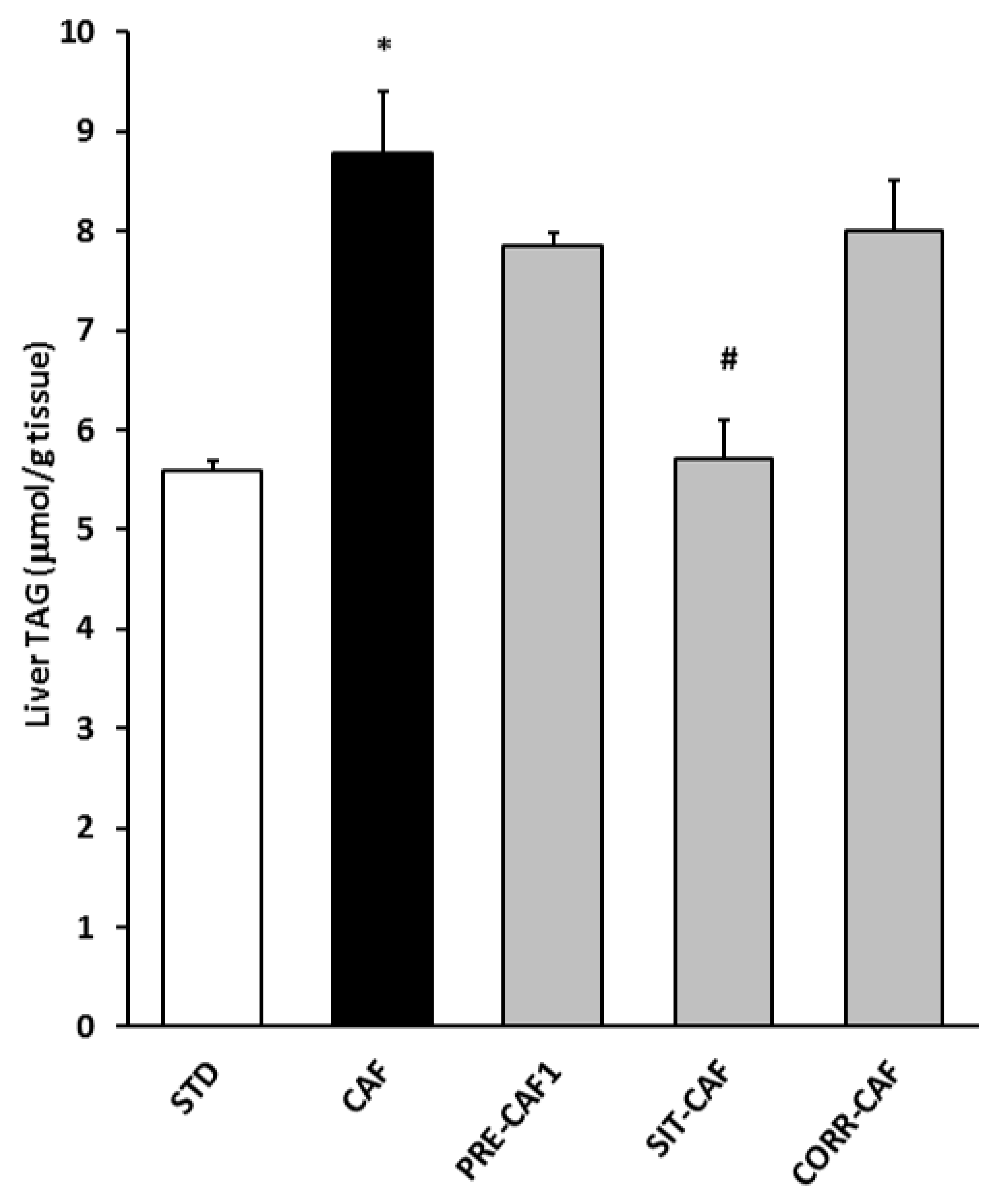
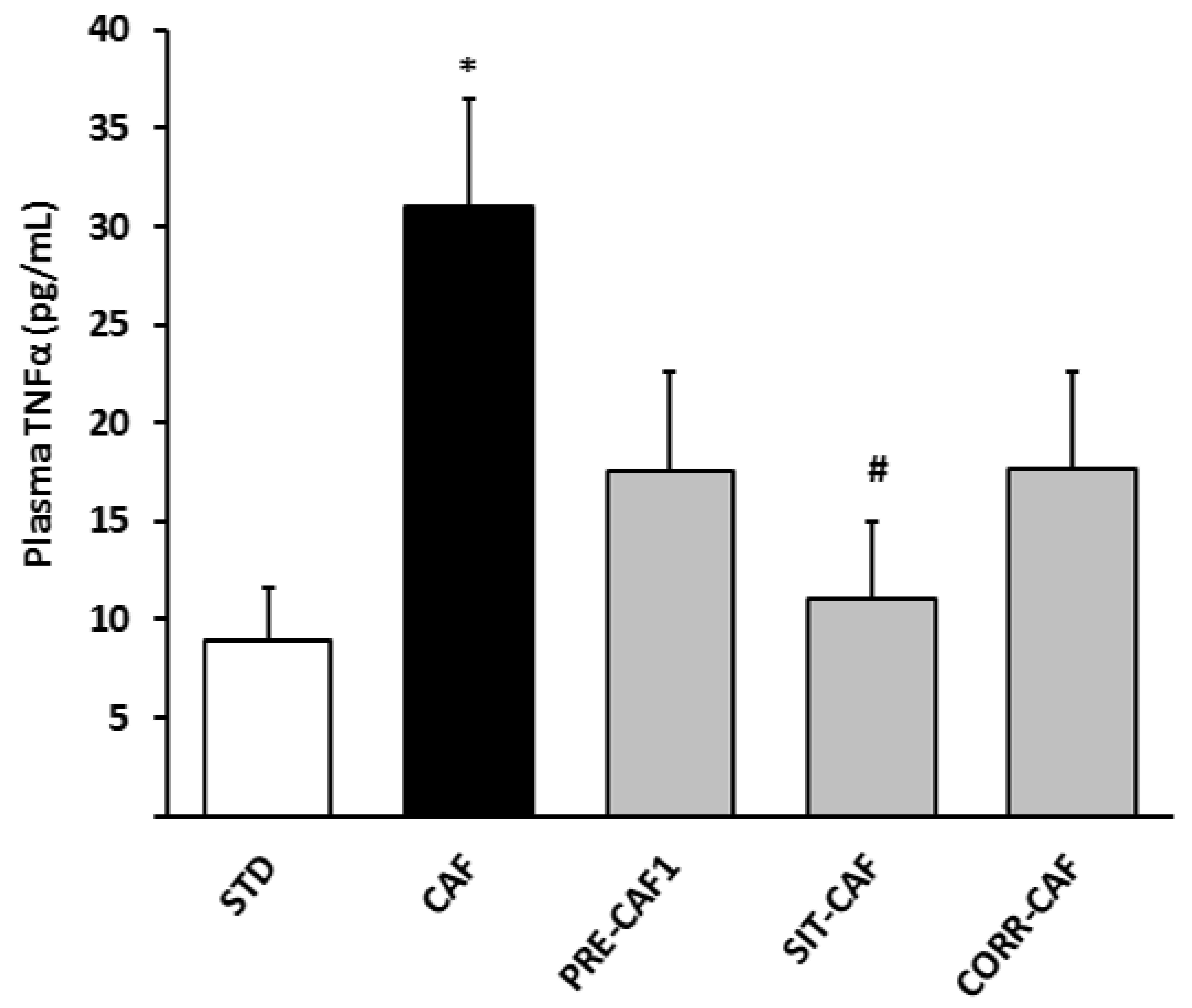
3.4. GSPE Pretreatment Prevents the Development of Metabolic Syndrome in Rats Fed a Cafeteria Diet
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.C.; Mcpherson, K.; Marsh, T.; Gortmaker, S.L.; Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2015, 378, 815–825. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight; Fact Sheet No. 311; World Health Organization Media Center: Geneva, Switzerland, 2015. [Google Scholar]
- Serrano, J.; Casanova-Martí, À.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Strategy for limiting food intake using food components aimed at multiple targets in the gastrointestinal tract. Trends Food Sci. Technol. 2017, 68, 113–129. [Google Scholar] [CrossRef]
- Salvadó, M.J.; Casanova, E.; Fernández-Iglesias, A.; Arola, L.; Bladé, C. Roles of proanthocyanidin rich extracts in obesity. Food Funct. 2015, 6, 1053–1071. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Arola, L.; Salvadó, M.-J.; Salvado, M.; Bladé, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Mcdougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Blay, M.; Ardevol, A.; Pinent, M. Grape-seed procyanidins prevent the cafeteria diet-induced decrease of glucagon-like peptide-1 production. J. Agric. Food Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Martí, À.; Serrano, J.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Acute selective bioactivity of grape seed proanthocyanidins on enteroendocrine secretions in the gastrointestinal tract. Food Nutr. Res. 2017, 61, 1321347. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2016, 56, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Blay, M.; Terra, X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016, 29, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Gender-related similarities and differences in the body distribution of grape seed flavanols in rats. Mol. Nutr. Food Res. 2016, 60, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Priego, T.; Palou, M.; Tobaruela, A.; Palou, A.; Picó, C.; Sa, J.; Priego, T.; Palou, M.; Tobaruela, A.; et al. Oral Supplementation with Physiological Doses of Leptin During Lactation in Rats Improves Insulin Sensitivity and Affects Food Preferences Later in Life. Endocrinology 2008, 149, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Montagut, G.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.L.; Pinent, M.; Ardévol, A.; Blade, C.; et al. Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance. J. Nutr. Biochem. 2010, 21, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Arola-Arnal, A.; Bladé, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.; del Bas, J.M.; Pajuelo, D.; Díaz, S.; Fernandez-Larrea, J.; Pinent, M.; Arola, L.; Salvadó, M.J.; Bladé, C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int. J. Obes. (Lond.) 2009, 33, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. BioFactors 2012, 38, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lajous, M.; Rossignol, E.; Fagherazzi, G.; Perquier, F.; Scalbert, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Flavonoid intake and incident hypertension in women. Am. J. Clin. Nutr. 2016, 103, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Casanova-Martí, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ardévol, A.; Bladé, C.; Salvadó, M.J.; Arola, L. Changes in lipolysis and hormone-sensitive lipase expression caused by procyanidins in 3T3-L1 adipocytes. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Bladé, M.; Salvadó, M.J.; Blay, M.; Pujadas, G.; Fernàndez-Larrea, J.; Arola, L.; Ardévol, A.; Bladé, C.; Salvadó, M.J.; et al. Procyanidin effects on adipocyte-related pathologies. Crit. Rev. Food Sci. Nutr. 2006, 46, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; del Bas, J.M.; Crescenti, A.; Arola, L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int. J. Obes. (Lond.) 2013, 37, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Vadillo, M.; Ardévol, A.; Fernández-Larrea, J.; Pujadas, G.; Bladé, C.; Salvadó, M.J.; Arola, L.; Blay, M. Moderate red-wine consumption partially prevents body weight gain in rats fed a hyperlipidic diet. J. Nutr. Biochem. 2006, 17, 139–142. [Google Scholar] [PubMed]
- Dorenkott, M.R.; Griffin, L.E.; Goodrich, K.M.; Thompson-Witrick, K.A.; Fundaro, G.; Ye, L.; Stevens, J.R.; Ali, M.; O’Keefe, S.F.; Hulver, M.W.; et al. Oligomeric Cocoa Procyanidins Possess Enhanced Bioactivity Compared to Monomeric and Polymeric Cocoa Procyanidins for Preventing the Development of Obesity, Insulin Resistance, and Impaired Glucose Tolerance during High-Fat Feeding. J. Agric. Food Chem. 2014, 62, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, À.; Blay, M.; Terra, X.; Ardévol, A.; Pinent, M. Defining conditions for optimal inhibition of food intake in rats by a grape-seed derived proanthocyanidin extract. Nutrients 2016, 8, 652. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Dai, X.; Ding, Y.; Jiang, Y.; Li, Y.; Zhang, Z.; Li, Y. Grape seed proanthocyanidin extracts ameliorate podocyte injury by activating peroxisome proliferator-activated receptor-γ coactivator 1α in low-dose streptozotocin-and high-carbohydrate/high-fat diet-induced diabetic rats. Food Funct. 2014, 5, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, J.D.; Verpeut, J.L.; Yeomans, B.L.; Yang, J.A.; Yasrebi, A.; Roepke, T.A.; Bello, N.T. Intermittent Fasting Promotes Fat Loss With Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology 2016, 157, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, M.; Tricò, D.; Natali, A. The impact of triglycerides on glucose tolerance: Lipotoxicity revisited. Diabetes Metab. 2017, 43, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cardoso, K.; Ginés, I.; Pinent, M.; Ardévol, A.; Terra, X.; Blay, M.; Gines, I.; Pinent, M.; Ardevol, A.; Terra, X.; et al. A cafeteria diet triggers intestinal inflammation and oxidative stress in obese rats. Br. J. Nutr. 2017, 117, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Garcia, S.; Del Bas, J.M.; Caimari, A.; Escorihuela, R.M.; Arola, L.; Suarez, M. Impact of a cafeteria diet and daily physical training on the rat serum metabolome. PLoS ONE 2017, 12, e0171970. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Gil-cardoso, K.; Blay, M.; Terra, X. Chronic supplementation with dietary proanthocyanidins protects from diet-induced intestinal. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J. Satiating Properties of a Grape Seed Proanthocyanidin Extract. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2017. [Google Scholar]

| kJ/g | % Energy (CH) | % Energy (Protein) | % Energy (Lipid) | |
|---|---|---|---|---|
| STD Chow | 12.13 | 72.5 | 19.3 | 8.2 |
| PRE-CAF1 | 20.73 | 52.0 | 14.1 | 33.9 |
| PRE-CAF2-HFHS | 17.66 | 77.3 | 16.2 | 6.5 |
| PRE-CAF2-CAF | 24.1 | 54.0 | 11.0 | 36.0 |
| Variable | STD | CAF | PRE-CAF1 | SIT-CAF | CORR-CAF |
|---|---|---|---|---|---|
| Morphometric measurements | |||||
| n | 9 | 10 | 10 | 7 | 9 |
| Initial body weight(g) | 220.7 ± 4.5 | 216.6 ± 3.5 | 214.2 ± 3.7 | 213.04 ± 5.2 | 219.6 ± 3.3 |
| Final body weight (g) | 273.7 ± 7.8 | 346.2 ± 12.0 * | 316.8 ± 9.2 | 297.3 ± 9.8 # | 331.5 ± 12.6 |
| mWAT (g) | 4.2 ± 0.4 | 11.3 ± 1.3 * | 9.5 ± 0.9 | 6.1 ± 0.7 # | 9.8 ± 1.0 |
| oWAT (g) | 7.5 ± 0.9 | 19.5 ± 2.0 * | 15.9 ± 1.4 | 12.7 ± 1.4 # | 18.5 ± 2.1 |
| rWAT (g) | 3.8 ± 0.4 | 10.7 ± 1.2 * | 9.8 ± 0.9 | 6.3 ± 0.6 # | 9.7 ± 1.1 |
| Total visceral WAT (g) | 15.4 ± 1.6 | 41.5 ± 3.8 * | 35.1 ± 2.7 | 25.1 ± 2.6 # | 38.1 ± 3.9 |
| BAT (g) | 0.5 ± 0.1 | 1.0 ± 0.1 * | 0.9 ± 0.1 | 0.8 ± 0.1 # | 1.0 ± 0.1 |
| % visceral adiposity | 5.6 ± 0.5 | 11.8 ± 0.8 * | 11.0 ± 0.6 | 8.4 ± 0.6 # | 11.3 ± 0.8 |
| % total adiposity (14 week) | 9.0 ± 0.9 | 24.7 ± 1.6 * | 21.1 ± 1.6 | 14.5 ± 1.6 # | |
| Liver (g) | 7.7 ± 0.3 | 8.9 ± 0.4 * | 8.5 ± 0.3 | 8.5 ± 0.6 | 9.3 ± 0.3 |
| Pancreas (g) | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.2 |
| Spleen (g) | 0.5 ± 0.1 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.1 | 0.7 ± 0.0 |
| Thymus (g) | 0.2 ± 0.0 | 0.3 ± 0.0 * | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.0 |
| Kidney (g) | 1.5 ± 0.0 | 1.7 ± 0.1 * | 1.6 ± 0.0 | 1.7 ± 0.1 | 1.8 ± 0.0 |
| Plasma biochemical parameters | |||||
| Triglycerides (mM) | 0.41 ± 0.07 | 0.56 ± 0.06 | 0.42 ± 0.05 | 0.35 ± 0.05 # | 0.45 ± 0.06 |
| Cholesterol (mM) | 1.6 ± 0.16 | 2.1 ± 0.17 * | 1.9 ± 0.14 | 1.5 ± 0.13 # | 1.7 ± 0.13 |
| Free Fatty Acids (mM) | 0.21 ± 0.04 | 0.42 ± 0.09 | 0.33 ± 0.03 | 0.24 ± 0.09 | 0.44 ± 0.11 |
| Glucose (mM) | 8.9 ± 0.7 | 10.2 ± 4.0 | 10.0 ± 6.0 | 9.7 ± 6.0 | 10.4 ± 5.0 |
| Insulin (µg/L) | 5.6 ± 0.9 | 5.0 ± 0.8 | 6.4 ± 1.0 | 3.8 ± 0.8 | 9.7 ± 1.2 |
| Variable | CAF | PRE-CAF2 |
|---|---|---|
| Morphometric variables | ||
| n | 7 | 7 |
| Initial body weight(g) | 225.6 ± 2.2 | 223.4 ± 2.1 |
| Final body weight (g) | 307.7 ± 6.7 | 281.4 ± 4.0 # |
| mWAT (g) | 9.3 ± 1.0 | 6.0 ± 0.6 # |
| oWAT (g) | 17.3 ± 1.1 | 14.5 ± 1.0 |
| rWAT (g) | 12.7 ± 0.9 | 9.8 ± 0.6 # |
| Total WAT (g) | 40.2 ± 2.1 | 31.0 ± 1.7 # |
| BAT (g) | 0.9 ± 0.1 | 0.8 ± 0.0 # |
| % visceral adiposity | 13.1 ± 0.5 | 11.0 ± 0.8 # |
| % total adiposity | 24.7 ± 1.2 | 19.5 ± 1.4 # |
| Plasma biochemical parameters | ||
| Triglycerides (mM) | 0.49 ± 0.04 | 0.40 ± 0.03 |
| Cholesterol (mM) | 3.09 ± 0.07 | 2.80 ± 0.12 |
| Free Fatty Acids (mM) | 0.28 ± 0.03 | 0.32 ± 0.05 |
| Glucose (mM) | 10.2 ± 0.3 | 10.4 ± 0.4 |
| Insulin (µg/L) | 5.1 ± 0.4 | 3.9 ± 0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginés, I.; Gil-Cardoso, K.; Serrano, J.; Casanova-Martí, À.; Blay, M.; Pinent, M.; Ardévol, A.; Terra, X. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients 2018, 10, 315. https://doi.org/10.3390/nu10030315
Ginés I, Gil-Cardoso K, Serrano J, Casanova-Martí À, Blay M, Pinent M, Ardévol A, Terra X. Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients. 2018; 10(3):315. https://doi.org/10.3390/nu10030315
Chicago/Turabian StyleGinés, Iris, Katherine Gil-Cardoso, Joan Serrano, Àngela Casanova-Martí, MTeresa Blay, Montserrat Pinent, Anna Ardévol, and Ximena Terra. 2018. "Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats" Nutrients 10, no. 3: 315. https://doi.org/10.3390/nu10030315
APA StyleGinés, I., Gil-Cardoso, K., Serrano, J., Casanova-Martí, À., Blay, M., Pinent, M., Ardévol, A., & Terra, X. (2018). Effects of an Intermittent Grape-Seed Proanthocyanidin (GSPE) Treatment on a Cafeteria Diet Obesogenic Challenge in Rats. Nutrients, 10(3), 315. https://doi.org/10.3390/nu10030315








