Serum Nutrient Levels and Aging Effects on Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Data
2.2. Demographics
2.3. Clinical Parameters
2.4. Environmental Variables
2.5. Statistical Approaches
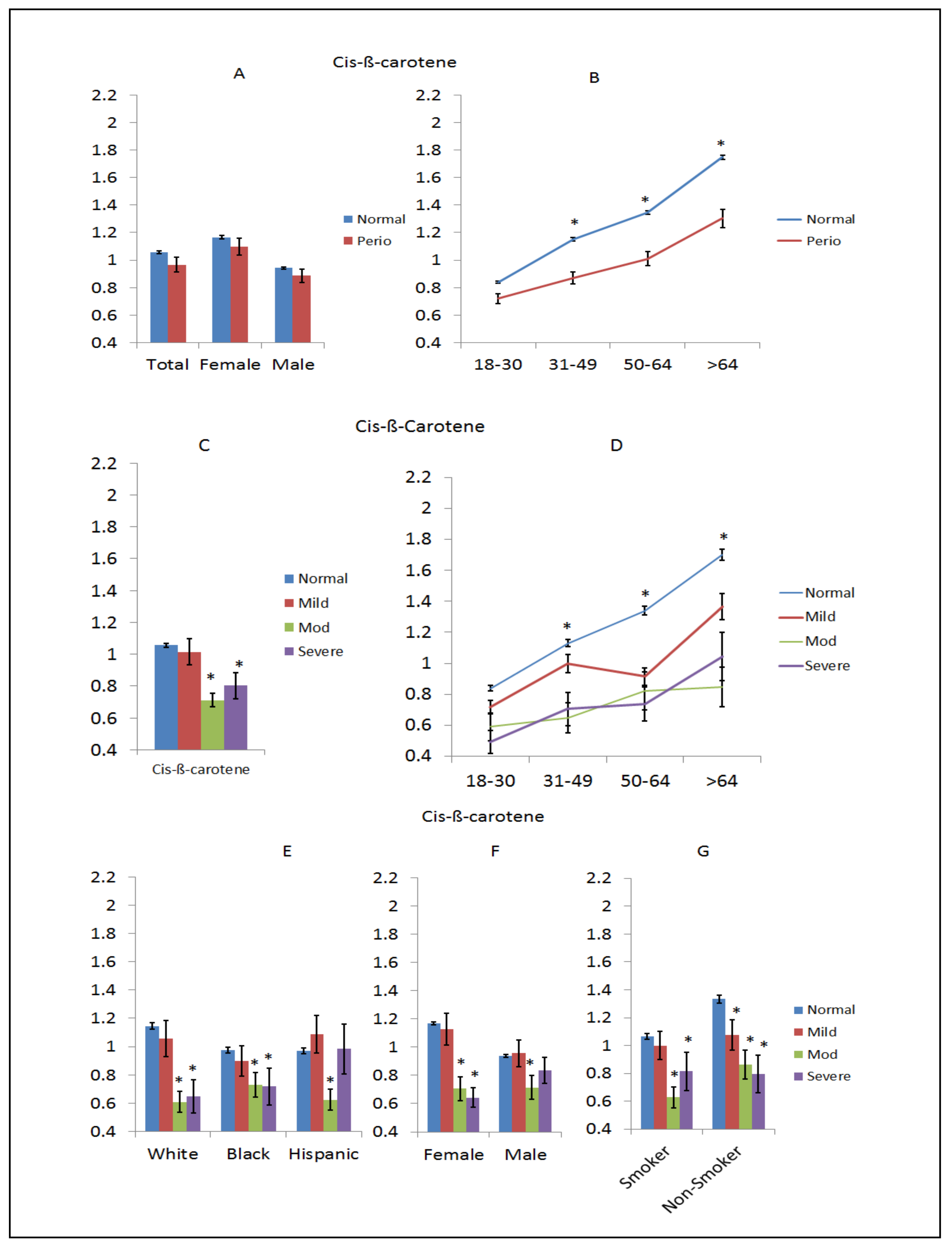
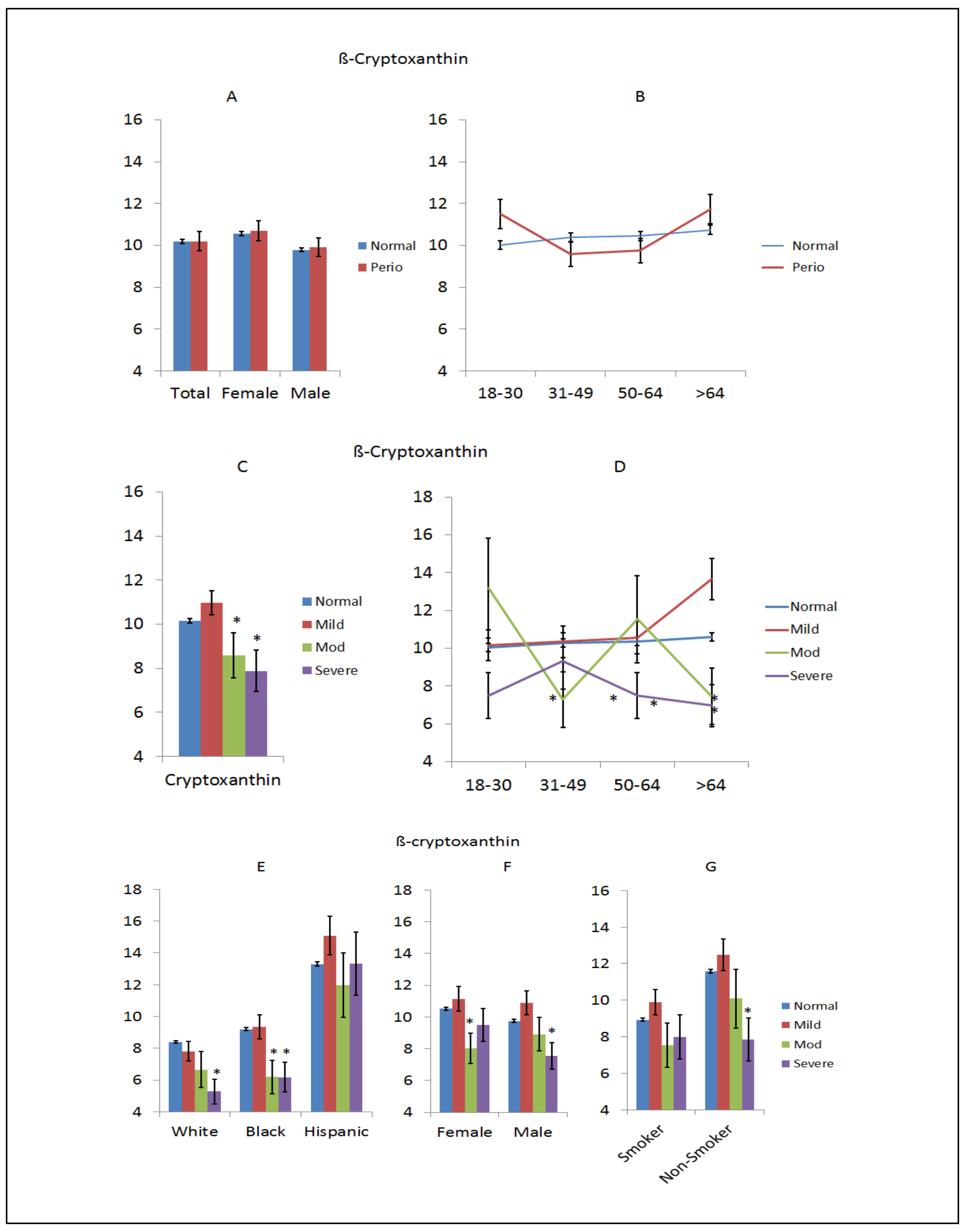
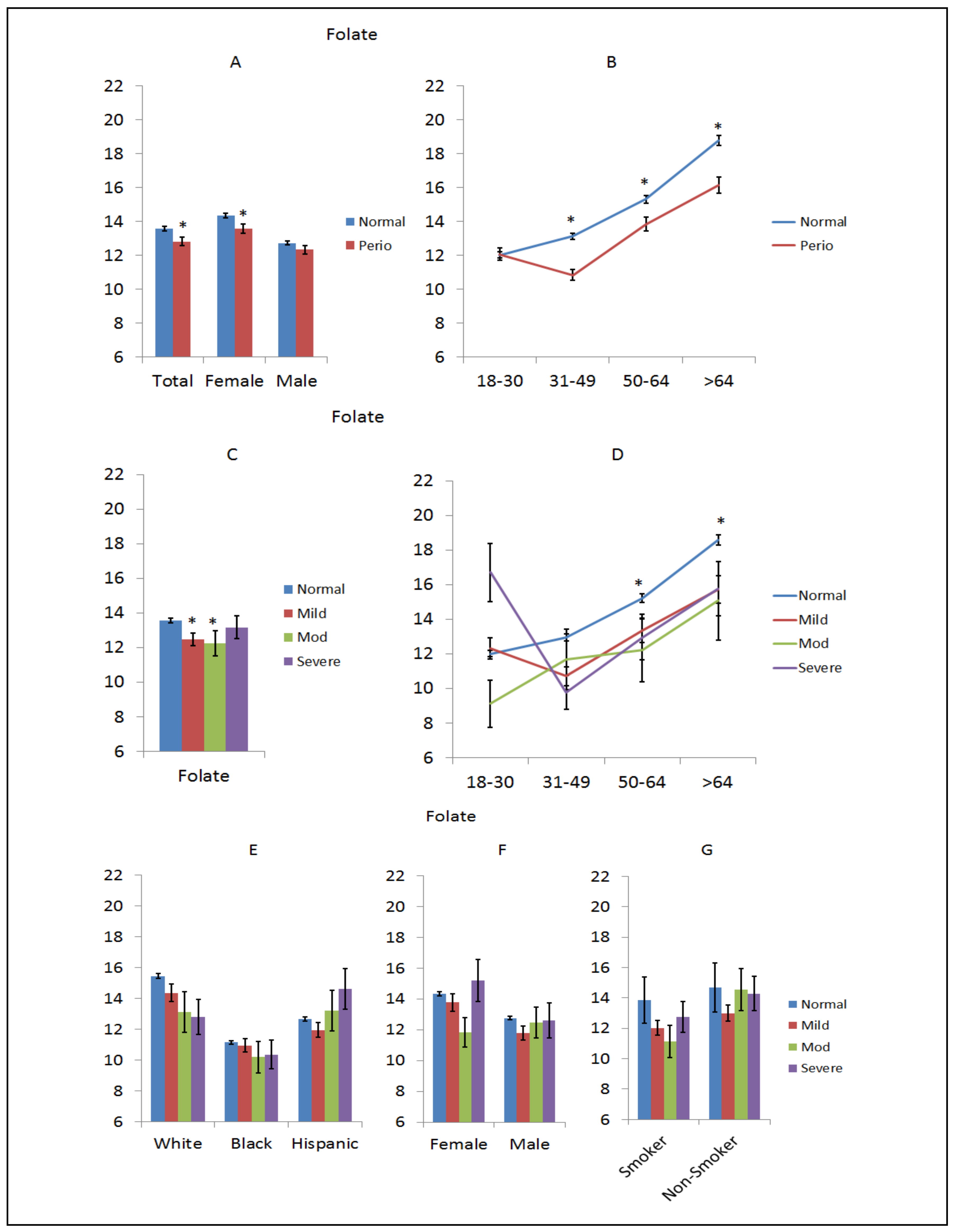
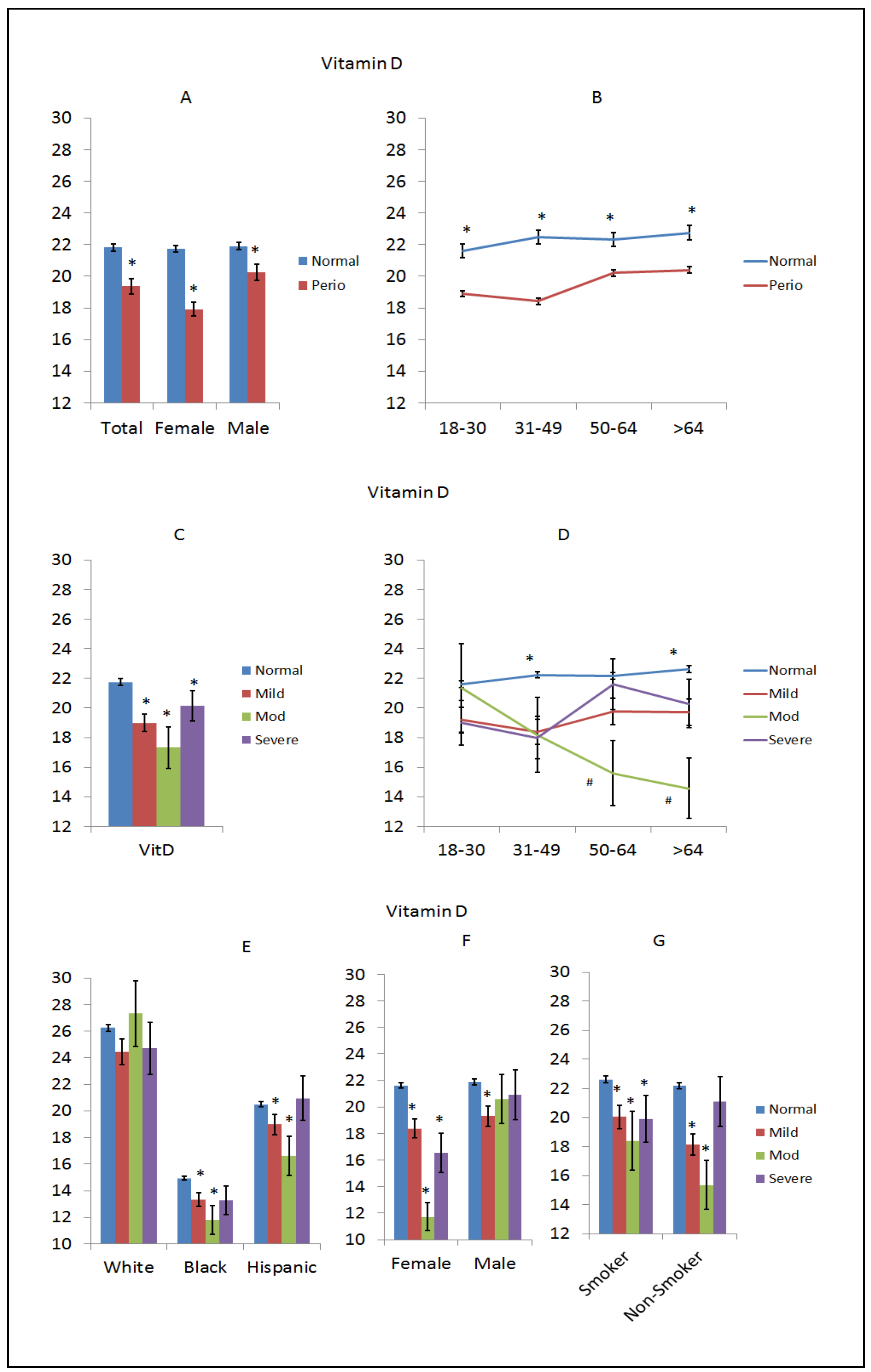
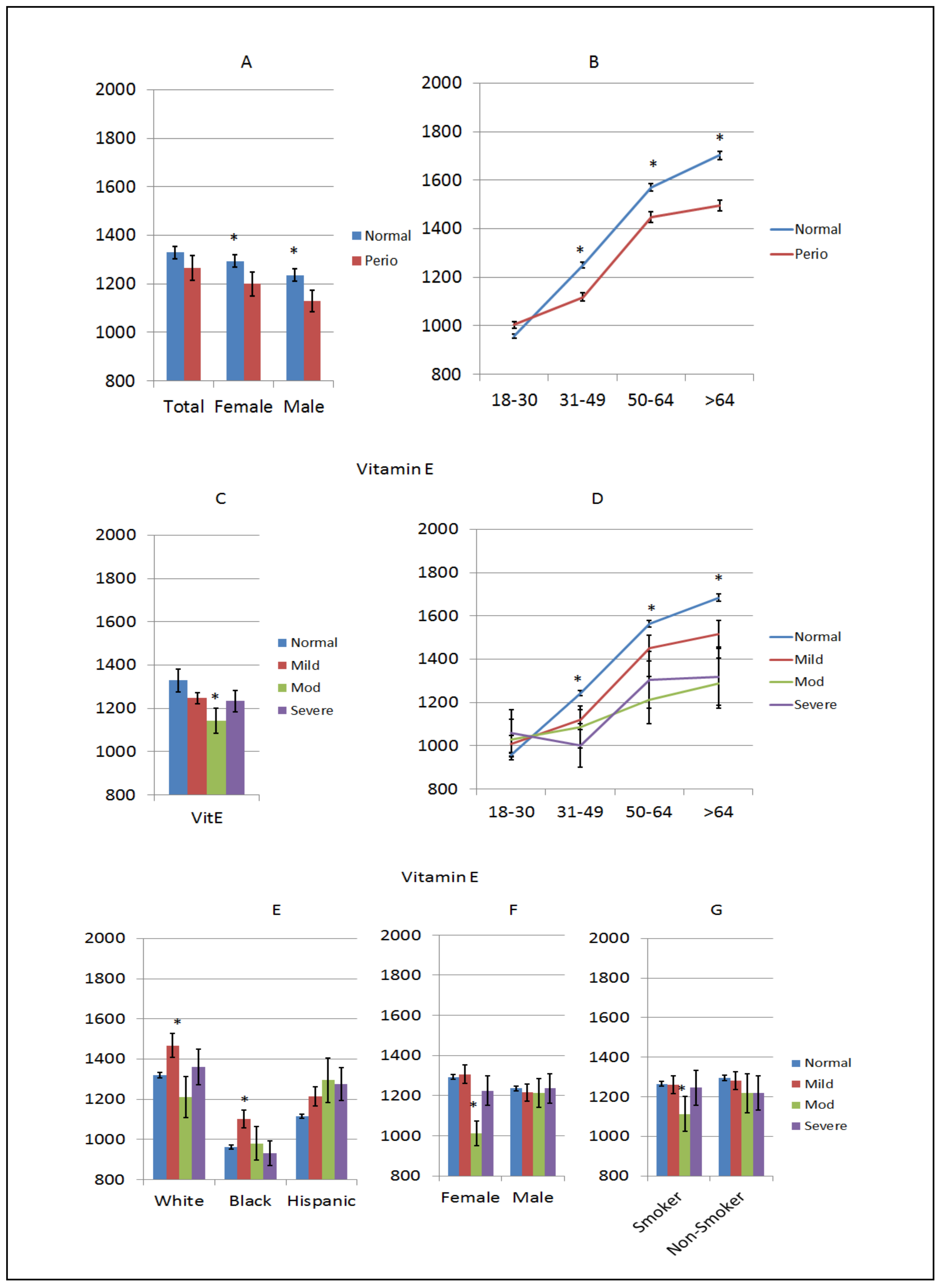
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, R.M. Oral health: The silent epidemic. Public Health Rep. 2010, 125, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Grossi, S.G.; Zambon, J.J.; Ho, A.W.; Koch, G.; Dunford, R.G.; Machtei, E.E.; Norderyd, O.M.; Genco, R.J. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J. Periodontol. 1994, 65, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.A.; Darveau, R.P. Microbial protection and virulence in periodontal tissue as a function of polymicrobial communities: Symbiosis and dysbiosis. Periodontology 2000 2015, 69, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. Strengthening the prevention of periodontal disease: The who approach. J. Periodontol. 2005, 76, 2187–2193. [Google Scholar] [CrossRef]
- Bartold, P.M.; Cantley, M.D.; Haynes, D.R. Mechanisms and control of pathologic bone loss in periodontitis. Periodontology 2000 2010, 53, 55–69. [Google Scholar] [CrossRef]
- Jansson, H.; Wahlin, A.; Johansson, V.; Akerman, S.; Lundegren, N.; Isberg, P.E.; Norderyd, O. Impact of periodontal disease experience on oral health-related quality of life. J. Periodontol. 2014, 85, 438–445. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Bergstrom, J.; Preber, H. Tobacco use as a risk factor. J. Periodontol. 1994, 65, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Walter, C.; Oluwagbemigun, K.; Bergmann, M.; Pischon, T.; Pischon, N.; Boeing, H. Smoking, smoking cessation, and risk of tooth loss: The epic-potsdam study. J. Dent. Res. 2015, 94, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, G.; Zeller, T.; Seedorf, H.; Reissmann, D.R.; Heydecke, G.; Schaefer, A.S.; Seedorf, U. Genetic susceptibility contributing to periodontal and cardiovascular disease. J. Dent. Res. 2017, 96, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Willenborg, C.; Richter, G.M.; Jockel-Schneider, Y.; Graetz, C.; Staufenbiel, I.; Wellmann, J.; Berger, K.; Krone, B.; Hoffmann, P.; et al. A genome-wide association study identifies nucleotide variants at SIGLEC5 and DEFA1A3 as risk loci for periodontitis. Hum. Mol. Genet. 2018, 27, 941–942. [Google Scholar] [CrossRef] [PubMed]
- Nashef, A.; Qabaja, R.; Salaymeh, Y.; Botzman, M.; Munz, M.; Dommisch, H.; Krone, B.; Hoffmann, P.; Wellmann, J.; Laudes, M.; et al. Integration of murine and human studies for mapping periodontitis susceptibility. J. Dent. Res. 2018, 97, 537–546. [Google Scholar] [CrossRef]
- de Coo, A.; Quintela, I.; Blanco, J.; Diz, P.; Carracedo, A. Assessment of genotyping tools applied in genetic susceptibility studies of periodontal disease: A systematic review. Arch. Oral Biol. 2018, 92, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Offenbacher, S.; Lomicronpez, N.J.; Chen, D.; Wang, H.Y.; Rogus, J.; Zhou, J.; Beck, J.; Jiang, S.; Bao, X.; et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J. Periodontal Res. 2015, 50, 52–61. [Google Scholar] [CrossRef]
- Ding, C.; Ji, X.; Chen, X.; Xu, Y.; Zhong, L. Tnf-alpha gene promoter polymorphisms contribute to periodontitis susceptibility: Evidence from 46 studies. J. Clin. Periodontol. 2014, 41, 748–759. [Google Scholar] [CrossRef]
- Rhodin, K.; Divaris, K.; North, K.E.; Barros, S.P.; Moss, K.; Beck, J.D.; Offenbacher, S. Chronic periodontitis genome-wide association studies: Gene-centric and gene set enrichment analyses. J. Dent. Res. 2014, 93, 882–890. [Google Scholar] [CrossRef]
- Kinane, D.F.; Shiba, H.; Hart, T.C. The genetic basis of periodontitis. Periodontology 2000 2005, 39, 91–117. [Google Scholar] [CrossRef]
- Hart, T.C.; Hart, P.S.; Michalec, M.D.; Zhang, Y.; Marazita, M.L.; Cooper, M.; Yassin, O.M.; Nusier, M.; Walker, S. Localisation of a gene for prepubertal periodontitis to chromosome 11q14 and identification of a cathepsin C gene mutation. J. Med. Genet. 2000, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.C.; Kornman, K.S. Genetic factors in the pathogenesis of periodontitis. Periodontology 2000 1997, 14, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, M.; Hulsmann, C.; Hoffmann, P.; Papapanou, P.N. Genome-wide analysis of periodontal and peri-implant cells and tissues. Methods Mol. Biol. 2017, 1537, 307–326. [Google Scholar] [PubMed]
- Divaris, K.; Monda, K.L.; North, K.E.; Olshan, A.F.; Reynolds, L.M.; Hsueh, W.C.; Lange, E.M.; Moss, K.; Barros, S.P.; Weyant, R.J.; et al. Exploring the genetic basis of chronic periodontitis: A genome-wide association study. Hum. Mol. Genet. 2013, 22, 2312–2324. [Google Scholar] [CrossRef] [PubMed]
- Divaris, K.; Monda, K.L.; North, K.E.; Olshan, A.F.; Lange, E.M.; Moss, K.; Barros, S.P.; Beck, J.D.; Offenbacher, S. Genome-wide association study of periodontal pathogen colonization. J. Dent. Res. 2012, 91, 21S–28S. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Offenbacher, S. Modifiable risk factors in periodontal disease: Epigenetic regulation of gene expression in the inflammatory response. Periodontology 2000 2014, 64, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Thorbert-Mros, S.; Rymo, L.; Berglundh, T. Influence of epigenetic modifications of the interleukin-10 promoter on IL10 gene expression. Eur. J. Oral Sci. 2012, 120, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L. Current concepts of epigenetics and its role in periodontitis. Curr. Oral Health Rep. 2017, 4, 286–293. [Google Scholar] [CrossRef]
- Schulz, S.; Immel, U.D.; Just, L.; Schaller, H.G.; Glaser, C.; Reichert, S. Epigenetic characteristics in inflammatory candidate genes in aggressive periodontitis. Hum. Immunol. 2016, 77, 71–75. [Google Scholar] [CrossRef]
- Larsson, L.; Castilho, R.M.; Giannobile, W.V. Epigenetics and its role in periodontal diseases: A state-of-the-art review. J. Periodontol. 2015, 86, 556–568. [Google Scholar] [CrossRef]
- Reynolds, M.A. Modifiable risk factors in periodontitis: At the intersection of aging and disease. Periodontology 2000 2014, 64, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Lod, S.; Johansson, T.; Abrahamsson, K.H.; Larsson, L. The influence of epigenetics in relation to oral health. Int. J. Dent. Hyg. 2014, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The role of nutrition in periodontal health: An update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, A.; Gomes-Filho, I.S.; Stellrecht, E.; Scannapieco, F.A. Role of periodontal therapy in management of common complex systemic diseases and conditions: An update. Periodontol 2000 2018, 78, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, E.M.; Reis, C.; Manzanares-Cespedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral dysbiotic communities and their implications in systemic diseases. Dent. J. (Basel) 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Mariotti, A. The future of periodontal-systemic associations: Raising the standards. Curr. Oral Health Rep. 2017, 4, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. 1), S183–S203. [Google Scholar] [CrossRef]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher intakes of fruits and vegetables, beta-carotene, vitamin c, alpha-tocopherol, epa, and dha are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary antioxidants and their indispensable role in periodontal health. J. Food Drug Anal. 2016, 24, 239–246. [Google Scholar] [CrossRef]
- Nanri, H.; Yamada, Y.; Itoi, A.; Yamagata, E.; Watanabe, Y.; Yoshida, T.; Miyake, M.; Date, H.; Ishikawa-Takata, K.; Yoshida, M.; et al. Frequency of fruit and vegetable consumption and the oral health-related quality of life among Japanese elderly: A cross-sectional study from the Kyoto-Kameoka study. Nutrients 2017, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Bouchard, P.; Cagetti, M.G.; Campus, G.; Carra, M.C.; Cocco, F.; Nibali, L.; Hujoel, P.; Laine, M.L.; Lingstrom, P.; et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S39–S51. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L. Potential mechanisms underpinning the nutritional modulation of periodontal inflammation. J. Am. Dent. Assoc. 2009, 140, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Ishikado, A.; Morino, K.; Nishio, Y.; Ugi, S.; Kajiwara, S.; Kurihara, M.; Iwakawa, H.; Nakao, K.; Uesaki, S.; et al. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: A pilot study. Nutr. Res. (New York, N.Y.) 2014, 34, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.S.; Bissada, N.F.; Borawski, E.A. Diet and periodontitis. J. Int. Acad. Periodontol. 2005, 7, 21–26. [Google Scholar] [PubMed]
- Kulkarni, V.; Bhatavadekar, N.B.; Uttamani, J.R. The effect of nutrition on periodontal disease: A systematic review. J. Calif. Dent. Assoc. 2014, 42, 302–311. [Google Scholar] [PubMed]
- Huja Emecen, P.; Li, H.; Ebersole, J.L.; Lambert, J.; Bush, H. The exposome and periodontal disease: Epidemiologic evaluation of nhanes within smoking classification. PLoS ONE 2018, in press. [Google Scholar]
- Zhuang, X.; Ni, A.; Liao, L.; Guo, Y.; Dai, W.; Jiang, Y.; Zhou, H.; Hu, X.; Du, Z.; Wang, X.; et al. Environment-wide association study to identify novel factors associated with peripheral arterial disease: Evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis 2018, 269, 172–177. [Google Scholar] [CrossRef]
- Choi, J.E.; Ainsworth, B.E. Associations of food consumption, serum vitamins and metabolic syndrome risk with physical activity level in middle-aged adults: The National Health and Nutrition Examination Survey (NHANES) 2005–2006. Public Health Nutr. 2016, 19, 1674–1683. [Google Scholar] [CrossRef]
- Linden, G.J.; McClean, K.M.; Woodside, J.V.; Patterson, C.C.; Evans, A.; Young, I.S.; Kee, F. Antioxidants and periodontitis in 60–70-year-old men. J. Clin. Periodontol. 2009, 36, 843–849. [Google Scholar] [CrossRef]
- NHANES. Available online: https://www.cdc.gov/nchs/nhanes/) (accessed on 15 October 2018).
- Demmer, R.T.; Squillaro, A.; Papapanou, P.N.; Rosenbaum, M.; Friedewald, W.T.; Jacobs, D.R., Jr.; Desvarieux, M. Periodontal infection, systemic inflammation, and insulin resistance: Results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care 2012, 35, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.A.; Nowjack-Raymer, R.; Barker, L.K.; Nunn, J.H.; Steele, J.G.; Tan, S.; Lewis, B.G.; Beltran-Aguilar, E.D. Overview and quality assurance for the oral health component of the National Health and Nutrition Examination Survey (NHANES), 2003–04. J. Public Health Dent. 2008, 68, 218–226. [Google Scholar] [CrossRef]
- Dye, B.A.; Barker, L.K.; Selwitz, R.H.; Lewis, B.G.; Wu, T.; Fryar, C.D.; Ostchega, Y.; Beltran, E.D.; Ley, E. Overview and quality assurance for the National Health and Nutrition Examination Survey (NHANES) oral health component, 1999–2002. Community Dent. Oral Epidemiol. 2007, 35, 140–151. [Google Scholar] [CrossRef]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A. Accuracy of NHANES periodontal examination protocols. J. Dent. Res. 2010, 89, 1208–1213. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: https://www.R-project.org (accessed on 1 January 2017).
- NHANES. Available online: http://www.cdc.gov/nchs/tutorials/Nhanes/SurveyDesign/Weighting/Task2.htm (accessed on 30 March 2018).
- Lambert, J.; Gong, L.; Elliot, C. rFSA: Feasible Soultion Algorithm for Finding Best Subsets and Interations. R Package Version 0.9.2. 2018. Available online: https://CRAN.R-project.org/package=rFSA (accessed on 5 October 2018).
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Dawson, D., 3rd; Emecen-Huja, P.; Nagarajan, R.; Howard, K.; Grady, M.E.; Thompson, K.; Peyyala, R.; Al-Attar, A.; Lethbridge, K.; et al. The periodontal war: Microbes and immunity. Periodontology 2000 2017, 75, 52–115. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.; Faustoferri, R.C.; Quivey, R.G., Jr. What are we learning and what can we learn from the human oral microbiome project? Curr. Oral Health Rep. 2016, 3, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Madupu, R.; Szpakowski, S.; Nelson, K.E. Microbiome in human health and disease. Sci. Prog. 2013, 96, 153–170. [Google Scholar] [CrossRef]
- Loos, B.G.; Papantonopoulos, G.; Jepsen, S.; Laine, M.L. What is the contribution of genetics to periodontal risk? Dent. Clin. N. Am. 2015, 59, 761–780. [Google Scholar] [CrossRef]
- Scapoli, L.; Girardi, A.; Palmieri, A.; Martinelli, M.; Cura, F.; Lauritano, D.; Pezzetti, F.; Carinci, F. Interleukin-6 gene polymorphism modulates the risk of periodontal diseases. J. Biol. Regul. Homeost. Agents 2015, 29, 111–116. [Google Scholar] [PubMed]
- Zhang, S.; Barros, S.P.; Niculescu, M.D.; Moretti, A.J.; Preisser, J.S.; Offenbacher, S. Alteration of ptgs2 promoter methylation in chronic periodontitis. J. Dent. Res. 2010, 89, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk indicators for periodontitis in US adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Babin, A.; Saciat, C.; Teixeira, M.; Troussard, J.P.; Motreuil, S.; Moreau, J.; Moret, Y. Limiting immunopathology: Interaction between carotenoids and enzymatic antioxidant defences. Dev. Comp. Immunol. 2015, 49, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; Northrop-Clewes, C.A.; Knowles, J. The use of adjustment factors to address the impact of inflammation on vitamin a and iron status in humans. J. Nutr. 2015, 145, 1137S–1143S. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar]
- Cao, Y.; Wittert, G.; Taylor, A.W.; Adams, R.; Appleton, S.; Shi, Z. Nutrient patterns and chronic inflammation in a cohort of community dwelling middle-aged men. Clin. Nutr. 2017, 36, 1040–1047. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Andreeva, V.A.; Ducros, V.; Jeandel, C.; Julia, C.; Hercberg, S.; Galan, P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br. J. Nutr. 2014, 111, 915–923. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary beta-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Horie, T.; Fukasawa, K.; Ozaki, K.; Onishi, Y.; Kanayama, T.; Iezaki, T.; Kaneda, K.; Sugiura, M.; Hinoi, E. Amelioration of the development of osteoarthritis by daily intake of beta-cryptoxanthin. Biol. Pharm. Bull. 2017, 40, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Sahin, N.; Yilmaz, I.; Juturu, V. Beta-cryptoxanthin ameliorates metabolic risk factors by regulating NF-kappab and NRF2 pathways in insulin resistance induced by high-fat diet in rodents. Food Chem. Toxicol. 2017, 107, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, C.; Ashida, N.; Yokoyama, S.; Tominari, T.; Hirata, M.; Ogawa, K.; Sugiura, M.; Yano, M.; Inada, M.; Miyaura, C. The protective effects of beta-cryptoxanthin on inflammatory bone resorption in a mouse experimental model of periodontitis. Biosci. Biotechnol. Biochem. 2013, 77, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, M.; Yamamoto, T.; Ichioka, H.; Honjo, K.; Yamamoto, K.; Oseko, F.; Kita, M.; Mazda, O.; Kanamura, N. Beta-cryptoxanthin regulates bone resorption related-cytokine production in human periodontal ligament cells. Arch. Oral Biol. 2013, 58, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Toti, E.; Chen, C.O.; Palmery, M.; Villano Valencia, D.; Peluso, I. Non-provitamin a and provitamin a carotenoids as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar] [CrossRef] [PubMed]
- Hennig, B.; Petriello, M.C.; Gamble, M.V.; Surh, Y.J.; Kresty, L.A.; Frank, N.; Rangkadilok, N.; Ruchirawat, M.; Suk, W.A. The role of nutrition in influencing mechanisms involved in environmentally mediated diseases. Rev. Environ Health 2018, 33, 87–97. [Google Scholar] [CrossRef]
- Sun, S.; He, M.; Wang, Y.; Yang, H.; Al-Abed, Y. Folic acid derived-p5779 mimetics regulate damp-mediated inflammation through disruption of HMGB1:TLR4:MD-2 axes. PLoS ONE 2018, 13, e0193028. [Google Scholar] [CrossRef]
- Ma, J.; Zhen, X.; Huang, X.; Jiang, X. Folic acid supplementation repressed hypoxia-induced inflammatory response via ROS and JAK2/STAT3 pathway in human promyelomonocytic cells. Nutr. Res. 2018, 53, 40–50. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Van Denburgh, M.; Zaharris, E.; Albert, C.M.; Manson, J.E. Effect of combined treatment with folic acid, vitamin B6, and vitamin B12 on plasma biomarkers of inflammation and endothelial dysfunction in women. J. Am. Heart Assoc. 2018, 7, e008517. [Google Scholar] [CrossRef]
- Erdemir, E.O.; Bergstrom, J. Relationship between smoking and folic acid, vitamin B12 and some haematological variables in patients with chronic periodontal disease. J. Clin. Periodontol. 2006, 33, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Al-Shammari, K.; Nociti, F.H., Jr.; Soehren, S.; Wang, H.L. Effects of vitamin-B complex supplementation on periodontal wound healing. J. Periodontol. 2005, 76, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Neiva, R.F.; Steigenga, J.; Al-Shammari, K.F.; Wang, H.L. Effects of specific nutrients on periodontal disease onset, progression and treatment. J. Clin. Periodontol. 2003, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Staudte, H.; Kranz, S.; Volpel, A.; Schutze, J.; Sigusch, B.W. Comparison of nutrient intake between patients with periodontitis and healthy subjects. Quintessence Int. 2012, 43, 907–916. [Google Scholar]
- Yu, Y.H.; Kuo, H.K.; Lai, Y.L. The association between serum folate levels and periodontal disease in older adults: Data from the National Health and Nutrition Examination Survey 2001/02. J. Am. Geriatr. Soc. 2007, 55, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Jiang, L. Status of vitamin d, antimicrobial peptide cathelicidin and t helper-associated cytokines in patients with diabetes mellitus and pulmonary tuberculosis. Exp. Ther. Med. 2015, 9, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Abreu, O.J.; Tatakis, D.N.; Elias-Boneta, A.R.; Lopez Del Valle, L.; Hernandez, R.; Pousa, M.S.; Palacios, C. Low vitamin D status strongly associated with periodontitis in puerto rican adults. BMC Oral Health 2016, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Nagrale, A.V.; Joseraj, M.G.; Pradeep Kumar, K.M.; Kaziyarakath, J.A.; Chandini, R. Low levels of serum vitamin D in chronic periodontitis patients with type 2 diabetes mellitus: A hospital-based cross-sectional clinical study. J. Indian Soc. Periodontol. 2015, 19, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, G.N.; Knuuttila, M.; Niemela, O.; Raunio, T.; Karttunen, R.; Vainio, O.; Hedberg, P.; Ylostalo, P.; Tervonen, T. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J. Periodontal Res. 2015, 50, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gumus, P.; Ozturk, V.O.; Bozkurt, E.; Emingil, G. Evaluation of the gingival inflammation in pregnancy and postpartum via 25-hydroxy-vitamin D3, prostaglandin E2 and TNF-alpha levels in saliva. Arch. Oral Biol. 2016, 63, 1–6. [Google Scholar] [CrossRef]
- Lee, H.J.; Je, D.I.; Won, S.J.; Paik, D.I.; Bae, K.H. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015, 43, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.; Giovannucci, E.; Krall Kaye, E.; Joshipura, K.J.; Dietrich, T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014, 17, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Li, H.; Zhang, P.P.; Wang, S.M. Association between vitamin D receptor polymorphisms and periodontitis: A meta-analysis. J. Periodontol. 2012, 83, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, X.; Tian, Y.; Zhang, X.; Lu, R.; Meng, H. Gc gene polymorphisms and vitamin d-binding protein levels are related to the risk of generalized aggressive periodontitis. Int. J. Endocrinol. 2016, 2016, 5141089. [Google Scholar] [CrossRef] [PubMed]
- Chantarangsu, S.; Sura, T.; Mongkornkarn, S.; Donsakul, K.; Torrungruang, K. Vitamin d receptor gene polymorphism and smoking in the risk of chronic periodontitis. J. Periodontol. 2016, 87, 1343–1351. [Google Scholar] [CrossRef]
- Bashutski, J.D.; Eber, R.M.; Kinney, J.S.; Benavides, E.; Maitra, S.; Braun, T.M.; Giannobile, W.V.; McCauley, L.K. The impact of vitamin d status on periodontal surgery outcomes. J. Dent. Res. 2011, 90, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin d-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Rigo, I.; McMahon, L.; Dhawan, P.; Christakos, S.; Yim, S.; Ryan, L.K.; Diamond, G. Induction of triggering receptor expressed on myeloid cells (TREM-1) in airway epithelial cells by 1,25(OH)(2) vitamin D(3). Innate Immun. 2012, 18, 250–257. [Google Scholar] [CrossRef]
- Dhawan, P.; Wei, R.; Sun, C.; Gombart, A.F.; Koeffler, H.P.; Diamond, G.; Christakos, S. C/EBPalpha and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J. Cell. Physiol. 2015, 230, 464–472. [Google Scholar] [CrossRef]
- Grenier, D.; Morin, M.P.; Fournier-Larente, J.; Chen, H. Vitamin d inhibits the growth of and virulence factor gene expression by porphyromonas gingivalis and blocks activation of the nuclear factor kappa B transcription factor in monocytes. J. Periodontal Res. 2016, 51, 359–365. [Google Scholar] [CrossRef]
- Xu, Q.A.; Li, Z.F.; Zhang, P.; Cao, L.H.; Fan, M.W. Effects of 1,25-dihydroxyvitamin D3 on macrophage cytokine secretion stimulated by porphyromonas gingivalis. Jpn. J. Infect. Dis. 2016, 69, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Caton, J.G.; Albandar, J.M.; Bissada, N.F.; Bouchard, P.; Cortellini, P.; Demirel, K.; de Sanctis, M.; Ercoli, C.; Fan, J.; et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. 1), S2372–S248. [Google Scholar]
- Al-Khalidi, B.; Rotondi, M.A.; Kimball, S.M.; Ardern, C.I. Clinical utility of serum 25-hydroxyvitamin d in the diagnosis of insulin resistance and estimation of optimal 25-hydroxyvitamin D in U.S. Adults. Diabetes Res. Clin. Pract. 2017, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Forno, E.; Celedon, J.C. Vitamin D insufficiency and asthma in a US nationwide study. J. Allergy Clin. Immunol. Pract. 2017, 5, 790.e1–796.e1. [Google Scholar] [CrossRef] [PubMed]
- Daraghmeh, A.H.; Bertoia, M.L.; Al-Qadi, M.O.; Abdulbaki, A.M.; Roberts, M.B.; Eaton, C.B. Evidence for the vitamin D hypothesis: The NHANES III extended mortality follow-up. Atherosclerosis 2016, 255, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Llewellyn, D.; Isaac, M.; Tabet, N. Vitamin e for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2017, 4, CD002854. [Google Scholar] [PubMed]
- Kim, S.H.; Park, Y.M.; Choi, B.Y.; Kim, M.K.; Roh, S.; Kim, K.; Yang, Y.J. Associations of serum levels of vitamins A, C, and E with the risk of cognitive impairment among elderly Koreans. Nutr. Res. Pract. 2018, 12, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.; Hoseini, Z.; Sahebkar, A.; Mirzaei, H. Anti-atherosclerotic effects of vitamins D and E in suppression of atherogenesis. J. Cell. Physiol. 2017, 232, 2968–2976. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin e and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2018, 1–23. [Google Scholar] [CrossRef]
- Zong, G.; Scott, A.E.; Griffiths, H.R.; Zock, P.L.; Dietrich, T.; Newson, R.S. Serum alpha-tocopherol has a nonlinear inverse association with periodontitis among US adults. J. Nutr. 2015, 145, 893–899. [Google Scholar] [CrossRef]
- Muniz, F.W.; Nogueira, S.B.; Mendes, F.L.; Rosing, C.K.; Moreira, M.M.; de Andrade, G.M.; Carvalho Rde, S. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch. Oral Biol. 2015, 60, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin e supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B.; Wright, H.J.; Scott, A.E.; Griffiths, H.R.; Grant, M.M. Ascorbate and alpha-tocopherol differentially modulate reactive oxygen species generation by neutrophils in response to fcgammar and tlr agonists. Innate Immun. 2013, 19, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Derradjia, A.; Alanazi, H.; Park, H.J.; Djeribi, R.; Semlali, A.; Rouabhia, M. Alpha-tocopherol decreases interleukin-1beta and -6 and increases human beta-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J. Periodontal Res. 2016, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Milward, M.R.; Ling-Mountford, N.; Weston, P.; Carter, K.; Askey, K.; Dallal, G.E.; De Spirt, S.; Sies, H.; Patel, D.; et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double-blind RCT. J. Clin. Periodontol. 2012, 39, 62–72. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebersole, J.L.; Lambert, J.; Bush, H.; Huja, P.E.; Basu, A. Serum Nutrient Levels and Aging Effects on Periodontitis. Nutrients 2018, 10, 1986. https://doi.org/10.3390/nu10121986
Ebersole JL, Lambert J, Bush H, Huja PE, Basu A. Serum Nutrient Levels and Aging Effects on Periodontitis. Nutrients. 2018; 10(12):1986. https://doi.org/10.3390/nu10121986
Chicago/Turabian StyleEbersole, Jeffrey L., Joshua Lambert, Heather Bush, Pinar Emecen Huja, and Arpita Basu. 2018. "Serum Nutrient Levels and Aging Effects on Periodontitis" Nutrients 10, no. 12: 1986. https://doi.org/10.3390/nu10121986
APA StyleEbersole, J. L., Lambert, J., Bush, H., Huja, P. E., & Basu, A. (2018). Serum Nutrient Levels and Aging Effects on Periodontitis. Nutrients, 10(12), 1986. https://doi.org/10.3390/nu10121986





