Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs?
Abstract
1. Introduction
2. Materials and Methods
2.1. AA Solutions and Complete Mixtures for Parenteral Nutrition
2.2. Patients
2.3. Statistical Analysis
3. Results
3.1. AA Solutions and Complete Mixtures for Parenteral Nutrition
3.2. Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Research Involving Human Participants and Informed Consent
Conflicts of Interest
Abbreviations
| AA | amino acid |
| BCAA | branched-chain amino acid |
| BIA | bioelectrical impedance analysis |
| BW | body weight |
| EAA | essential amino acid |
| FAO | Food and Agriculture Organization of the United Nations |
| IAAO | indicator amino acid oxidation |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NEAA | nonessential amino acid |
| PN | parenteral nutrition |
| SBS | short bowel syndrome |
| SMM | skeletal muscle mass |
| TAA | total amino acid |
| UNU | United Nations University |
| WHO | World Health Organization |
References
- Singer, P.; Berger, M.M.; Van den Berghe, G.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C.; et al. ESPEN Guidelines on parenteral nutrition: Intensive care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hébuterne, X.; Pertkiewicz, M.; et al. ESPEN Guidelines on Parenteral Nutrition: Home parenteral nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, L.; Schneider, S.M.; Cederholm, T.; Cederholm, T.; Krznaric, Z.; Shenkin, A.; Stanga, Z.; Toigo, G.; Vandewoude, M.; Volkert, D.; et al. ESPEN Guidelines on Parenteral Nutrition: Geriatrics. Clin. Nutr. 2009, 28, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yarandi, S.S.; Zhao, V.M.; Hebbar, G.; Ziegler, T.R. Amino acid composition in parenteral nutrition: What is the evidence? Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 75–82. [Google Scholar] [CrossRef]
- Hoffer, L.J. Parenteral Nutrition: Amino Acids. Nutrients 2017, 9, 257. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Campillo, B.; Kondrup, J.; Marchesini, G.; Schütz, T.; Shenkin, A.; Wendon, J. ESPEN Guidelines on Parenteral Nutrition: Hepatology. Clin. Nutr. 2009, 28, 436–444. [Google Scholar] [CrossRef]
- Stein, J.; Boehles, H.J.; Blumenstein, I.; Goeters, C.; Schulz, R. Amino acids: Guidelines on parenteral nutrition, chapter 4. Ger. Med. Sci. 2009, 7, 1–8. [Google Scholar]
- Koletzko, B.; Goulet, O.; Hunt, J.; Parenteral Nutrition Guidelines Working Group; European Society for Clinical Nutrition and Metabolism; European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN); European Society of Paediatric Research (ESPR). Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of PaediatricResearch (ESPR). J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. 2), S1–S87. [Google Scholar]
- Doig, G.S.; Simpson, F.; Delaney, A. A review of the true methodological quality of nutritional support trials conducted in the critically ill: Time for improvement. Anesth. Analg. 2005, 100, 527–533. [Google Scholar] [CrossRef]
- Debaveye, Y.; Van den Berghe, G. Risks and benefits of nutritional support during critical illness. Annu. Rev. Nutr. 2006, 26, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Sitren, H.S.; Fisher, H.; Ali, R. Comparison of two amino acid solutions for total parenteral nutrition of normal and traumatized rats. J. Nutr. 1975, 105, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- McLaughlan, J.M.; Illman, W.I. Use of free plasma amino acid levels for estimating amino acid requirements of the growing rat. J. Nutr. 1967, 93, 21–24. [Google Scholar] [CrossRef]
- Heird, W.C.; Dell, R.B.; Helms, R.A.; Greene, H.L.; Ament, M.E.; Karna, P.; Storm, M.C. Amino acid mixture designed to maintain normal plasma amino acid patterns in infants and children requiring parenteral nutrition. Pediatrics 1987, 80, 401–408. [Google Scholar] [PubMed]
- Iapichino, G.; Radrizzani, D.; Veschi, G.; Bonetti, G.; Cesari, R.; Ciceri, R.; Guarnerio, C.; Quarenghi, E.; Rigoli, A.; Ronzoni, G.; et al. Kinetic study during amino acid infusion in catabolic patients. Comparison between two solutions. Minerva Anestesiol. 1991, 57, 83–90. [Google Scholar] [PubMed]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Pennings, B.; Groen, B.; de Lange, A.; Gijsen, A.P.; Zorenc, A.H.; Senden, J.M.; van Loon, L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E992–E999. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Deutz, N.E.; Memelink, R.G.; Verlaan, S.; Wolfe, R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014, 13, 9. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organ Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Indicator amino acid oxidation: Concept and application. J. Nutr. 2008, 138, 243–246. [Google Scholar] [CrossRef] [PubMed]
- FAO. Nutritional Studies: Amino-Acid Content of Foods and Biological Data on Proteins; FAO Nutritional Studies; FAO: Rome, Italy, 1970; Volume 24, pp. 1–285. [Google Scholar]
- Tengvall, M.; Ellegård, L.; Malmros, V.; Bosaeus, N.; Lissner, L.; Bosaeus, I. Body composition in the elderly: Reference values and bioelectrical impedance spectroscopy to predict total body skeletal muscle mass. Clin. Nutr. 2009, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Wolfe, R.R. Is there a maximal anabolic response to protein intake with a meal? Clin. Nutr. 2013, 32, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Deutz, N.E.P.; Wolfe, R.R. Update on maximal anabolic response to dietary protein. Clin. Nutr. 2018, 37, 411–418. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Ren, M.; Qiao, S.; He, P.; Li, D.; Zeng, X. Effects of isoleucine on glucose uptake through the enhancement of muscular membrane concentrations of GLUT1 and GLUT4 and intestinal membrane concentrations of Na+/glucose co-transporter 1 (SGLT-1) and GLUT2. Br. J. Nutr. 2016, 116, 593–602. [Google Scholar] [CrossRef] [PubMed]
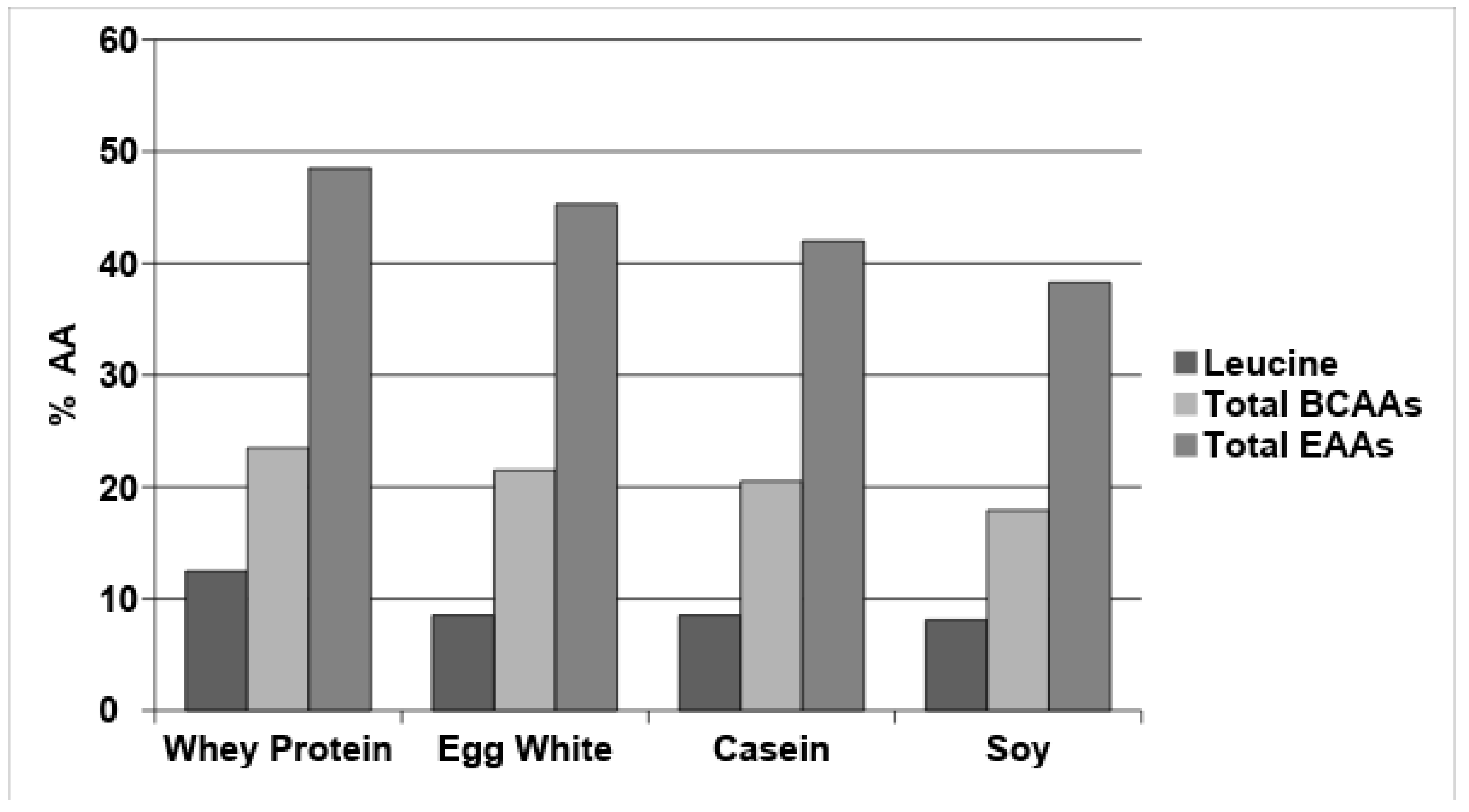
| EAA (mg) | mg EAA/kg BW/d | mg EAA/g TAAs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult EAA Requirement a | Whey Protein | Commercially Available IV Standard Amino Acid Solutions | |||||||||
| WHO/FAO/UNU (2007 Report) | IAAO Method | Glamin® 13.4% | Aminoven® 10% | Sintamin® 10% | Isopuramin® 10% | Parentamin® 10% | Amixal®10% | Freamine®10% | Travasol®10% | ||
| Leucine | 39 | - | 125 | 58 | 74 | 95 | 106 | 118 | 89 | 94 | 73 |
| Isoleucine | 20 | - | 60 | 41 | 50 | 73 | 80 | 55 | 50 | 71 | 60 |
| Valine | 26 | - | 50 | 53 | 62 | 69 | 116 | 97 | 62 | 68 | 58 |
| Total BCAAs | 85 | 144 | 235 | 152 | 186 | 237 | 302 | 270 | 201 | 233 | 191 |
| Lysine | 30 | 36 | 105 | 66 | 66 | 77 | 115 | 153 | 28 | 75 | 58 |
| Methionine + Cysteine | 15 | 12.6 | 55 | 41 | 43 | 57 | 73 | 28 | 44 | 57 | 40 |
| Threonine | 15 | 19 | 45 | 41 | 44 | 42 | 95 | 58 | 42 | 41 | 42 |
| Histidine | 10 | - | 20 | 50 | 30 | 35 | 50 | 21 | 30 | 29 | 48 |
| Phenylalanine + Tyrosine | 25 | 42 | 75 | 60 | 55 | 60 | 82 | 37 | 51 | 58 | 96 |
| Tryptophan | 4 | 4 | 25 | 14 | 20 | 16 | 32 | 17 | 16 | 15 | 18 |
| Total EAAs | 184 | 250 | 560 | 424 | 444 | 524 | 749 | 584 | 412 | 508 | 493 |
| Total NEAAs | - | - | 440 | 576 | 556 | 476 | 251 | 416 | 588 | 492 | 507 |
| EAA (mg) | mg EAA/Kg BW/d | mg EAA/g TAAs | ||||||
|---|---|---|---|---|---|---|---|---|
| Adult EAA Requirement a | Commercially Available IV Complete Parenteral Mixtures | |||||||
| WHO/FAO/UNU (2007 Report) | IAAO Method | Whey Protein | Periven® Kabiven® | Krinuven® Smofkabiven® Aminomix® | Nutriplus® Nutrispecial® Nutriperi® Basalflex® Periflex® Plusflex® Specialflex® | Clinimix® Oliclinomel® | Olimel® | |
| Leucine | 39 | - | 125 | 71 | 76 | 78 | 73 | 69 |
| Isoleucine | 20 | - | 60 | 49 | 52 | 59 | 60 | 50 |
| Valine | 26 | - | 50 | 64 | 62 | 65 | 58 | 64 |
| Total BCAAs | 85 | 144 | 235 | 184 | 190 | 202 | 191 | 183 |
| Lysine | 30 | 36 | 105 | 80 | 49 | 57 | 58 | 79 |
| Methionine + Cysteine | 15 | 12.6 | 55 | 49 | 43 | 49 | 40 | 50 |
| Threonine | 15 | 19 | 45 | 49 | 45 | 45 | 42 | 50 |
| Histidine | 10 | - | 20 | 60 | 30 | 31 | 48 | 60 |
| Phenylalanine + Tyrosine | 25 | 42 | 75 | 73 | 55 | 88 | 60 | 72 |
| Tryptophan | 4 | 4 | 25 | 17 | 20 | 14 | 18 | 17 |
| Total EAAs | 184 | 250 | 560 | 512 | 433 | 486 | 457 | 511 |
| Total NEAAs | - | - | 440 | 488 | 567 | 514 | 543 | 489 |
| Mean ± SD | Min-Max | |
|---|---|---|
| Age (y) | 49 ± 17 | 24-75 |
| Weight (kg) | 55 ± 12 | 42-81 |
| Height (cm) | 162 ± 10 | 141-182 |
| Delta SMM (kg) | 0.3 ± 0.9 | −1.5-2.2 |
| Delta Time (days) | 143 ± 94 | 30-356 |
| TAAs/kg BW/day (g) | 1.15 ± 0.36 | 0.53-1.98 |
| EAAs/kg BW/day (g) | 0.54 ± 0.17 | 0.28-0.98 |
| BCAAs/kg BW/day(g) | 0.24 ± 0.08 | 0.13-0.44 |
| Leucine/kg BW/day (g) | 0.09 ± 0.03 | 0.05-0.18 |
| Isoleucine/kg BW/day (g) | 0.07 ± 0.02 | 0.04-0.14 |
| Valine/kg BW/day (g) | 0.07 ± 0.02 | 0.04-0.13 |
| Lysine/kg BW/day (g) | 0.07 ± 0.02 | 0.04-0.14 |
| Methionine/kg BW/day (g) | 0.06 ± 0.02 | 0.03-0.11 |
| Phenylalanine/kg BW/day (g) | 0.07 ± 0.03 | 0.03-0.15 |
| Threonine/kg BW/day (g) | 0.05 ± 0.02 | 0.02-0.09 |
| Tryptophan/kg BW/day (g) | 0.02 ± 0.01 | 0.01-0.04 |
| Histidine/kg BW/day (g) | 0.04±0.01 | 0.02-0.07 |
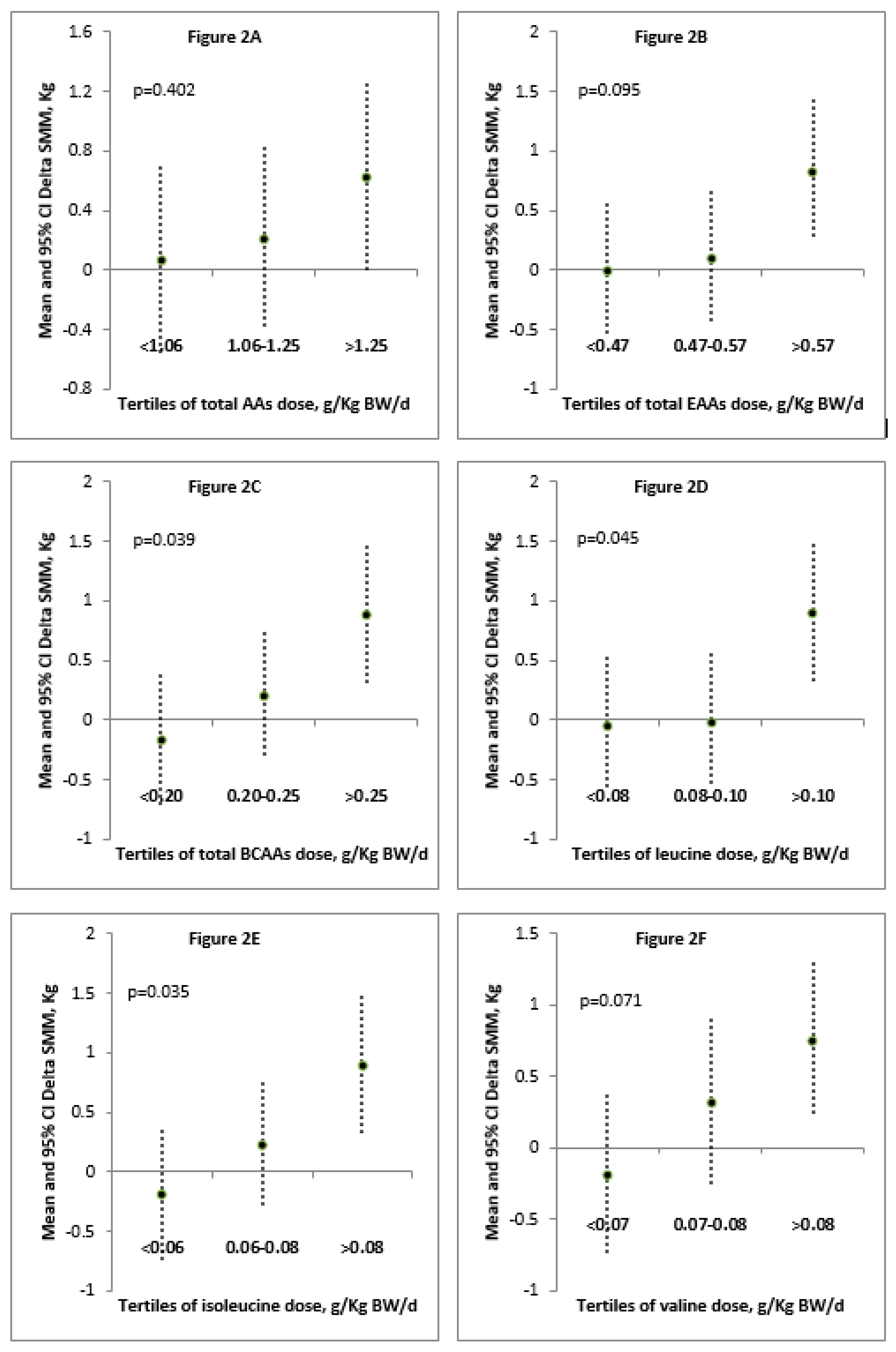
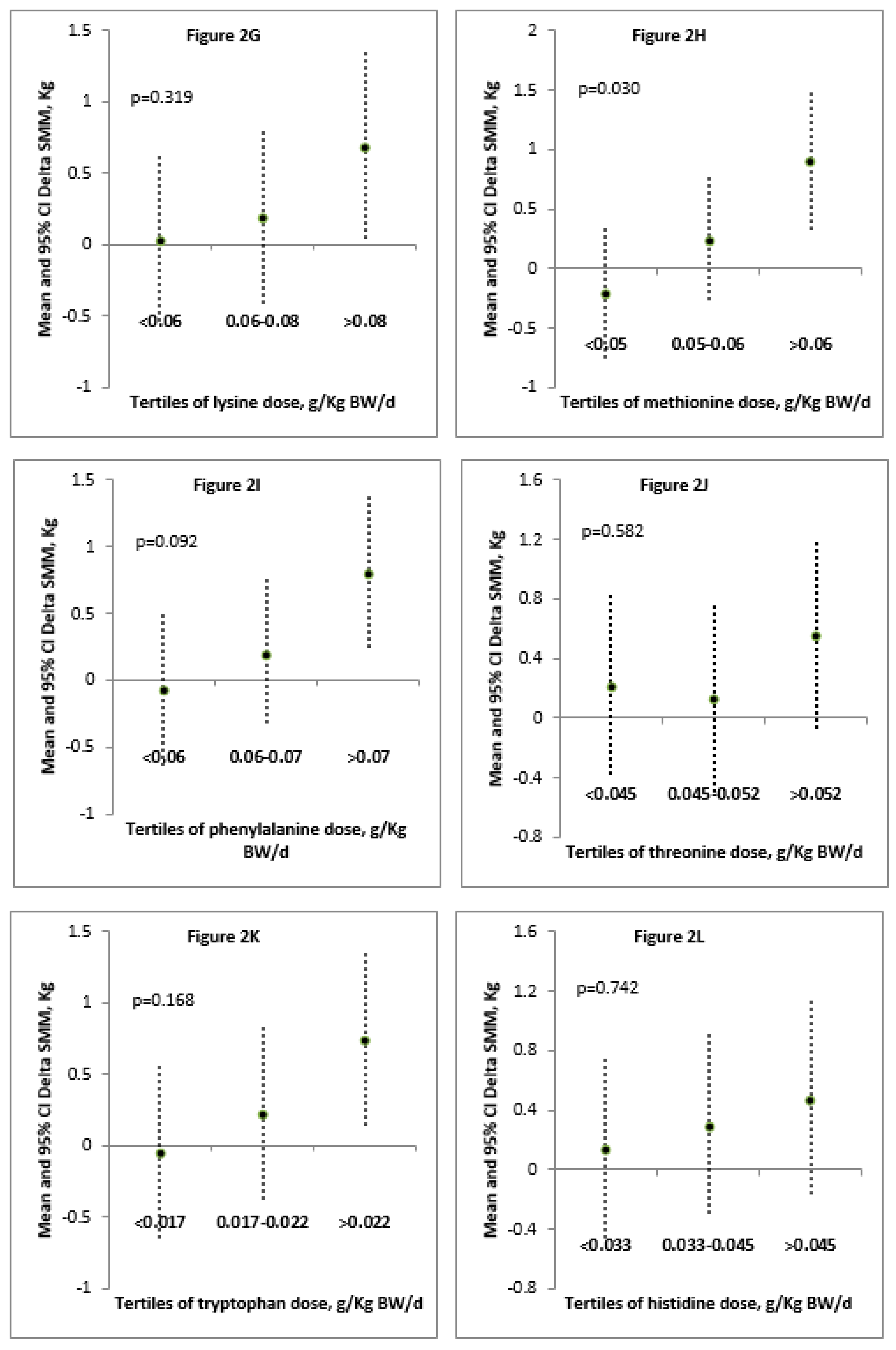
| Correlation between Delta SMM (kg) and Daily Dose (g) of: | rho | p |
|---|---|---|
| TAAs/kg BW/day | 0.350 | 0.080 |
| EAAs/kg BW/day | 0.398 | 0.044 * |
| BCAAs/kg BW/day | 0.460 | 0.018 * |
| Leucine/kg BW/day | 0.463 | 0.017 * |
| Isoleucine/kg BW/day | 0.434 | 0.027 * |
| Valine/kg BW/day | 0.439 | 0.025 * |
| Lysine/kg BW/day | 0.348 | 0.081 |
| Methionine/kg BW/day | 0.447 | 0.022 * |
| Phenylalanine/kg BW/day | 0.359 | 0.072 |
| Threonine/kg BW/day | 0.311 | 0.122 |
| Tryptophan/kg BW/day | 0.338 | 0.091 |
| Histidine/kg BW/day | 0.258 | 0.204 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacone, R.; Scanzano, C.; Santarpia, L.; Alfonsi, L.; Marra, M.; Pagano, M.C.; D’Isanto, A.; Frangipane, I.; Vitalone, A.; D’Angeli, M.; et al. Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs? Nutrients 2018, 10, 1937. https://doi.org/10.3390/nu10121937
Iacone R, Scanzano C, Santarpia L, Alfonsi L, Marra M, Pagano MC, D’Isanto A, Frangipane I, Vitalone A, D’Angeli M, et al. Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs? Nutrients. 2018; 10(12):1937. https://doi.org/10.3390/nu10121937
Chicago/Turabian StyleIacone, Roberto, Clelia Scanzano, Lidia Santarpia, Lucia Alfonsi, Maurizio Marra, Maria Carmen Pagano, Anna D’Isanto, Ignazio Frangipane, Andrea Vitalone, Mariana D’Angeli, and et al. 2018. "Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs?" Nutrients 10, no. 12: 1937. https://doi.org/10.3390/nu10121937
APA StyleIacone, R., Scanzano, C., Santarpia, L., Alfonsi, L., Marra, M., Pagano, M. C., D’Isanto, A., Frangipane, I., Vitalone, A., D’Angeli, M., Contaldo, F., & Pasanisi, F. (2018). Essential Amino Acid Profile in Parenteral Nutrition Mixtures: Does It Meet Needs? Nutrients, 10(12), 1937. https://doi.org/10.3390/nu10121937








