The Healing-Promoting Properties of Selected Cyclitols—A Review
Abstract
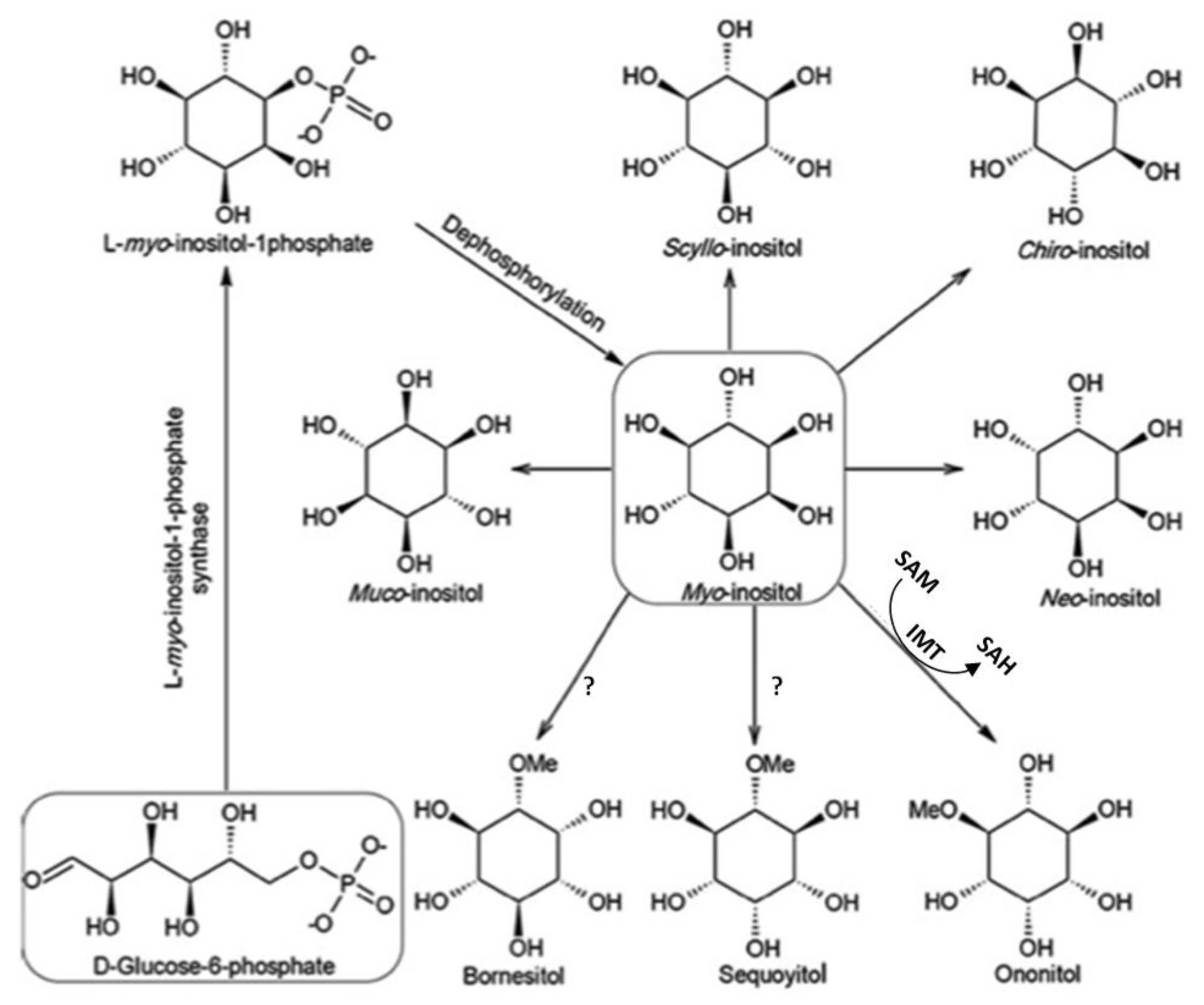
1. Background
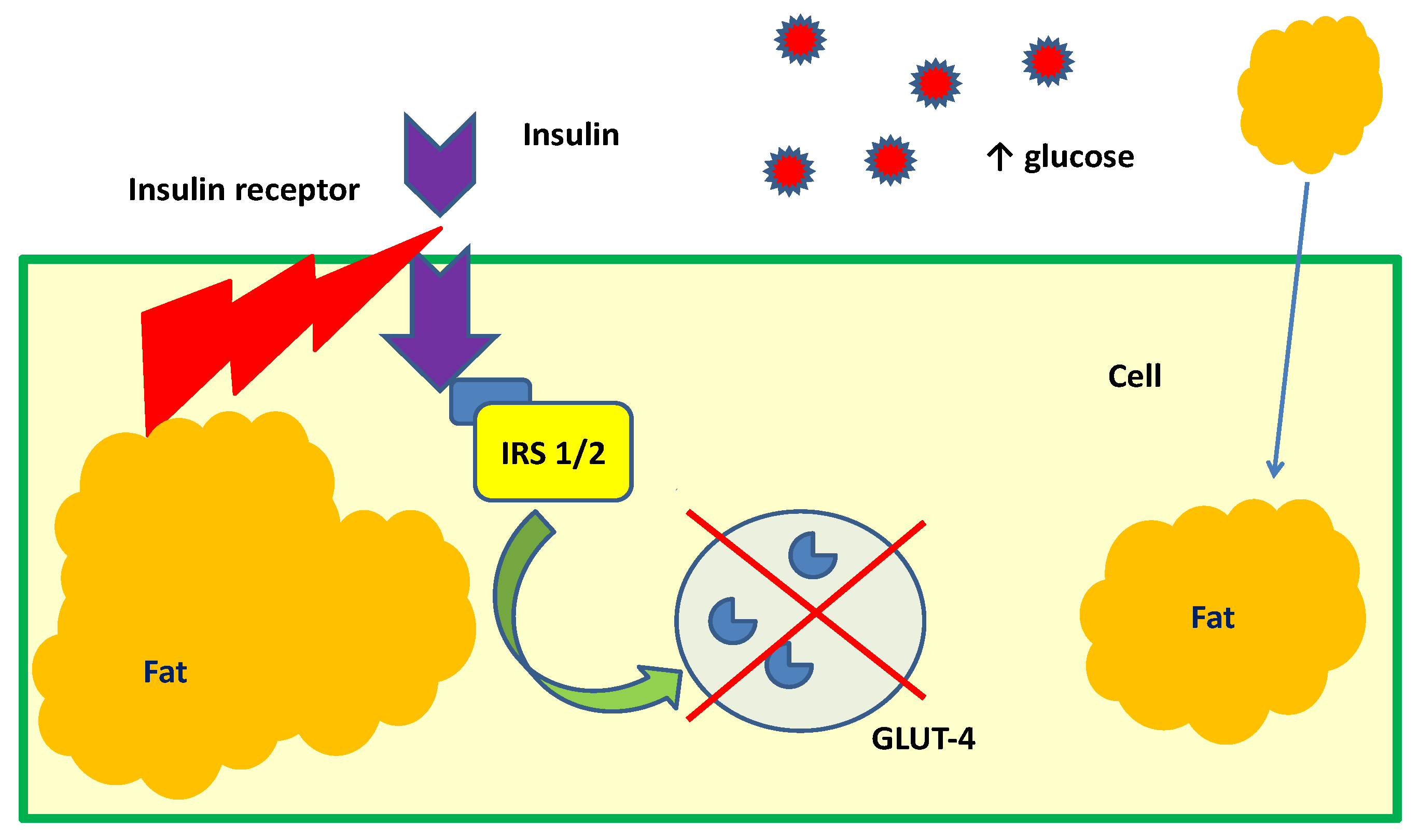
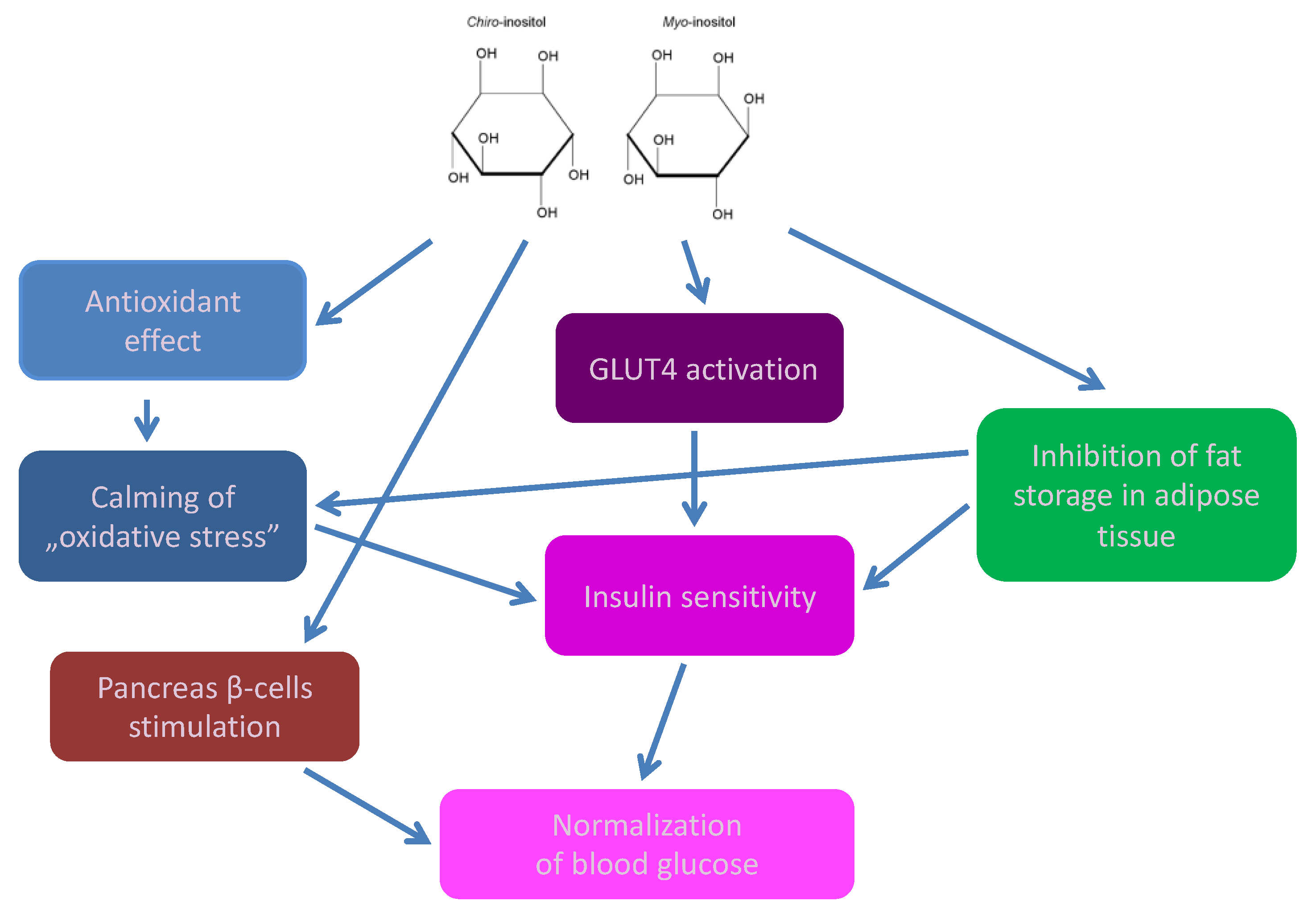
2. Cyclitols, Insulin Resistance, and Diabetes
- Abnormal, low DCI levels in urine, plasma, and tissues (liver, muscles, fat),
- Higher urinary excretion of MI
- MI deficiency in tissues insensitive to insulin (kidneys, sciatic nerve, lens, retina) [13].
3. Cyclitols, Polycystic ovary syndrome (PCOS), and Fertility
4. Cyclitols and Hypertension
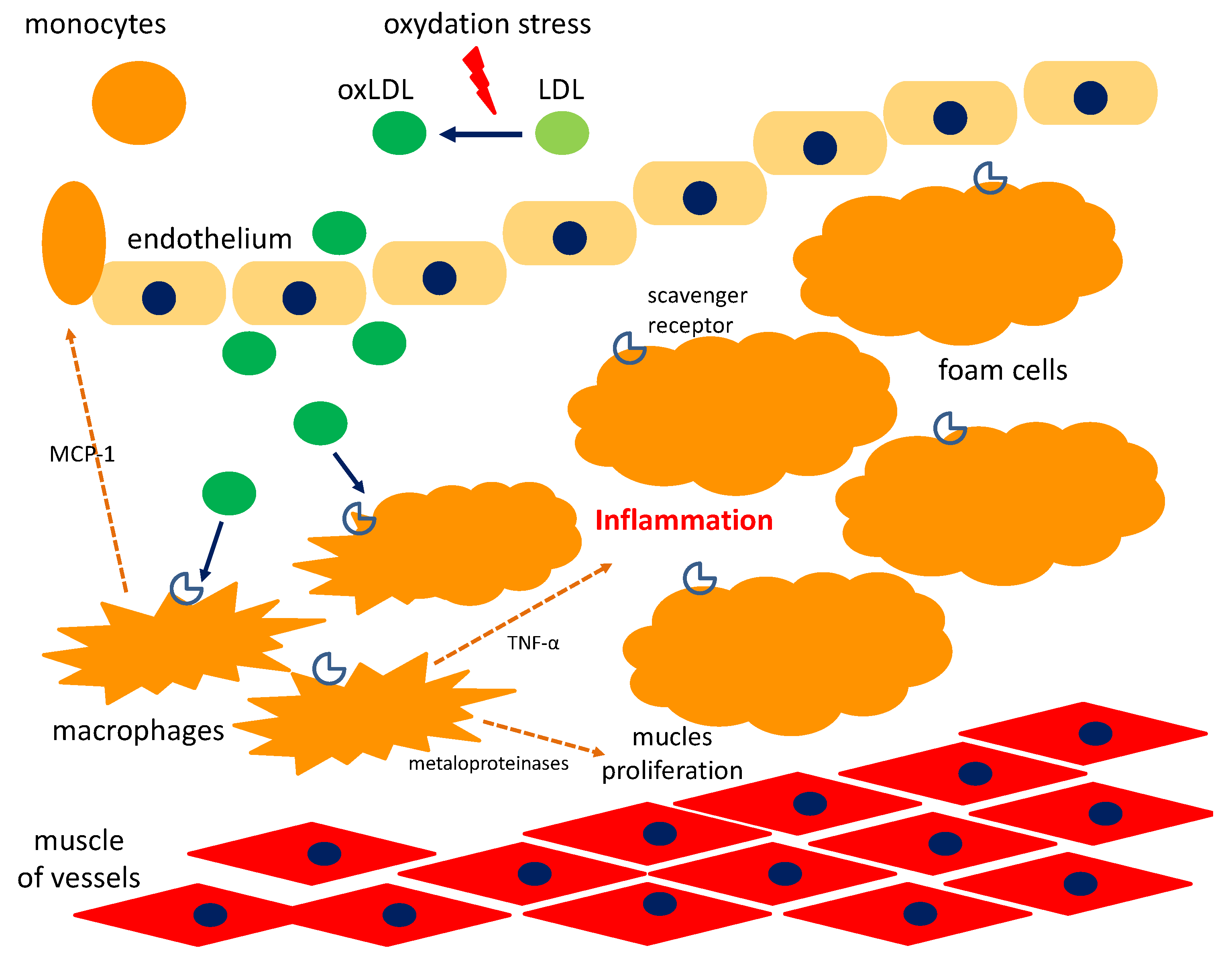
5. Cyclitols and Atherosclerosis
6. Cyclitols for Nervous System Diseases
7. Cyclitols and Carcinogenesis
8. Cyclitols and Allergic Diseases
9. Adverse Effect of Cyclitols
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Al-Suod, H.; Ligor, M.; Rațiu, I.-A.; Rafińska, K.; Górecki, R.; Buszewski, B. A window on cyclitols: Characterization and analytics of inositols. Phytochem. Lett. 2017, 20, 507–519. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy, P.P. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Lackey, K.H. Expression of 1L-Myoinositol-1-Phosphate synthase in organelles. Plant Physiol. 2003, 132, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Özturan, A.; Arslan, S.; Kocaadam, B.; Elibol, E.; İmamoğlu, İ.; Karadağ, M.G. Effect of inositol and its derivatives on diabetes: A systematic review. Crit. Rev. Food Sci. Nutr. 2017. [Google Scholar] [CrossRef]
- Campbell, J.A.; Goheen, S.C.; Donald, P. Extraction and analysis of inositols and other carbohydrates from soybean plant tissues. In Recent Trends for Enhancing the Diversity and Quality of Soybean Products; Krezhova, D., Ed.; In-Tech Open Access Publisher: Rijeka, Croatia, 2011; pp. 281–304. [Google Scholar] [CrossRef]
- Thomas, M.P.; Mills, S.J.; Potter, B.V.L. The “other” inositols and their phosphates: Synthesis, biology, and medicine (with recent advances in myo-inositol chemistry). Angew. Chem. Int. Ed. Engl. 2016, 55, 1614–1650. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Goswami, L.; Sangma, S.; Mukherjee, A.; Mukherjee, R.; Roy, N.; Basak, P.; Majumder, A.L. Manipulation of inositol metabolism for improved plant survival under stress: A “network engineering approach”. J. Plant Biochem. Biotechnol. 2012, 21, 15–23. [Google Scholar] [CrossRef]
- Sengupta, S.; Mukherjee, S.; Basak, P.; Majumder, A.L. Significance of galactinol and raffinose family oligosaccharide synthesis in plants. Front. Plant Sci. 2015, 6, 656. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Lahuta, L.B.; Placek, W.; Górecki, R.J. The potential benefits of plant cyclitols in the treatment of psoriasis. Pol. Ann. Med. 2018, 25, 166–171. [Google Scholar] [CrossRef]
- Ishitani, M.; Majumder, A.L.; Bornhouser, A.; Michalowski, C.B.; Jensen, R.G.; Bohnert, H.J. Coordinate transcriptional induction of myo-inositol metabolism during environmental stress. Plant J. 1996, 9, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Szkodziak, P.; Paszkowski, T. Statement of the Polish Gynecological Society on the application of myo-inozytol in patients with PCOS (polycystic ovary syndrome). Ginekol. Pol. 2014, 85, 158–160. [Google Scholar]
- Larner, J. D-Chiro-Inositol—Its functional role in insulin action and its deficit in insulin resistance. Int. J. Exp. Diab. Res. 2002, 3, 47–60. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Bray, D.; Hopkin, K.; Johnson, A.; Lewis, J.; Raff, M.; Rober, K. Chapter 16. Cell signaling. In Essential Cell Biology, 4th ed.; Alberts, B., Bray, D., Hopkin, K., Johnson, A., Lewis, J., Raff, M., Rober, K., Eds.; Garland Science: New York, NY, USA, 2013; pp. 525–555. ISBN 9780815344544. [Google Scholar]
- Bizzarri, M.; Carlomagno, G. Inositol: History of an effective therapy for Polycystic Ovary Syndrome. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1896–1903. [Google Scholar] [PubMed]
- Do, G.-M.; Choi, M.-S.; Kim, H.-J.; Woo, M.-N.; Lee, M.-K.; Jeon, S.-M. Soy pinitol acts partly as an insulin sensitizer or insulin mediator in 3T3-L1 preadipocytes. Genes Nutr. 2008, 2, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Subramanian, S. D-pinitol attenuates the impaired activities of hepatic key enzymes in carbohydrate metabolism of streptozotocin-induced diabetic rats. Gen. Physiol. Biophys. 2009, 28, 233–241. [Google Scholar] [CrossRef]
- Shen, H.; Shao, M.; Cho, K.W.; Wang, S.; Chen, Z.; Sheng, L.; Wang, T.; Liu, Y.; Rui, L. Herbal constituent sequoyitol improves hyperglycemia and glucose intolerance by targeting hepatocytes, adipocytes, and β-cells. Am. J. Physiol. Endocrinol. Metabol. 2012, 302, E932–E940. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Chung, S.K.; Miao, D.; Lau, K.S.; Chan, A.W.; Kung, A.W. Sodium/myo-inositol cotransporter 1 and myo-inositol are essential for osteogenesis and bone formation. J. Bone Miner. Res. 2011, 26, 582–590. [Google Scholar] [CrossRef]
- Liu, S.-C.; Chuang, S.-M.; Tang, C.-H. D-pinitol inhibits RANKL-induced osteoclastogenesis. Int. Immunopharmacol. 2012, 12, 494–500. [Google Scholar] [CrossRef]
- Nascimento, N.R.F.; Lessa, L.M.A.; Kerntopf, M.R.; Sousa, C.M.; Alves, R.S.; Queiroz, M.G.R.; Price, J.; Heimark, D.B.; Larner, J.; Du, X. Inositols prevent and reverse endothelial dysfunction in diabetic rat and rabbit vasculature metabolically and by scavenging superoxide. Proc. Natl. Acad. Sci. USA 2006, 103, 218–223. [Google Scholar] [CrossRef]
- Sivakumar, S.; Palsamy, P.; Subramanian, S.P. Impact of d-pinitol on the attenuation of proinflammatory cytokines, hyperglycemia-mediated oxidative stress and protection of kidney tissue ultrastructure in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2010, 188, 237–245. [Google Scholar] [CrossRef]
- Lin, T.-H.; Tan, T.-W.; Tsai, T.-H.; Chen, C.-C.; Hsieh, T.-F.; Lee, S.-S.; Liu, H.-H.; Chen, W.-C.; Tang, C.-H. D-pinitol inhibits prostate cancer metastasis through inhibition of αVβ3 integrin by modulating FAK, c-Src and NF-κB pathways. IJMS 2013, 14, 9790–9802. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, T.; Nandakumar, N.; Rajendran, P.; Ganesh, M.K.; Balasubramanian, M.P.; Nishigaki, I. D-pinitol mitigates tumor growth by modulating interleukins and hormones and induces apoptosis in rat breast carcinogenesis through inhibition of NF-κB. J. Physiol. Biochem. 2015, 71, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor- B activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Therap. 2018, 7, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.T.; Mukai, R.; Yoshida, K.; Ashida, H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef]
- Satoh, T. Molecular mechanisms for the regulation of insulin-stimulated glucose uptake by small guanosine triphosphatases in skeletal muscle and adipocytes. IJMS 2014, 15, 18677–18692. [Google Scholar] [CrossRef]
- Lin, X.; Ma, L.; Fitzgerald, R.L.; Ostlund, R.E. Human sodium/inositol cotransporter 2 (SMIT2) transports inositols but not glucose in L6 cells. Arch. Biochem. Biophys. 2009, 481, 197–201. [Google Scholar] [CrossRef]
- Saleem, F.; Rizvi, S.W. New therapeutic approaches in obesity and metabolic syndrome associated with polycystic ovary syndrome. Cureus 2017, 9, e1844. [Google Scholar] [CrossRef]
- Pascente, R.; Frigerio, F.; Rizzi, M.; Porcu, L.; Boido, M.; Davids, J.; Zaben, M.; Tolomeo, D.; Filibian, M.; Gray, W.P. Cognitive deficits and brain myo-Inositol are early biomarkers of epileptogenesis in a rat model of epilepsy. Neurobiol. Dis. 2016, 93, 146–155. [Google Scholar] [CrossRef]
- Fouka, P.; Alexopoulos, H.; Chatzi, I.; Dedos, S.G.; Samiotaki, M.; Panayotou, G.; Politis, P.; Tzioufas, A.; Dalakas, M.C. Antibodies to inositol 1,4,5-triphosphate receptor 1 in patients with cerebellar disease. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e306. [Google Scholar] [CrossRef]
- Paul, C.; Laganà, A.S.; Maniglio, P.; Triolo, O.; Brady, D.M. Inositol’s and other nutraceuticals’ synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: State-of-the-art and future perspectives. Gynecol. Endocrinol. 2016, 32, 431–438. [Google Scholar] [CrossRef]
- Greene, N.D.; Leung, K.-Y.; Copp, A.J. Inositol, neural tube closure and the prevention of neural tube defects. Birth Defects Res. 2017, 109, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Unfer, V.; Facchinetti, F.; Orrù, B.; Giordani, B.; Nestler, J. Myo-inositol effects in women with PCOS: A meta-analysis of randomized controlled trials. Endocr. Connect. 2017, 6, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Jones, R.B.; Bailey, C.J. Insulin-like effect of pinitol. Br. J. Pharmacol. 2000, 130, 1944–1948. [Google Scholar] [CrossRef]
- Hernández-Mijares, A.; Bañuls, C.; Peris, J.E.; Monzó, N.; Jover, A.; Bellod, L.; Victor, V.M.; Rocha, M. A single acute dose of pinitol from a naturally-occurring food ingredient decreases hyperglycaemia and circulating insulin levels in healthy subjects. Food Chem. 2013, 141, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Cubedo, J.; Padró, T.; Vilahur, G.; López-Bernal, S.; Rocha, M.; Hernández-Mijares, A.; Badimon, L. Effects of a carob-pod-derived sweetener on glucose metabolism. Nutrients 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Lazarenko, R.; Geisler, J.; Bayliss, D.; Larner, J.; Li, C. D-chiro-inositol glycan stimulates insulin secretion in pancreatic β cells. Mol. Cell. Endocrinol. 2014, 387, 1–7. [Google Scholar] [CrossRef]
- Baquer, N.Z.; Kumar, P.; Taha, A.; Kale, R.; Cowsik, S.; McLean, P. Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J. Biosci. 2011, 36, 383–396. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, J.I.; Yoon, S.Y.; Kim, J.C.; Cha, I.J. Pinitol from soybeans reduces postprandial blood glucose in patients with type 2 diabetes mellitus. J. Med. Food. 2006, 9, 182–186. [Google Scholar] [CrossRef]
- Kim, J.-I.; Kim, J.C.; Kang, M.-J.; Lee, M.-S.; Kim, J.-J.; Cha, I.-J. Effects of pinitol isolated from soybeans on glycaemic control and cardiovascular risk factors in Korean patients with type II diabetes mellitus: A randomized controlled study. Eur. J. Clin. Nutr. 2005, 59, 456–458. [Google Scholar] [CrossRef]
- Müller, A.C.; Patnala, S.; Kis, O.; Bendayan, R.; Kanfer, I. Interactions between phytochemical components of Sutherlandia Frutescens and the antiretroviral, atazanavir in vitro: Implications for absorption and metabolism. J. Pharm. Pharm. Sci. 2018, 15, 221. [Google Scholar] [CrossRef]
- Shashkin, P.N.; Huang, L.C.; Larner, J.; Vandenhoff, G.E.; Katz, A. Fasting decreases the content of D-chiroinositol in human skeletal muscle. Int. J. Exp. Diabetes Res. 2002, 3, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Cheang, K.I.; Baillargeon, J.-P.; Essah, P.A.; Ostlund, R.E.; Apridonize, T.; Islam, L.; Nestler, J.E. Insulin-stimulated release of D-chiro-inositol–containing inositolphosphoglycan mediator correlates with insulin sensitivity in women with polycystic ovary syndrome. Metabolism 2008, 57, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, S.; Marinaro, F.; Tondelli, D.; Lui, J.; Xella, S.; Marsella, T.; Tagliasacchi, D.; Argento, C.; Tirelli, A.; Giulini, S. Modulation of gonadotrophin induced steroidogenic enzymes in granulosa cells by d-chiroinositol. Reprod. Biol. Endocrinol. 2016, 14, 52. [Google Scholar] [CrossRef]
- Shan, W.F.; Chen, B.Q.; Zhu, S.J.; Jiang, L.; Zhou, Y.F. Effects of GLUT4 expression on insulin resistance in patients with advanced liver cirrhosis. J. Zhejiang Univ. Sci. B 2011, 12, 677–682. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar] [PubMed]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- Altaf, Q.-A.; Barnett, A.H.; Tahrani, A.A. Novel therapeutics for type 2 diabetes: Insulin resistance. Diabetes Obes. Metab. 2015, 17, 319–334. [Google Scholar] [CrossRef]
- Pereira, A.C.; Pereira, A.B.D.; Moreira, C.C.; Botion, L.M.; Lemos, V.S.; Braga, F.C.; Cortes, S.F. Hancornia speciosa Gomes (Apocynaceae) as a potential anti-diabetic drug. J. Ethnopharmacol. 2015, 161, 30–35. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska, J.; Ciak, M.; Górecki, R.J. The occurrence and accumulation of D-pinitol in fenugreek (Trigonella foenum graecum L.). Acta Physiol. Plant. 2018, 40, 155. [Google Scholar] [CrossRef]
- Kawa, J.M.; Taylor, C.G.; Przybylski, R. Buckwheat concentrate reduces serum glucose in streptozotocin-diabetic rats. J. Agric. Food Chem. 2003, 51, 7287–7291. [Google Scholar] [CrossRef] [PubMed]
- Fonteles, M.; Almeida, M.; Larner, J. Antihyperglycemic effects of 3-o-methyl-d-chiro-inositol and d-chiro-inositol associated with manganese in streptozotocin diabetic rats. Horm. Metab. Res. 2000, 32, 129–132. [Google Scholar] [CrossRef]
- Pintaudi, B.; Di Vieste, G.; Bonomo, M. The effectiveness of myo-inositol and D-chiro inositol treatment in type 2 diabetes. Int. J. Endocrinol. 2016, 2016, 9132052. [Google Scholar] [CrossRef] [PubMed]
- Kingery, S.E.; Wu, Y.L.; Zhou, B.; Hoffman, R.P.; Yu, C.Y. Gene CNVs and protein levels of complement C4A and C4B as novel biomarkers for partial disease remissions in new-onset type 1 diabetes patients. Pediatr. Diabetes 2012, 13, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Capalbo, D.; Marcovecchio, M.L.; Sleigh, A.; Jørgensen, S.W.; Hill, N.R.; Mooslehner, K.; Yeo, G.S.H.; Bluck, L.; Juul, A. Low circulating levels of IGF-1 in healthy adults are associated with reduced β-cell function, increased intramyocellular lipid, and enhanced fat utilization during fasting. J. Clin. Endocrinol. Metabol. 2014, 99, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Domené, H.M.; Hwa, V.; Jasper, H.G.; Rosenfeld, R.G. Acid-labile subunit (ALS) deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Malvasi, A.; Kosmas, I.; Mynbaev, O.; Sparic, R.; Gustapane, S.; Guido, M.; Tinelli, A. Can trans resveratrol plus D-chiro-inositol and myo-inositol improve maternal metabolic profile in overweight pregnant patients? Clin. Ter. 2017, 168, e240–e247. [Google Scholar] [CrossRef] [PubMed]
- Dell’Edera, D.; Sarlo, F.; Allegretti, A.; Simone, F.; Lupo, M.G.; Epifania, A.A. The influence of D-chiro-inositol and D-myo-inositol in pregnant women with glucose intolerance. Biomed. Rep. 2017, 7, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Christiansen, M.; Horowitz, J.F.; Klein, S.; Hellerstein, M.K.; Ostlund, R.E. Effect of pinitol treatment on insulin action in subjects with insulin resistance. Diabetes Care 2000, 23, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. Myo-inositol and d-chiro inositol in improving insulin resistance in obese male children: Preliminary data. Int. J. Endocrinol. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Aja, S.; Ronnett, G.V.; Kim, E.-K. D-chiro-inositol glycan reduces food intake by regulating hypothalamic neuropeptide expression via AKT-FoxO1 pathway. Biochem. Biophys. Res. Commun. 2016, 470, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; Reamer, P.; Gunn, R.D.; Allan, G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. N. Engl. J. Med. 1999, 340, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.; Reilly, E.; Cheang, K.; Bachmann, L.; Downs, R., Jr. A pilot study: Effects of decreasing serum insulin with diazoxide on vitamin D levels in obese women with polycystic ovary syndrome. Trans. Am. Clin. Climatol. Assoc. 2012, 123, 209–220. [Google Scholar] [PubMed]
- Gerli, S.; Mignosa, M.; Di Renzo, G. Effects of inositol on ovarian function and metabolic factors in women with PCOS: A randomized double blind placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2003, 6, 151–159. [Google Scholar]
- Genazzani, A.D.; Santagni, S.; Rattighieri, E.; Chierchia, E.; Despini, G.; Marini, G.; Prati, A.; Simoncini, T. Modulatory role of D-chiro-inositol (DCI) on LH and insulin secretion in obese PCOS patients. Gynecol. Endocrinol. 2014, 30, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Focarelli, R.; Capaldo, A.; Stendardi, A.; Cappelli, V.; Cianci, A.; La Marca, A.; Luddi, A.; De Leo, V. Protein modification as oxidative stress marker in follicular fluid from women with polycystic ovary syndrome: The effect of inositol and metformin. J. Assist. Reprod. Genet. 2014, 31, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.Y.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharmacol. 2014, 54, 1079–1092. [Google Scholar] [CrossRef]
- Carlomagno, G.; Unfer, V.; Roseff, S. The D-chiro-inositol paradox in the ovary. Fertil. Steril. 2011, 95, 2515–2516. [Google Scholar] [CrossRef]
- Simi, G.; Genazzani, A.R.; Obino, M.E.R.; Papini, F.; Pinelli, S.; Cela, F.; Artini, P.G. Inositol and in vitro fertilization with embryo transfer. Int. J. Endocrinol. 2017, 2017, 5469409. [Google Scholar] [CrossRef]
- Ciotta, L.; Stracquadanio, M.; Pagano, I.; Carbonaro, A.; Palumbo, M.; Gulino, F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: A double blind trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 509–514. [Google Scholar]
- Benelli, E.; Del Ghianda, S.; Di Cosmo, C.; Tonacchera, M. A Combined therapy with myo-inositol and d-chiro-inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int. J. Endocrinol. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Ravanos, K.; Monastra, G.; Pavlidou, T.; Goudakou, M.; Prapas, N. Can high levels of D-chiro-inositol in follicular fluid exertdetrimental effects on blastocyst quality? Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Sortino, M.A.; Salomone, S.; Carruba, M.O.; Drago, F. Polycystic ovary syndrome: Insights into the therapeutic approach with inositols. Front. Pharmacol. 2018, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.; La Vignera, S.; Mongioi, L.; Vitale, S.; Lagana, A.; Cimino, L.; Calogero, A. Myo-inositol as a male fertility molecule: Speed them up! Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. 2), 30–35. [Google Scholar] [PubMed]
- Schulz, E.; Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.C.; Braga, F.C.; Lemos, V.S.; Cortes, S.F. Potent antihypertensive effect of Hancornia speciosa leaves extract. Phytomedicine 2016, 23, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Braga, F.; Lima, M.; Pesquero, J.; Lemos, V.; Cortes, S. Hancornia speciosa Gomes induces hypotensive effect through inhibition of ACE and increase on NO. J. Ethnopharmacol. 2011, 137, 709–713. [Google Scholar] [CrossRef]
- Ferreira, H.C.; Serra, C.P.; Lemos, V.S.; Braga, F.C.; Cortes, S.F. Nitric oxide-dependent vasodilatation by ethanolic extract of Hancornia speciosa via phosphatidyl-inositol 3-kinase. J. Ethnopharmacol. 2007, 109, 161–164. [Google Scholar] [CrossRef]
- Choi, M.-S.; Lee, M.-K.; Jung, U.J.; Kim, H.-J.; Do, G.-M.; Park, Y.B.; Jeon, S.-M. Metabolic response of soy pinitol on lipid-lowering, antioxidant and hepatoprotective action in hamsters fed-high fat and high cholesterol diet. Mol. Nutr. Food Res. 2009, 53, 751–759. [Google Scholar] [CrossRef]
- Choi, M.-S.; Lee, W.-H.; Kwon, E.-Y.; Kang, M.A.; Lee, M.-K.; Park, Y.B.; Jeon, S.-M. Effects of soy pinitol on the pro-inflammatory cytokines and scavenger receptors in oxidized low-density lipoprotein-treated THP-1 macrophages. J. Med. Food 2007, 10, 594–601. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2015, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Witztum, J. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J. Neurochem. 2002, 82, 736–754. [Google Scholar] [CrossRef] [PubMed]
- Frej, A.D.; Otto, G.P.; Williams, R.S. Tipping the scales: Lessons from simple model systems on inositol imbalance in neurological disorders. Eur. J. Cell Biol. 2017, 96, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Silverstone, P.H.; McGrath, B.M.; Kim, H. Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jevtic, S.; Markham-Coultes, K.; Ellens, N.P.; O’Reilly, M.A.; Hynynen, K.; Aubert, I.; McLaurin, J. Investigating the efficacy of a combination Aβ-targeted treatment in a mouse model of Alzheimer’s disease. Brain Res. 2018, 1678, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alokail, M.S.; Alkharfy, K.M.; Mohammed, A.K.; Abd-Alrahman, S.H.; Yakout, S.M.; Amer, O.E.; Krishnaswamy, S. Fenugreek extract as an inducer of cellular death via autophagy in human T lymphoma Jurkat cells. BMC Complement. Altern. Med. 2012, 12, 202. [Google Scholar] [CrossRef]
- Alsemari, A.; Alkhodairy, F.; Aldakan, A.; Al-Mohanna, M.; Bahoush, E.; Shinwari, Z.; Alaiya, A. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum. BMC Complement. Altern. Med. 2014, 14, 114. [Google Scholar] [CrossRef]
- Al-Ghamdi, H.; Sabbah, R.; Martin, J.; Patay, Z. Primary T-cell lymphoma of the brain in children: A case report and literature review. Pediatr. Hematol. Oncol. 2000, 17, 341–343. [Google Scholar] [CrossRef]
- Raju, J.; Paltolla, J.M.; Swamy, M.V.; Rao, C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1392–1398. [Google Scholar]
- Shabbeer, S.; Sobolewski, M.; Anchoori, R.K.; Kachhap, S.; Hidalgo, M.; Jimeno, A.; Davidson, N.E.; Carducci, M.; Khan, S.R. Fenugreek: A naturally occurring edible spice as an anticancer agent. Cancer Biol. Ther. 2009, 8, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.-J.; Shin, H.S.; Choi, D.-W.; Shon, D.-H. Antiallergic effect of Trigonella foenum-graecum L. extracts on allergic skin inflammation induced by trimellitic anhydride in BALB/c mice. J. Ethnopharmacol. 2012, 144, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Gupta, K.K.; Bani, S. The immunosuppressive effects of Agyrolobium roseum and pinitol in experimental animals. Int. Immunopharmacol. 2011, 11, 286–291. [Google Scholar] [CrossRef] [PubMed]
| NO. | Authors | Cyclitol/Source | Group | Effect |
|---|---|---|---|---|
| 1 | Pereira et al., 2014 [26] | Bornesitol/ (Hancornia speciosa) | epididymal adipocytes of Swiss mice | —Inhibition of alpha-glucosidase activity —Increase in glucose uptake in adipocytes |
| 2 | Sivakumar & Subramania, 2009 [12] | d-pinitol (chemical pure) | Rats with STZ, d-pinitol 50 mg/kg body weight, 30 days | —Decreasing of blood glucose, HgbA1, increasing of insulin levels and BMI —Increasing of hepatic gluconeogenesis enzymes activity and decreasing of glycogenolytic enzymes activity |
| 3 | Bates et al., 2000 [27] | d-pinitol/ (Bougainvillea spectabilis) | Obese mouse (ob/ob) with STZ, intraperitoneal and oral d-pinitol 100 mg/kg body weight | —Decreasing of blood glucose, without changes in insulin level —Increasing of glucose uptake by muscle cells |
| 4 | Dang et al., 2010 [28] | d-pinitol and myo-inositol | DP or MI 1 g/kg body weight orally in mice for 30 min before oral administration of glucose (2 g/kg of body weight) | —Increasing of GLUT4 translocation in skeletal muscle —Reduction of plasma glucose and insulin levels |
| 5 | Gao et al., 2015 [29] | d-pinitol | Insulin resistance induced by diet and STZ in Sprague Dawley rats, d-pinitol in two doses (30 and 60 mg/kg body weight) | —Decreasing of fasting blood glucose by 12.63% in the high-dose group —Improvement of oral glucose tolerance |
| 6 | Geethan et al., 2008 [30] | d-pinitol | Sprague Dawley rats (STZ)—DP 25, 50 and 100 mg/kg body weight | —Decreasing of Low-density lipoprotein (LDL) and Very-low-density lipoprotein (VLDL), a significant High-density lipoproteins (HDL) increasing in serum |
| 7 | Hannan et al., 2006 [31] | fraction of T. foenum-graecum | STZ rats, a soluble dietary fibre fraction of T. foenum-graecum (0.5 g/kg body weight) | —Decreasing of glucose in serum, increasing of glycogen content in the liver —Increasing of total antioxidant status —No effect on insulin secretion |
| 8 | Kawa et al., 2003 [32] | d-chiro-inositol/ chemically synthesized | STZ rats fed with buckwheat concentrate (10 and DCI 20 mg/kg body weight) | —Reduction of glucose by 12–19% after 90 and 120 min in the serum after administration of the concentrate |
| No. | Authors | Cyclitol/Source | Group | Effect |
|---|---|---|---|---|
| 1 | Davis et al., 2000 [33] | soybean-derived DP—20 mg/kg/day | 22 obese subjects (BMI 36.6) with diet-treated T2DM or glucose intolerance (HbA1 6.8%): 12 receive either DP or 10 placebo in a 28-day double-blinded trial | —No toxicity of DP was observed —Plasma levels of DP were 48-fold and DCI levels 14-fold greater in DP group compared with placebo |
| 2 | Kim et al., 2004 [34] | 0.6 g soybean-derived DP | 30 patients with T2DM received an oral dose of DP or placebo twice daily for 13 weeks | —DP significantly decreased fasting plasma glucose, insulin, fructosamine, HbA1c and HOMA-IR —DP significantly decreased total cholesterol, LDL, the LDL/HDL ratio, and systolic and diastolic blood pressure and increased HDL |
| 3 | Kang et al., 2006 [35] | 0.6 g DP from soybean | 15 subjects with tT2DM ingested cooked white rice containing 50 g of available carbohydrate with or without prior ingestion of DP (1 g dose at 0, 60, 120, or 180 min or as a 0.6 g dose at 60 min prior to rice ingestion) | —1.2 g of DP 60 min prior to rice consumption controlled blood glucose most effectively, significantly diminishing the postprandial increase in plasma glucose levels at 90 and 120 min after rice consumption —DP had no apparent effect on postprandial insulin levels |
| 4 | Hernández-Mijares et al., 2013 [36] | a nutritive beverage (Fruit Up®) containing 2.5, 4.0, or 6.0 g of DP | 31 healthy volunteers, BMI 20–30, fasting glycaemia <100 mg/dL; serum glucose and insulin levels were determined at 0, 15, 30, 45, 60, 90, and 120 min for each dose of DP | —Good tolerated of DP and no adverse events (including hypoglycaemic episodes) for any doses administered —Only a dose 6 g of DP reduced glucose and insulin levels at 45 and 60 min |
| 5 | Mancini et al., 2016 [37] | MI 1100 mg, DCI 27.6 m, folic acid 400 µg (Inofolic Combi, Lo.Li. Pharma, Rome, Italy) | 23 obese children, aged 7–15, with a mean BMI 29.8, 11 treated with normocaloric diet, physical activity and Inofolic and 12 were controls | —MI/DCI lowered more effective insulin increase after OGGT in children with higher basal insulin level —BMI after six months of supplementation was not significantly different from the baseline which emphasize only inositol role in glucose profile improvement |
| 6 | Pintaudi et al., 2016 [38] | MI 550 mg, DCI 13.8 mg, folic acid 400 µg (Inofolic Combi, Lo.Li. Pharma, Rome, Italy) | 20 subjects a combination of MI + DCI was suggested to be taken orally twice a day as add-on supplement to hypoglycemic treatment | —After 3 months of treatment fasting blood glucose and HbA1c levels significantly decreased compared to baseline —There was no significant difference in blood pressure, lipid profile, and BMI levels —None of the participants reported side effects |
| 7 | Lambert et al., 2017 [39] | a sweetened beverage with natural carbohydrates containing DP (PEB) compared to a sucrose-enriched beverage (SEB) | 40 healthy volunteers and 40 overweight volunteers with impaired glucose tolerance (IGT) and 38 T2DM patients, 6 weeks intake PEB or SEB | —A significant increase in two proteins involved in the insulin secretion pathway: IGF acid labile subunit and complement C4A in IGT subjects but not in healthy volunteers —PEB induces changes in the insulin secretion pathway that could help to reduce blood glucose levels by protecting β-cells and by stimulating the insulin secretion pathway |
| 8 | Malvasi et al., 2017 [40] | MI/DCI + Trans-resveratrol (Revifast®) | 104 pregnant (gestational age between the 24th and 28th week) were treated: 35 group I Revifast® + DCI/MI, 34 group II DCI/MI alone and placebo group-35 | —After 30 and 60 days of therapy no difference in systolic and diastolic parameters among 3 groups during study —All blood chemistry parameters improved compared to placebo at 30 days already, but significantly to 60 days —Group I demonstrates significantly improved lipid and glucose parameters than group II, which are at 30 to 60 days of treatment —None of the women reported an adverse reaction to therapy |
| 9 | Dell’ Edera et al., 2017 [41] | DCI 250 mg/d, MI 1.75 g/d, 12.5 mg/d zinc, 10 mg/d methylsulfonylmethane, 400 μg/d 5-methyltetrahydrofolic acid | 40 pregnant with the onset of GDM were treated DCI/MI, from the first trimester of pregnancy, with and 43 controls with only 400 μg/d folic acid | —At the 24th week of pregnancy, the incidence of maternal GDM was lower in the treated group (RR 3.35) —A significant difference was observed between treated and control groups in terms of risk of macrosomia —No significant difference was identified between two groups, regarding neonatal hypoglycemia and preterm delivery |
| Cyclitol | Application in Medicine |
|---|---|
| d-pinitol | —Reduction of hyperglycaemia and plasma glucose level —Improvement of lipid profile —Inhibition the formation of foam cells and oxLDL —Inhibition of inflammation and carcinogen-induced activation of NF-κB, which leads to reduction of proliferation, invasion, and angiogenesis in tumors —Stimulation of apoptosis and autophagy of lymphoma cells —Reduction of osteoclastogenesis by inhibiting NF-κB ligand receptor activator (RANKL) —In rats, protection against chemically induced liver damage |
| myo-inositol | —Treatment of type 2 diabetes and insulin resistance (induce translocation GLUT4 into the cell membrane, thereby increasing cellular uptake of glucose) —Stimulation of menstruation and ovulation in polycystic ovary syndrome (PCOS), improvement of oocyte and blastocyst quality —Improvement of osteogenesis —Disturbance of transformation in neurodegenerative diseases (increase in brain concentration) |
| d-chiro-inositol | —Treatment of type 2 diabetes and insulin resistance (participates in insulin signaling, stimulating enzymes involved in the regulation of glucose metabolism) —Improvement of lipid and carbohydrate profile in pregnant women, prophylaxis of gestational diabetes —Appetite regulation—Influence on hypothalamic insulin signaling —Treatment of insulin resistance, hyperandrogenism and infertility in PCOS —Inhibition of osteoclasts, improvement of osteogenesis |
| scyllo-inositol | —Reduction of the content of amyloid A (helps prevent the formation of insoluble amyloid fibrils) and increase the activity of microglia and the ability of phagocytosis in the brain —In rats, reduction of epileptic seizures induced by pentylenetetrazole |
| bornesitol | —Lowering of blood pressure, dilation of arteries vessels depending on the dose |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarczyk-Saczonek, A.; Lahuta, L.B.; Ligor, M.; Placek, W.; Górecki, R.J.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients 2018, 10, 1891. https://doi.org/10.3390/nu10121891
Owczarczyk-Saczonek A, Lahuta LB, Ligor M, Placek W, Górecki RJ, Buszewski B. The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients. 2018; 10(12):1891. https://doi.org/10.3390/nu10121891
Chicago/Turabian StyleOwczarczyk-Saczonek, Agnieszka, Lesław Bernard Lahuta, Magdalena Ligor, Waldemar Placek, Ryszard Józef Górecki, and Bogusław Buszewski. 2018. "The Healing-Promoting Properties of Selected Cyclitols—A Review" Nutrients 10, no. 12: 1891. https://doi.org/10.3390/nu10121891
APA StyleOwczarczyk-Saczonek, A., Lahuta, L. B., Ligor, M., Placek, W., Górecki, R. J., & Buszewski, B. (2018). The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients, 10(12), 1891. https://doi.org/10.3390/nu10121891








