Vitamin D Supplementation Modestly Reduces Serum Iron Indices of Healthy Arab Adolescents
Abstract
1. Introduction
2. Materials and Methods
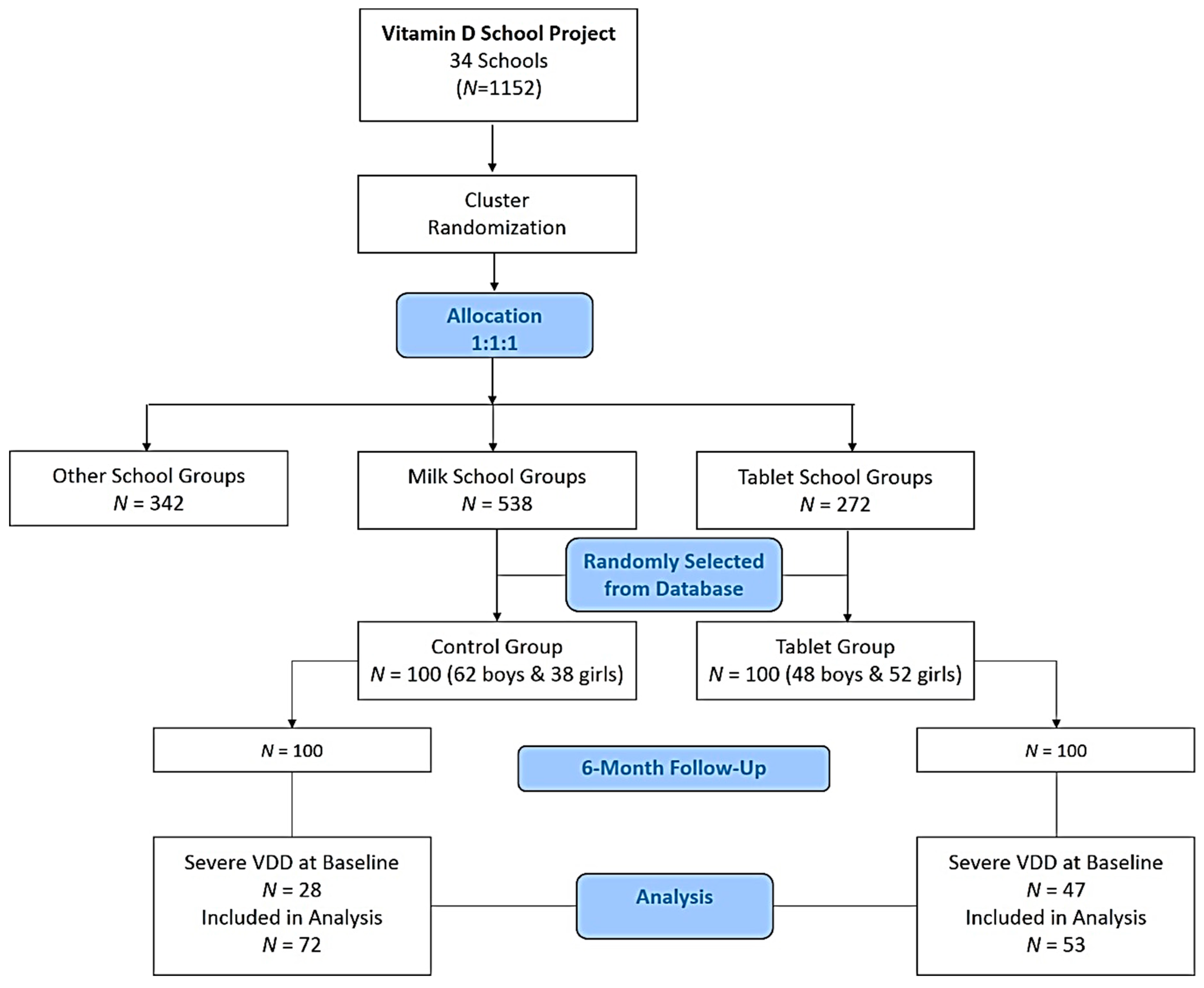
2.1. Study Design and Participants
2.2. Anthropometric and Biochemical Assessment
2.3. Vitamin D and Iron Indices
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, G.; Gannage-Yared, M.H.; Ezzedine, J.; Larijani, B.; Badawi, S.; Rached, A.; Zakroui, L.; Masri, B.; Azar, E.; Saba, E.; et al. Middle East and North Africa consensus on osteoporosis. J. Musculoskelet. Neuronal Interact. 2007, 7, 131–143. [Google Scholar]
- Chakhtoura, M.; Rahme, M.; Chamoun, M.; El-Hajj Fuleihan, G. Vitamin D in the Middle East and North Africa. Bone Rep. 2018, 8, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bassil, D.; Rahme, M.; Hoteit, M.; Fuleihan, G.E. Hypovitaminosis D in the Middle East and North Africa: Prevalence, risk factors and impact on outcomes. Dermatoendocrinol 2013, 5, 274–298. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Lac, P.T.; Liu, I.L.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D deficiency and anemia: A cross-sectional study. Ann. Hematol. 2010, 89, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Iron and Iron Deficiency. Nutrition for Everyone. 2012. Available online: http://www.cdc.gov/nutrition/everyone/basics/vitamins/iron.html (accessed on 8 November 2018).
- Assessing the Iron Status of Populations. Available online: http://www.who.int/nutrition/publications/micronutrients/anaemia_iron_deficiency/9789241596107/en (accessed on 8 November 2018).
- Global Target 2025. Available online: http://www.who.int/nutrition/global-target-2025/en/ (accessed on 8 November 2018).
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The prevalence of micronutrient deficiencies and inadequacies in the Middle-East and approaches to interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Lee, J.E.; Lee, D.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Nah, J.W.; Kim, S.K. Prevalence and Relationships of Iron Deficiency Anemia with Blood Cadmium and Vitamin D Levels in Korean Women. J. Korean Med. Sci. 2016, 31, 25–32. [Google Scholar]
- Azizi-Soleiman, F.; Vafa, M.; Abiri, M.; Safavi, M. Effects of iron on vitamin D metabolism: A systematic review. Int. J. Prev. Med. 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Saleh, Y.; Aljohani, N.; Sulimani, R.; Al-Othman, A.M.; Alfawaz, H.; Fouda, M.; Al-Amri, F.; Shahrani, A.; Alharbi, M.; et al. Vitamin D status correction in Saudi Arabia: An experts’ consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Arch. Osteoporos. 2017, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Ansari, M.G.A.; Sabico, S.; Al-Saleh, Y.; Aljohani, N.J.; Alfawaz, H.; Alharbi, M.; Alokail, M.S.; Wimalawansa, S.J. Efficacy of different modes of vitamin D supplementation strategies in Saudi adolescents. J. Steroid Biochem. Mol. Biol. 2018, 180, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Abd-Alrahman, S.H.; Panigrahy, A.; Al-Saleh, Y.; Aljohani, N.; Al-Attas, O.S.; Khattak, M.N.K.; Alokail, M. Efficacy of vitamin D interventional strategies in Saudi children and adults. J. Steroid Biochem. Mol. Biol. 2018, 180, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.M.; Almalki, M.H.; Aljohani, N.; Alzahrani, A.; Alsaleh, Y.; Holick, M.F. Vitamin D: Light side and best time of sunshine in Riyadh, Saudi Arabia. Dermatoendocrinol 2013, 5, 177–180. [Google Scholar] [CrossRef]
- Torgerson, D.J. Contamination in trials: Is cluster randomization the answer? BMJ 2001, 322, 355–357. [Google Scholar] [CrossRef]
- Doudin, A.; Becker, A.; Rothenberge, A.; Meyer, T. Relationship between serum 25-hydroxyvitamin D and red blood cell indices in German adolescents. Eur. J. Pediatr. 2018, 177, 583–591. [Google Scholar] [CrossRef]
- Madar, A.M.; Stene, L.C.; Meyer, H.E.; Brekke, M.; Lagerløv, P.; Knutsen, K.V. Effect of vitamin D3 supplementation on iron status: A randomized, double-blind, placebo-controlled trial among ethnic minorities living in Norway. Nutr. J. 2016, 15, 74. [Google Scholar] [CrossRef]
- Sooragonda, B.; Bhadada, S.K.; Shah, V.N.; Malhotra, P.; Ahluwalia, J.; Sachdeva, N. Effect of vitamin D replacement on hemoglobin concentration in subjects with concurrent iron-deficiency anemia and vitamin D deficiency: A randomized, single-blinded, placebo-controlled trial. Acta Haematol. 2015, 133, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-dose vitamin D3 reduces circulating hepcidin concentrations: A pilot, randomized, double-blind, placebo-controlled trial in healthy adults. Clin. Nutr. 2017, 36, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, M.; Kaczmarczyk, M.; Suarez, A.D.; Sanchez, G.F.L.; Jastrzebska, J.; Radziminski, L.; Jastrzebski, Z. Iron, hematological parameters and blood plasma lipid profile in vitamin D supplemented and non-supplemented young soccer players subjected to a high-intensity interval training. J. Nutr. Sci. Vitaminol. 2017, 63, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Jungvogel, A.; Prokop, A.; Kuhn, J.; Dreier, J.; Fuchs, U.; Schulz, U.; Gummert, J.F.; Borgermann, J. Vitamin D deficiency is an independent predictor of anemia in end-stage heart failure. Clin. Res. Cardiol. 2011, 100, 781–788. [Google Scholar] [CrossRef]
- Ernst, J.B.; Zittermann, A.; Pilz, S.; Kleber, M.E.; Scharnagl, H.; Brandenburg, V.M.; Konig, W.; Grammer, T.B.; Marz, W. Independent associations of vitamin D metabolites with anemia in patients referred to coronary angiography. Eur. J. Nutr. 2017, 56, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill-health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Perez-Granados, A.M.; Toxqui, L.; Zazo, P.; de la Piedra, C.; Vaquero, M.P. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur. J. Nutr. 2013, 52, 695–703. [Google Scholar] [CrossRef]
- Ma, Y.; Johnson, C.S.; Trump, D.L. Mechanistic insights of vitamin D anticancer effects. Vitam. Horm. 2016, 100, 395–491. [Google Scholar]
- Lonnerdal, B. Calcium and iron absorption—Mechanisms and public health relevance. Int. J. Vitam. Nutr. Res. 2010, 80, 293–299. [Google Scholar] [CrossRef]
- Grinder-Pedersen, L.; Bukhave, K.; Jensen, M.; Hojgaard, L.; Hansen, M. Calcium and milk or calcium fortified foods does not inhibit nonheme-iron absorption from a whole diet consumed over a 4-d period. Am. J. Clin. Nutr. 2004, 80, 404–409. [Google Scholar] [CrossRef]
- Maguire, J.L.; Lebovic, G.; Kandasamy, S.; Khovratovich, M.; Mamdani, M.; Birken, C.S.; Parkin, P.C. The relationship between cow’s milk and stores of vitamin D and iron in early childhood. Pediatrics 2013, 13, e144–e151. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Ali, M.; Al Elq, A.; Al-Farhan, M.; Sadat, N.A. Fortification with vitamin D: Comparative study in the Saudi Arabian and US markets. J. Family Community Med. 2013, 20, 49–52. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Saleh, Y.; Khan, N.; Sabico, S.; Aljohani, N.; Alfawaz, H.; Alsulaimani, M.; Al-Othman, A.M.; Alokail, M.S. Sun exposure, skin color and vitamin D status in Arab children and adolescents. J. Steroid Biochem. Mol. Biol. 2016, 164, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Atkinson, S.H.; Armitage, A.E.; Khandwala, S.; Veenemans, J.; Cox, S.E.; Eddowes, L.A.; Hayes, T.; Doherty, C.P.; Demir, A.Y.; et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci. Transl. Med. 2014, 6, 235re3. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the diagnosis of iron disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Altemose, K.E.; Kumar, J.; Portale, A.A.; Warady, B.A.; Furth, S.L.; Fadrowski, J.J.; Atkinson, M.A. Vitamin D insufficiency, hemoglobin, and anemia in children with chronic kidney disease. Pediatr. Nephrol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Icardi, A.; Paoletti, E.; De Nicola, L.; Mazzaferro, S.; Russo, R.; Cozzolino, M. Renal anaemia and EPO hyporesponsiveness is associated with vitamin D deficiency: The potential role of inflammation. Nephrol. Dial. Transplant. 2013, 28, 1672–1679. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Melamed, M.L.; Kumar, J.; Roy, C.N.; Miller, E.R., 3rd; Furth, S.L.; Fadrowski, J.J. Vitamin D, race and risk for anemia in children. J. Pediatr. 2014, 164, 153–158. [Google Scholar] [CrossRef]
- Monlezun, D.J.; Camargo, C.A., Jr.; Mullen, J.T.; Quraishi, S.A. Vitamin D status and the risk of anemia in community-dwelling adults: Results from the national health and nutrition examination survey 2001–2006. Medicine 2015, 94, 1799. [Google Scholar] [CrossRef]
| Parameter | Tablet | Control | p-Value |
|---|---|---|---|
| N | 53 | 72 | |
| Males (%) | 30 (56.6) | 40 (55.6) | 0.68 |
| Anthropometrics | |||
| Age (years) | 14.1 ± 1.0 | 14.8 ± 1.4 | 0.09 |
| BMI (kg/m2) | 22.8 ± 5.8 | 22.9 ± 6.2 | 0.96 |
| Waist circumference (cm) | 78.2 ± 15.8 | 80.1 ± 16.8 | 0.60 |
| Hip Circumference (cm) | 92.3 ± 13.8 | 94.5 ± 15.9 | 0.50 |
| Waist-Hip Ratio | 0.80 ± 0.1 | 0.80 ± 0.1 | 0.98 |
| Systolic Blood Pressure (mmHg) | 116.2 ± 12.9 | 122.5 ± 16.6 | 0.05 |
| Diastolic Blood Pressure (mmHg) | 70.6 ± 11.6 | 71.1 ± 13.6 | 0.88 |
| Routine Biochemical Indices | |||
| Glucose (mmol/L) | 5.2 ± 0.6 | 5.4 ± 0.7 | 0.17 |
| Triglycerides (mmol/L) | 1.2 ± 0.6 | 1.3 ± 0.6 | 0.43 |
| Total Cholesterol (mmol/L) | 4.7 ± 0.8 | 4.5 ± 1.0 | 0.22 |
| LDL-Cholesterol (mmol/L) | 2.9 ± 0.7 | 2.5 ± 0.8 | 0.06 |
| HDL-Cholesterol (mmol/L) | 1.1 ± 0.3 | 1.3 ± 0.3 | 0.10 |
| Calcium (mmol/L) | 2.0 ± 0.1 | 1.9 ± 0.3 | 0.11 |
| Vitamin D and Iron Indices | |||
| 25(OH)D (nmol/L) | 34.6 ± 6.4 | 37.2 ± 7.5 | 0.09 |
| Iron (µmol/L) # | 18.2 (3–41) | 21.5 (8–39) | 0.09 |
| Transferrin Iron-Binding Capacity (µmol/L) # | 83.4 (19–102) | 83.6 (28–99) | 0.35 |
| Transferrin Saturation (%) # | 23.9 (3–71) | 26.3 (2–70) | 0.91 |
| Parameter | Tablet | Control | Tablet Effects | ||||
|---|---|---|---|---|---|---|---|
| N | 53 | 72 | |||||
| Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | p-Value | |
| Anthropometrics | |||||||
| Weight (kg) | 55.9 ± 17.3 | 56.3 ± 18.6 | 0.65 | 62.1 ± 19.3 | 65.0 ± 22.9 | 0.006 | 0.07 |
| BMI (kg/m2) | 22.8 ± 5.8 | 22.9 ± 6.2 | 0.59 | 22.9 ± 6.2 | 23.9 ± 7.5 | 0.09 | 0.69 |
| Waist circumference (cm) | 78.2 ± 15.8 | 82.2 ± 17.1 | 0.01 | 80.1 ± 16.8 | 81.4 ± 18.0 | 0.29 | 0.87 |
| Hip circumference (cm) | 92.3 ± 13.8 | 91.0 ± 13.4 | 0.17 | 94.5 ± 15.9 | 92.4 ± 14.4 | 0.049 | 0.56 |
| Waist-Hip Ratio | 0.8 ± 0.1 | 0.9 ± 0.1 | <0.001 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.011 | 0.67 |
| SBP (mmHg) | 116.2 ± 12.9 | 113.9 ± 12.3 | 0.27 | 122.5 ± 16.6 | 121.4 ± 13.0 | 0.61 | 0.006 |
| DBP (mmHg) | 70.6 ± 11.6 | 69.8 ± 12.6 | 0.68 | 71.1 ± 13.6 | 69.9 ± 15.5 | 0.6 | 0.92 |
| Routine Biochemical Indices | |||||||
| Glucose (mmol/L) | 5.2 ± 0.6 | 5.0 ± 0.5 | 0.038 | 5.4 ± 0.7 | 5.2 ± 0.7 | 0.15 | 0.029 |
| Triglycerides (mmol/L) # | 1.0 (0.3–3.1) | 0.9 (0.3–2.3) | 0.015 | 1.2 (0.3–3.1) | 1.3 (0.4–3.0) | 0.45 | 0.059 |
| Total Cholesterol (mmol/L) | 4.7 ± 0.8 | 4.7 ± 0.8 | 0.84 | 4.5 ± 1.0 | 4.6 ± 1.0 | 0.22 | 0.33 |
| LDL-Cholesterol (mmol/L) | 2.9 ± 0.7 | 2.8 ± 0.7 | 0.62 | 2.4 ± 0.8 | 2.5 ± 0.7 | 0.53 | 0.74 |
| HDL-Cholesterol (mmol/L) | 1.1 ± 0.3 | 1.3 ± 0.3 | 0.06 | 1.3 ± 0.3 | 1.1 ± 0.2 | 0.008 | 0.005 |
| Calcium (mmol/L) | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.07 | 1.9 ± 0.5 | 1.8 ± 0.5 | 0.44 | 0.062 |
| Parameters | Tablet (N = 53) | Control (N = 72) | Intervention Effects | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | ||
| 25(OH)D (nmol/L) | 34.6 ± 6.4 | 51.9 ± 13.0 | <0.001 | 37.2 ± 7.5 | 37.9 ± 10.6 | 0.69 | 0.001 |
| Iron (µmol/L) # | 18.2 (2.1–40.9) | 11.5 (1.3–49.5) | 0.002 | 21.5 (8.1–39.5) | 21.7 (8.7–38.0) | 0.86 | <0.001 |
| TIBC (µmol/L) # | 83.4 (18.7–102.8) | 90.5 (78.9–102.5) | 0.01 | 83.6 (28.0–99.5) | 84.9 (52.7–99.5) | 0.90 | 0.42 |
| Transferrin Saturation (%) # | 23.9 (2.1–70.8) | 12.3 (1.4–48.7) | 0.001 | 26.3 (1.2–70.7) | 25.1 (10.3–80.3) | 0.70 | 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masoud, M.S.; Alokail, M.S.; Yakout, S.M.; Khattak, M.N.K.; AlRehaili, M.M.; Wani, K.; Al-Daghri, N.M. Vitamin D Supplementation Modestly Reduces Serum Iron Indices of Healthy Arab Adolescents. Nutrients 2018, 10, 1870. https://doi.org/10.3390/nu10121870
Masoud MS, Alokail MS, Yakout SM, Khattak MNK, AlRehaili MM, Wani K, Al-Daghri NM. Vitamin D Supplementation Modestly Reduces Serum Iron Indices of Healthy Arab Adolescents. Nutrients. 2018; 10(12):1870. https://doi.org/10.3390/nu10121870
Chicago/Turabian StyleMasoud, Mohammad S., Majed S. Alokail, Sobhy M. Yakout, Malak Nawaz K. Khattak, Marwan M. AlRehaili, Kaiser Wani, and Nasser M. Al-Daghri. 2018. "Vitamin D Supplementation Modestly Reduces Serum Iron Indices of Healthy Arab Adolescents" Nutrients 10, no. 12: 1870. https://doi.org/10.3390/nu10121870
APA StyleMasoud, M. S., Alokail, M. S., Yakout, S. M., Khattak, M. N. K., AlRehaili, M. M., Wani, K., & Al-Daghri, N. M. (2018). Vitamin D Supplementation Modestly Reduces Serum Iron Indices of Healthy Arab Adolescents. Nutrients, 10(12), 1870. https://doi.org/10.3390/nu10121870






