Does Village Chicken-Keeping Contribute to Young Children’s Diets and Growth? A Longitudinal Observational Study in Rural Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Population
2.2. Ethical Approval
2.3. Data Collection
2.3.1. Questionnaires
2.3.2. Household Visits
2.3.3. Anthropometry
2.3.4. Rainfall Data
2.4. Data Analysis
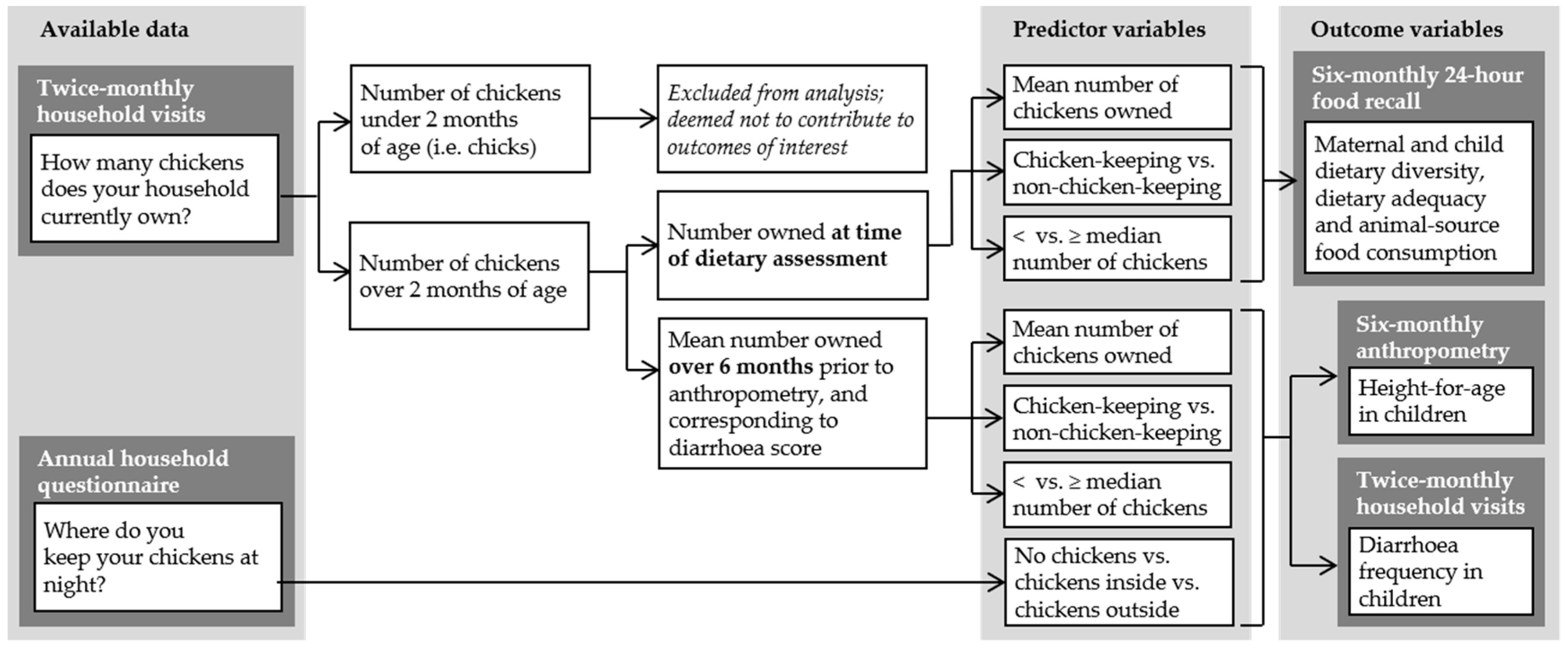
2.4.1. Defining Variables
- (a)
- as a bivariate categorical variable (i.e., yes/no);
- (b)
- relative to the median number of animals in this population (i.e., </≥ median number); and,
- (c)
- in terms of the number of animals owned.
- (a)
- no livestock;
- (b)
- chickens only;
- (c)
- small ruminants, with or without chickens; and,
- (d)
- cattle, with or without other livestock.
2.4.2. Descriptive Statistics
2.4.3. Univariable and Multivariable Models
- (a)
- maternal and child dietary diversity, dietary adequacy and the consumption of ASF;
- (b)
- child HAZ and probability of the stunting; and,
- (c)
- children’s “diarrhea score”.
3. Results
3.1. Characterizing the Population
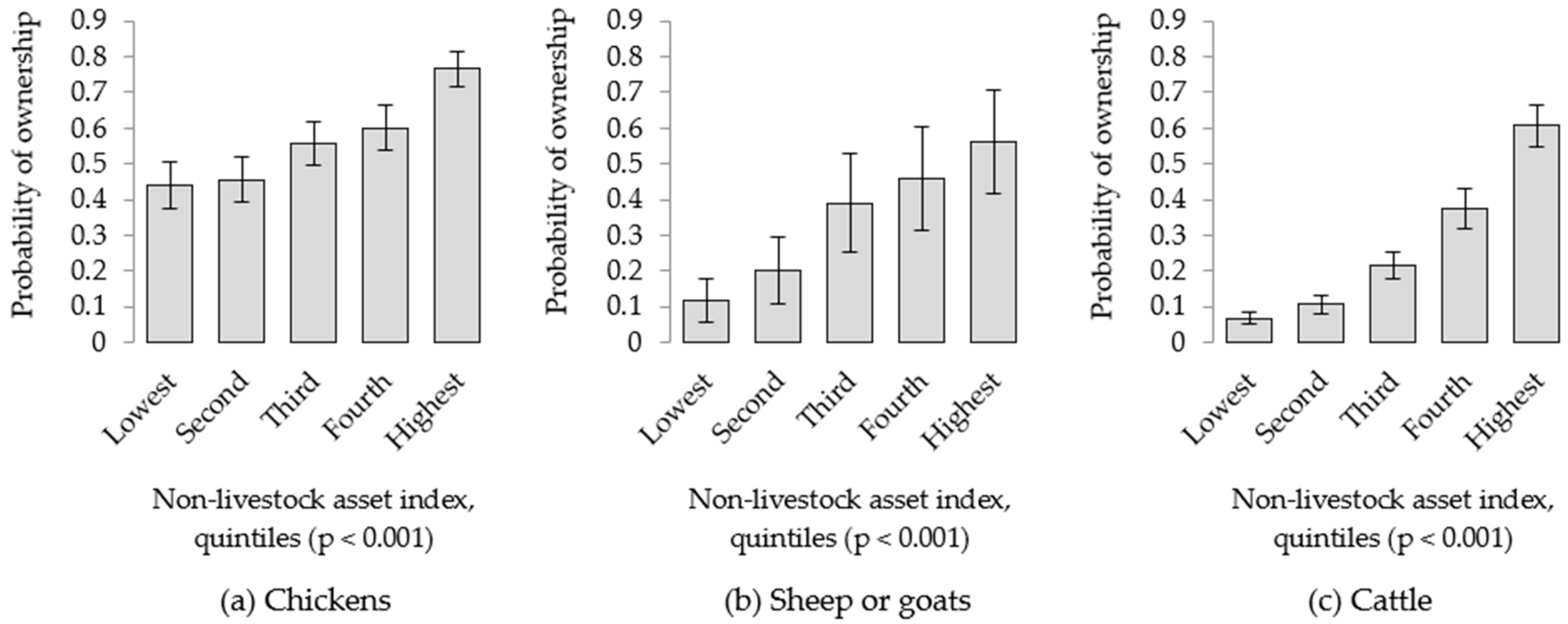
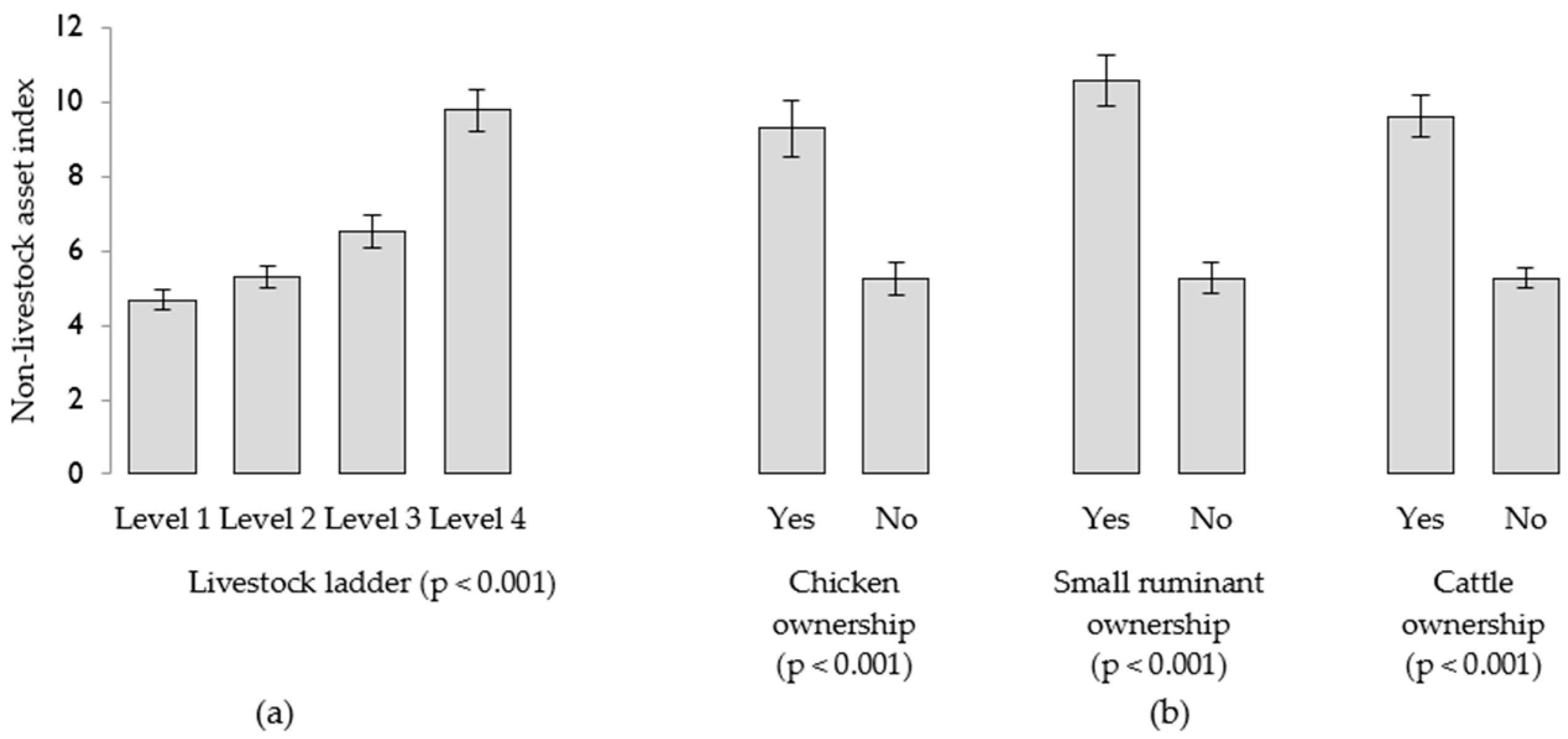
3.2. Livestock and Household Wealth
3.3. Maternal and Child Diets
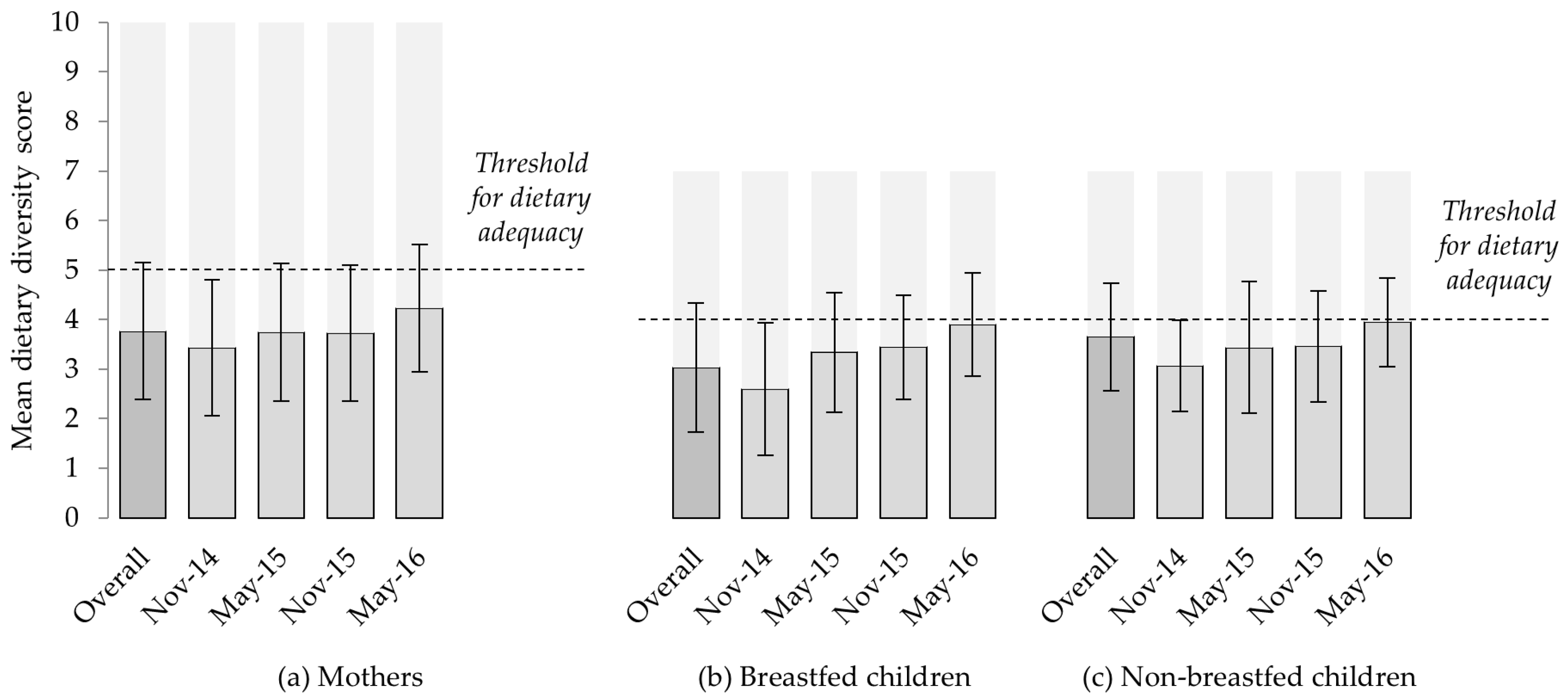
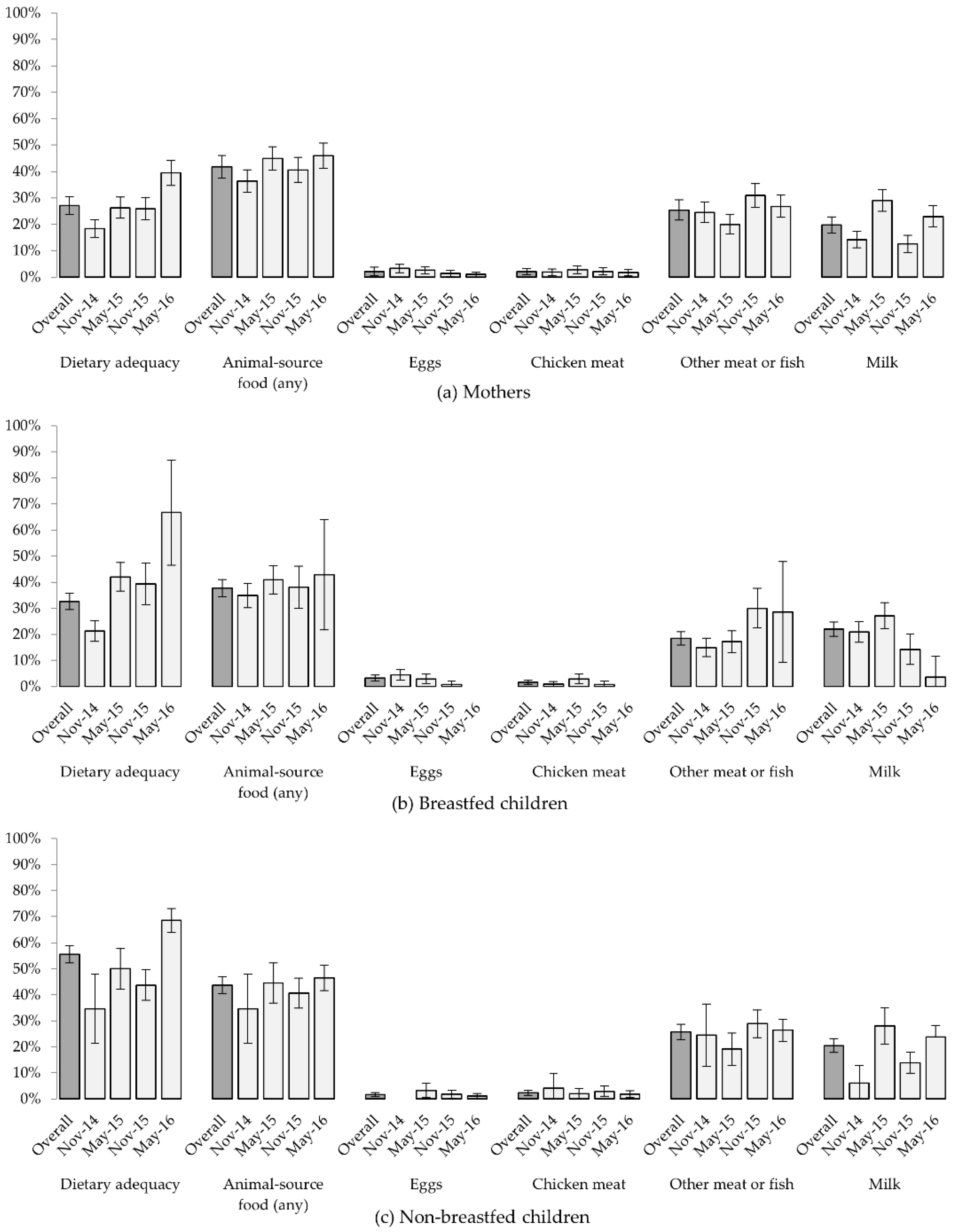
3.3.1. Dietary Diversity and Animal-Source Food Consumption
3.3.2. Univariable and Multivariable Models
3.4. Height-for-Age and Diarrhea in Children
3.4.1. Prevalence of Stunting
3.4.2. Univariable and Multivariable Models
4. Discussion
4.1. Seasonal and Temporal Variation in Diets
4.2. Chicken-Keeping and Diets
4.3. Livestock and Child Growth
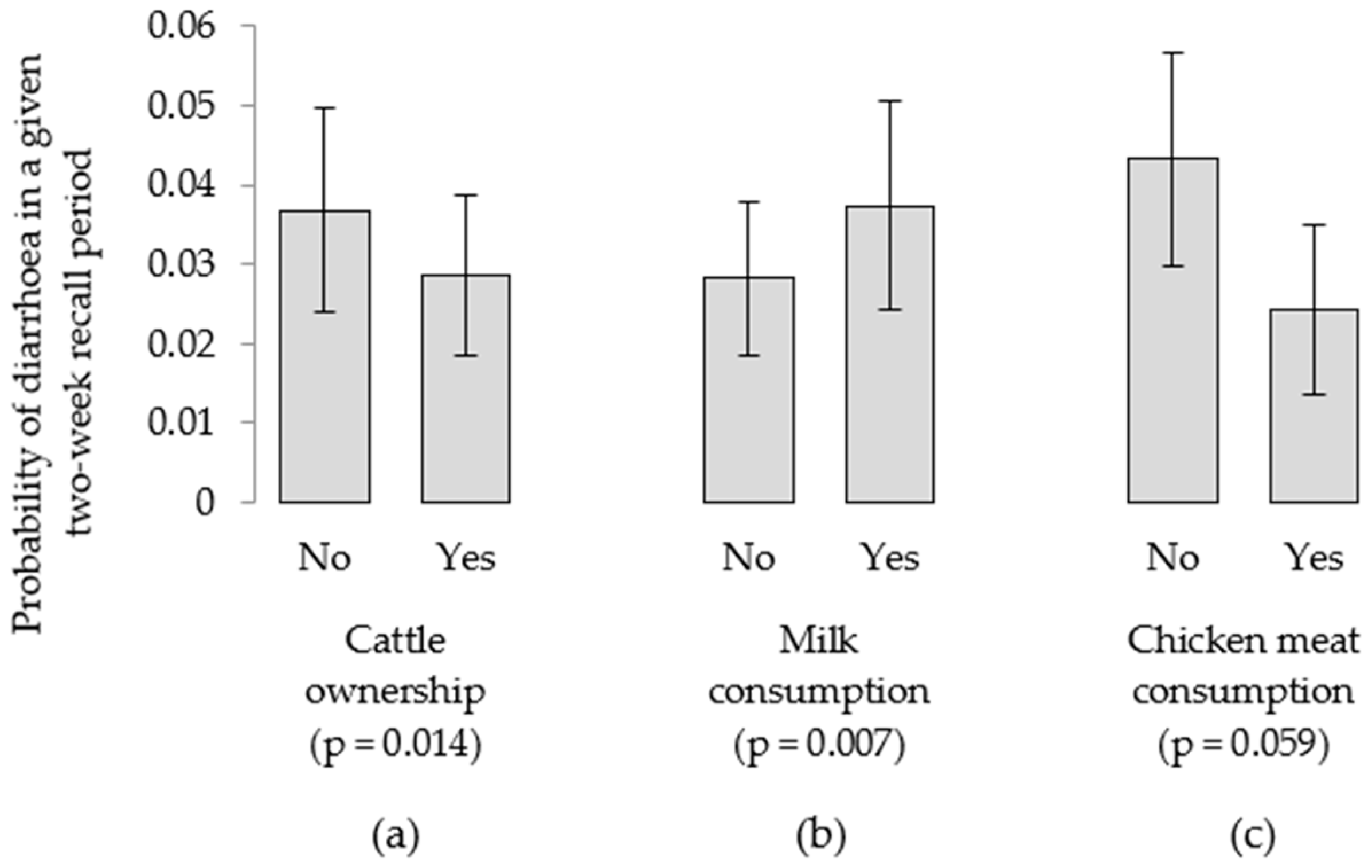
4.4. Livestock and Child Diarrhea
4.5. Gender and Nutrition
4.6. Limitations of This Study
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EED | environmental enteric dysfunction |
| HAZ | height-for-age |
| mo | months of age |
| DD | dietary diversity |
| MDD-W | Minimum Dietary Diversity for Women of Reproductive Age |
| IYCMDD | Infant and Young Child Minimum Dietary Diversity |
| HDAI | Household Domestic Asset Index |
| NLAI | Non-Livestock Asset Index |
| LMIC | low- to middle-income country |
Appendix A
| Variable(s) | Description | |
|---|---|---|
| Child | Age |
|
| Gender |
| |
| HAZ |
| |
| Diarrhea score |
| |
| Mother and child | Dietary diversity | |
| Dietary adequacy | ||
| ASF consumption |
| |
| Household demographics | Maternal age |
|
| Maternal formal education |
| |
| Household size |
| |
| Household head |
| |
| Household language group |
| |
| Water and sanitation | Water source |
|
| Toilet facility |
| |
| Socioeconomic status | Household Domestic Assets Index (HDAI) |
|
| Non-Livestock Asset Index (NLAI) |
| |
| Livestock ownership | Livestock ownership |
|
| Cattle ownership |
| |
| Small ruminant ownership |
| |
| Chicken ownership | For dietary models, based on chicken ownership during the month of dietary assessment. For stunting and diarrhea models, based on ownership over the six-month periods preceding each round of anthropometry. In both cases:
|
Appendix B
| Predictor Variables | Dietary Adequacy | DD Score b | ASF Consumption | |||
|---|---|---|---|---|---|---|
| Any | Chicken | Egg | Milk | |||
| (a) Mothers | ||||||
| Month of dietary assessment, May | <0.001 (+) | <0.001 (+) | <0.001(+) | 0.694 | 0.343 | <0.001 (+) |
| Maternal age | 0.393 | 0.299 | 0.043 (−) | 0.976 | 0.423 | 0.157 |
| Maternal formal education, yes | 0.090 (+) | 0.095 (+) | 0.939 | 0.465 | 0.959 | 0.239 |
| Breastfeeding, yes | <0.001 (−) | < 0.001 (−) | 0.022 (−) | 0.887 | 0.018 (+) | 0.377 |
| Sex of household head, female | 0.502 | 0.725 | 0.284 | 0.428 | 0.015 (+) | 0.004 (−) |
| Number of household members | 0.815 | 0.944 | 0.864 | 0.213 | 0.689 | 0.016 (+) |
| Language group, Sukuma | 0.484 | 0.118 | 0.001 (+) | 0.120 | 0.486 | <0.001 (+) |
| Household domestic asset index c,d | <0.001 (+) | <0.001 (+) | <0.001 (+) | 0.024 (+) | 0.005 (+) | <0.001 (+) |
| Chickens owned, yes | 0.004 (+) | 0.003 (+) | 0.003 (+) | 0.036 (+) | 0.685 | 0.011 (+) |
| Chickens, above median number | <0.001 (+) | 0.009 (+) | 0.011 (+) | 0.463 | 0.148 | 0.003 (+) |
| Chickens, number owned c | <0.001 (+) | <0.001 (+) | <0.001 (+) | 0.013 (+) | 0.159 | <0.001 (+) |
| Cattle owned, yes | 0.003 (+) | 0.002 (+) | <0.001 (+) | 0.251 | 0.146 | <0.001 (+) |
| Cattle, above median number | 0.167 | 0.053 (+) | 0.003 (+) | 0.418 | 0.305 | <0.001 (+) |
| Cattle, number owned c | 0.003 (+) | 0.001 (+) | <0.001 (+) | 0.137 | 0.070 (+) | <0.001 (+) |
| (b) Breastfed children | ||||||
| Month of dietary assessment, May | <0.001 (+) | <0.001 (+) | 0.655 | 0.149 | 0.458 | 0.021 |
| Child age | <0.001 (+) | <0.001 (+) | 0.043 (+) | 0.224 | 0.158 | 0.152 |
| Sex of child, female | 0.196 | 0.998 | 0.425 | 0.929 | 0.865 | 0.304 |
| Child height-for-age Z-score | 0.518 | 0.192 | 0.623 | 0.256 | 0.588 | 0.372 |
| Maternal formal education, yes | 0.673 | 0.517 | 0.460 | 0.134 | 0.326 | 0.680 |
| Sex of household head, female | 0.656 | 0.518 | 0.559 | 0.513 | 0.106 | 0.113 |
| Number of household members | 0.075 (+) | 0.142 | 0.361 | 0.591 | 0.391 | 0.018 (+) |
| Language group, Sukuma | 0.015 (+) | 0.103 | 0.002 (+) | 0.260 | 0.127 | <0.001 (+) |
| Household domestic asset index c,d | <0.001 (+) | 0.002 (+) | <0.001 (+) | 0.162 | 0.214 | <0.001 (+) |
| Chickens owned, yes | 0.012 (+) | 0.004 (+) | 0.008 (+) | 0.104 | 0.242 | 0.013 (+) |
| Chickens, above median number | 0.005 (+) | <0.001 (+) | 0.004 (+) | 0.162 | 0.178 | 0.003 (+) |
| Chickens, number owned c | 0.003 (+) | <0.001 (+) | <0.001 (+) | 0.016 (+) | 0.104 | 0.008 (+) |
| Cattle owned, yes | 0.004 (+) | 0.025 (+) | <0.001 (+) | 0.181 | N/A | <0.001 (+) |
| Cattle, above median number | <0.001 (+) | 0.020 (+) | <0.001 (+) | 0.289 | 0.704 | <0.001 (+) |
| Cattle, number owned c | <0.001 (+) | 0.004 (+) | <0.001 (+) | 0.182 | 0.048 (+) | <0.001 (+) |
| (c) Non-breastfed children | ||||||
| Month of dietary assessment, May | <0.001 (+) | <0.001 (+) | 0.003 (+) | 0.849 | 0.834 | <0.001 (+) |
| Child age | 0.138 | 0.023 (+) | 0.884 | 0.163 | 0.045 (−) | 0.950 |
| Sex of child, female | 0.123 | 0.180 | 0.015 (−) | 0.799 | 0.328 | 0.288 |
| Child height-for-age Z-score | 0.044 (+) | 0.060 (+) | 0.568 | 0.434 | 0.976 | 0.323 |
| Maternal formal education, yes | 0.165 | 0.237 | 0.332 | 0.536 | 0.968 | 0.212 |
| Sex of household head, female | 0.307 | 0.892 | 0.565 | 0.482 | 0.266 | 0.082 (−) |
| Number of household members | 0.789 | 0.709 | 0.924 | 0.080 (+) | 0.185 | 0.116 |
| Language group, Sukuma | 0.289 | 0.063 (+) | 0.003 (+) | 0.231 | 0.751 | <0.001 (+) |
| Household domestic asset index c,d | 0.667 | 0.098 (+) | 0.287 | 0.365 | 0.075 (+) | 0.004 (+) |
| Chickens owned, yes | 0.215 | 0.119 | 0.375 | 0.402 | 0.654 | 0.145 |
| Chickens, above median number | 0.725 | 0.097 (+) | 0.169 | 0.998 | 0.432 | 0.021 (+) |
| Chickens, number owned c | 0.361 | 0.025 (+) | 0.056 (+) | 0.521 | 0.103 | 0.007 (+) |
| Cattle owned, yes | 0.491 | 0.111 | 0.090 (+) | 0.600 | 0.629 | <0.001 (+) |
| Cattle, above median number | 0.768 | 0.531 | 0.051 (+) | N/A | 0.554 | <0.001 (+) |
| Cattle, number owned c | 0.780 | 0.124 | 0.024 (+) | 0.846 | 0.517 | <0.001 (+) |
| Predictor Variables | HAZ | Stunting | Diarrhea |
|---|---|---|---|
| Child age | <0.001 (−) | <0.001 (+) | <0.001 (−) |
| Sex of child, female | 0.006 (+) | 0.003 (−) | 0.681 |
| Diarrhea frequency | 0.004 (−) | 0.558 | N/A |
| Height-for-age Z-score | N/A | N/A | 0.641 |
| Month of data collection, May c | <0.001 (−) | 0.011 (+) | 0.031 (−) |
| Sex of household head, female | 0.126 | 0.495 | 0.995 |
| Number of household members | 0.761 | 0.504 | 0.808 |
| Maternal formal education, yes | 0.794 | 0.305 | 0.351 |
| Household language group, Sukuma | <0.001 (+) | 0.001 (−) | 0.512 |
| Improved water source | 0.312 | 0.627 | 0.298 |
| Improved toilet facility | 0.555 | 0.945 | 0.618 |
| Household domestic asset index | |||
| Livestock and non-livestock assets d | 0.205 | 0.061 (−) | 0.072 (−) |
| Non-livestock assets only d | 0.076 (+) | <0.001 (−) | 0.828 |
| Non-livestock assets only, quintiles | <0.001 (+) | <0.001 (−) | 0.558 |
| Livestock | |||
| Livestock owned, yes | 0.086 (−) | 0.368 | 0.327 |
| “Livestock ladder” e | 0.134 | 0.467 | 0.259 |
| Chickens owned, yes | 0.214 | 0.925 | 0.128 |
| Chickens, above median | 0.002 (−) | 0.109 | 0.479 |
| Chickens, number owned d | 0.007 (−) | 0.424 | 0.252 |
| Chickens, location of overnight housing | 0.651 | 0.692 | 0.101 |
| Sheep or goats owned, yes | 0.618 | 0.919 | 0.302 |
| Sheep or goats, above median | 0.121 | 0.035 (−) | 0.398 |
| Sheep or goats, number owned d | 0.100 (+) | 0.260 | 0.513 |
| Cattle owned, yes | 0.392 | 0.541 | 0.046 (−) |
| Cattle, above median | 0.340 | 0.060 (−) | 0.385 |
| Cattle, number owned d | 0.125 | 0.223 | 0.151 |
| Children’s diet, previous 24 h | |||
| ASF consumption, yes | 0.075 (−) | 0.405 | 0.367 |
| Chicken meat consumption, yes | 0.181 | 0.324 | 0.050 (−) |
| Other meat or fish consumption, yes | 0.001 (−) | 0.531 | 0.473 |
| Egg consumption, yes | 0.587 | 0.814 | 0.584 |
| Milk consumption, yes | 0.084 (+) | 0.554 | 0.042 (+) |
References
- Thornton, P.K.; Herrero, M. Adapting to climate change in the mixed crop and livestock farming systems in sub-Saharan Africa. Nat. Clim. Chang. 2015, 5, 830–836. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Seo, S.N. Is an integrated farm more resilient against climate change? A micro-econometric analysis of portfolio diversification in African agriculture. Food Policy 2010, 35, 32–40. [Google Scholar] [CrossRef]
- Murphy, S.P.; Allen, L.H. Nutritional importance of animal source foods. J. Nutr. 2003, 133, 3932S–3935S. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.G.; Harris, D.M.; Rogers, L.M. Contribution of animal source foods in improving diet quality and function in children in the developing world. Nutr. Res. 2002, 22, 193–220. [Google Scholar] [CrossRef]
- Randolph, T.F.; Ruel, M.; Schelling, E.; Grace, D.; Nicholson, C.F.; Leroy, J.L.; Cole, D.C.; Demment, M.W.; Omore, A.; Zinsstag, J. Invited review: Role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007, 85, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Penakalapati, G.; Swarthout, J.; Delahoy, M.J.; McAliley, L.; Wodnik, B.; Levy, K.; Freeman, M.C. Exposure to animal feces and human health: A systematic review and proposed research priorities. Environ. Sci. Technol. 2017, 51, 11537–11552. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.D.; Levy, K.; Menezes, N.P.; Freeman, M.C. Human diarrhea infections associated with domestic animal husbandry: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.J.; Jones, K.D.; Berkley, J.A. Environmental enteric dysfunction: An overview. Food Nutr. Bull. 2015, 36, S76–S87. [Google Scholar] [CrossRef] [PubMed]
- Mbuya, M.N.; Humphrey, J.H. Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: An opportunity for stunting reduction in developing countries. Matern. Child Nutr. 2016, 12, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Ngure, F.M.; Reid, B.M.; Humphrey, J.H.; Mbuya, M.N.; Pelto, G.; Stoltzfus, R.J. Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: Making the links. Ann. N. Y. Acad. Sci. 2014, 1308, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.M.; Mutasa, M.; Prendergast, A.J.; Humphrey, J.; Manges, A.R. Environmental enteric dysfunction pathways and child stunting: A systematic review. PLoS Negl. Trop. Dis. 2018, 12, e0006205. [Google Scholar] [CrossRef] [PubMed]
- Fierstein, J.L.; Eliasziw, M.; Rogers, B.L.; Forrester, J.E. Nonnative cattle ownership, diet, and child height-for-age: Evidence from the 2011 Uganda Demographic and Health Survey. Am. J. Trop. Med. Hyg. 2017, 96, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Glatz, P.; Pym, R. Poultry housing and management in developing countries. In Poultry Development Review; FAO: Rome, Italy, 2013; pp. 24–28. ISBN 978-92-5-108067-2. [Google Scholar]
- Guèye, E.F. Village egg and fowl meat production in Africa. World’s Poult. Sci. J. 1998, 54, 73–86. [Google Scholar] [CrossRef]
- Aini, I. Indigenous chicken production in South-East Asia. World’s Poult. Sci. J. 1990, 46, 51–57. [Google Scholar] [CrossRef]
- Sonaiya, E.B. Direct assessment of nutrient resources in free-range and scavenging systems. World’s Poult. Sci. J. 2004, 60, 523–535. [Google Scholar] [CrossRef]
- Bagnol, B. Gender issues in small-scale family poultry production: Experiences with Newcastle Disease and Highly Pathogenic Avian Influenza control. World’s Poult. Sci. J. 2009, 65, 231–240. [Google Scholar] [CrossRef]
- Guèye, E.F. Gender aspects in family poultry management systems in developing countries. World’s Poult. Sci. J. 2005, 61, 39–46. [Google Scholar] [CrossRef]
- Guèye, E.F. Women and family poultry production in rural Africa. Dev. Pract. 2000, 10, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.T.; Alderman, H. Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013, 382, 536–551. [Google Scholar] [CrossRef]
- World Bank. From Agriculture to Nutrition: Pathways, Synergies and Outcomes; World Bank: Washington, DC, USA, 2007. [Google Scholar]
- Headey, D.; Hirvonen, K. Is exposure to poultry harmful to child nutrition? An observational analysis for rural Ethiopia. PLoS ONE 2016, 11, e0160590. [Google Scholar] [CrossRef] [PubMed]
- Headey, D.; Nguyen, P.; Kim, S.; Rawat, R.; Ruel, M.; Menon, P. Is exposure to animal feces harmful to child nutrition and health outcomes? A multicountry observational analysis. Am. J. Trop. Med. Hyg. 2017, 96, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, J.B.; Wiethoelter, A.K.; Negin, J.; Mor, S.M. Livestock ownership, animal source foods and child nutritional outcomes in seven rural village clusters in Sub-Saharan Africa. Agric. Food Secur. 2017, 6, 9. [Google Scholar] [CrossRef]
- Schmidt, W.P.; Boisson, S.; Routray, P.; Bell, M.; Cameron, M.; Torondel, B.; Clasen, T. Exposure to cows is not associated with diarrhoea or impaired child growth in rural Odisha, India: A cohort study. Epidemiol. Infect. 2016, 144, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Headey, D.; Hirvonen, K. Exploring Child Health Risks of Poultry Keeping in Ethiopia: Insights from the 2015 Feed the Future Survey. In Essp ii Research Note 43; International Food Policy Research Institute and Ethiopian Development Research Institute: Washington, DC, USA; Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Mosites, E.M.; Rabinowitz, P.M.; Thumbi, S.M.; Montgomery, J.M.; Palmer, G.H.; May, S.; Rowhani-Rahbar, A.; Neuhouser, M.L.; Walson, J.L. The relationship between livestock ownership and child stunting in three countries in Eastern Africa using national survey data. PLoS ONE 2015, 10, e0136686. [Google Scholar] [CrossRef] [PubMed]
- Chilonda, P.; Otte, J. Indicators to monitor trends in livestock production at national, regional and international levels. Livest. Res. Rural Dev. 2006, 18, 117. Available online: http://www.lrrd.org/lrrd18/8/chil18117.htm (accessed on 1 August 2017).
- Jahnke, H.E. Livestock Production Systems and Livestock Development in Tropical Africa; Kieler Wissenschaftsverlag Vauk: Kiel, Germany, 1982. [Google Scholar]
- Dolberg, F. A livestock development approach that contributes to poverty alleviation and widespread improvement of nutrition among the poor. Livestock Res. Rural Dev. 2001, 13, 41. Available online: http://www.lrrd.org/lrrd13/5/dolb135.htm (accessed on 1 August 2017).
- Maass, B.L.; Chiuri, W.L.; Zozo, R.; Katunga-Musale, D.; Metre, T.K.; Birachi, E. Using the ‘livestock ladder’ as a means for poor crop–livestock farmers to exit poverty in Sud Kivu province, eastern DR Congo. In Agro-Ecological Intensification of Agricultural Systems in the African Highlands; Vanlauwe, B., Asten, P.V., Blomme, G., Eds.; Earthscan, Routledge: London, UK, 2013; pp. 145–155. ISBN 978-0-415-53273-0. [Google Scholar]
- Todd, H. Women climbing out of poverty through credit; or what do cows have to do with it? Livest. Res. Rural Dev. 1998, 10, 45–63. [Google Scholar]
- Akinola, L.A.F.; Essien, A. Relevance of rural poultry production in developing countries with special reference to Africa. World’s Poult. Sci. J. 2011, 67, 697–705. [Google Scholar] [CrossRef]
- Alders, R.G.; Pym, R.A.E. Village poultry: Still important to millions, eight thousand years after domestication. World’s Poult. Sci. J. 2009, 65, 181–190. [Google Scholar] [CrossRef]
- Doran, M.H.; Low, A.R.C.; Kemp, R.L. Cattle as a store of wealth in Swaziland: Implications for livestock development and overgrazing in Eastern and Southern Africa. Am. J. Agric. Econ. 1979, 61, 41–47. [Google Scholar] [CrossRef]
- Moll, H.A.J. Costs and benefits of livestock systems and the role of market and nonmarket relationships. Agric. Econ. 2005, 32, 181–193. [Google Scholar] [CrossRef]
- Alders, R.; Aongola, A.; Bagnol, B.; de Bruyn, J.; Kimboka, S.; Kock, R.; Li, M.; Maulaga, W.; McConchie, R.; Mor, S.; et al. Using a one health approach to promote food and nutrition security in Tanzania and Zambia. GRF Davos Planet@Risk 2014, 2, 187–190. [Google Scholar]
- Lema, M.; Majule, A. Impacts of climate change, variability and adaptation strategies on agriculture in semi arid areas of Tanzania: The case of Manyoni District in Singida Region, Tanzania. Afr. J. Environ. Sci. Technol. 2009, 3, 206–209. [Google Scholar]
- FAO and FHI 360. Minimum Dietary Diversity for Women: A Guide for Measurement; FAO and FHI 360: Rome, Italy, 2016. [Google Scholar]
- WHO. Diarrhoeal Disease (Fact Sheet No. 330); WHO: Geneva, Switzerland, 2013. [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Pædiatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- WHO. WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices. Part 1: Definitions; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Bandoh, D.A.; Kenu, E. Dietary diversity and nutritional adequacy of under-fives in a fishing community in the central region of Ghana. BMC Nutr. 2017, 3, 2. [Google Scholar] [CrossRef]
- Alkire, S.; Meinzen-Dick, R.S.; Peterman, A.; Quisumbing, A.R.; Seymour, G.; Vaz, A. The Women’s Empowerment in Agriculture Index. Oxford Poverty and Human Development Initiative Working Paper No. 58; University of Oxford: Oxford, UK, 2013. [Google Scholar]
- Njuki, J.; Poole, J.; Johnson, N.; Baltenweck, I.; Pali, P.; Lokman, Z.; Mburu, S. Gender, Livestock and Livelihood Indicators; International Livestock Research Institute: Nairobi, Kenya, 2011. [Google Scholar]
- Mabilia, M. Beliefs and practices in infant feeding among the Wagogo of Chigongwe (Dodoma Rural District), Tanzania: I. Breastfeeding. Ecol. Food Nutr. 1996, 35, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Selemani, I.S.; Eik, L.O.; Holand, Ø.; Ådnøy, T.; Mtengeti, E.; Mushi, D. The role of indigenous knowledge and perceptions of pastoral communities on traditional grazing management in north-western Tanzania. Afr. J. Agric. Res. 2012, 7, 5537–5547. [Google Scholar]
- WHO; UNICEF. Core Questions on Drinking-Water and Sanitation for Household Surveys; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Allegretti, A. ‘Being Maasai’ in markets and trade: The role of ethnicity-based institutions in the livestock market of northern Tanzania. Nomadic Peoples 2017, 21, 63–86. [Google Scholar] [CrossRef]
- Savy, M.; Martin-Prevel, Y.; Traissac, P.; Eymard-Duvernay, S.; Delpeuch, F. Dietary diversity scores and nutritional status of women change during the seasonal food shortage in rural Burkina Faso. J. Nutr. 2006, 136, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Buza, J.J.; Mwamuhehe, H.A. Country report: Tanzania. In Proceedings of SADC Planning Workshop on Newcastle Disease Control in Village Chickens, Maputo, Mozambique, 6–9 March 2000; Alders, R.G., Spradbrow, P.B., Eds.; Australian Centre for International Agricultural Researc: Canberra, Australia, 2001; pp. 38–42. [Google Scholar]
- Ministry of Health Community Development Gender Elderly and Children [Tanzania Mainland] (MoHCDGEC); Ministry of Health [Zanzibar] (MoH). Tanzania Demographic and Health Survey and Malaria Indicator Survey 2015–16; MoHCDGEC, MoH, NBS, OCGS and ICF: Dar es Salaam, Tanzania; Rockville, MD, USA, 2016.
- Guèye, E.F. Employment and income generation through family poultry in low-income food-deficit countries. World’s Poult. Sci. J. 2002, 58, 541–557. [Google Scholar] [CrossRef]
- Pym, R.A.E.; Guerne Bleich, E.; Hoffman, I. The Relative Contribution of Indigenous Chicken Breeds to Poultry Meat and Egg Production and Consumption in the Developing Countries of Africa and Asia. Presentation at XII European Poultry Conference, Verona, Italy, 10–14 September 2006; CABI: Wallingford, UK, 2006. [Google Scholar]
- de Bruyn, J.; Bagnol, B.; Darnton-Hill, I.; Maulaga, W.; Thomson, P.C.; Alders, R. Characterising infant and young child feeding practices and the consumption of poultry products in rural Tanzania: A mixed methods approach. Matern. Child Nutr. 2017, 14, e12550. [Google Scholar] [CrossRef] [PubMed]
- Udo, H.M.J.; Aklilu, H.A.; Phong, L.T.; Bosma, R.H.; Budisatria, I.G.S.; Patil, B.R.; Samdup, D.; Bebe, B.O. Impact of intensification of different types of livestock production in smallholder crop-livestock systems. Livest. Sci. 2011, 139, 22–29. [Google Scholar] [CrossRef]
- Neumann, C.G.; Murphy, S.P.; Gewa, C.; Grillenberger, M.; Bwibo, N.O. Meat supplementation improves growth, cognitive, and behavioral outcomes in Kenyan children. J. Nutr. 2007, 137, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.S.; Ikenaka, Y.; Nakayama, S.M.M.; Ishizuka, M. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 2014, 76, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Gizachew, D.; Szonyi, B.; Tegegne, A.; Hanson, J.; Grace, D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control 2016, 59, 773–779. [Google Scholar] [CrossRef]
- Knight-Jones, T.; Hang’ombe, M.; Songe, M.; Sinkala, Y.; Grace, D. Microbial contamination and hygiene of fresh cow’s milk produced by smallholders in Western Zambia. Int. J. Environ. Res. Public Health 2016, 13, 737. [Google Scholar] [CrossRef] [PubMed]
- Budlender, D. The debate about household headship. Soc. Dyn. 2003, 29, 48–72. [Google Scholar] [CrossRef]
- Rosenhouse Persson, S. Identifying the Poor: Is “Headship” a Useful Concept? Living Standards Measurement Study Working Paper No. 58; World Bank: Washington, DC, USA, 1989. [Google Scholar]
- Kennedy, E.; Haddad, L. Are pre-schoolers from female-headed households less malnourished? A comparative analysis of results from Ghana and Kenya. J. Dev. Stud. 1994, 30, 680–695. [Google Scholar] [CrossRef]
- Cronk, L. Preferential parental investment in daughters over sons. Hum. Nat. 1991, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.D. Social class, gender and intrahousehold food allocations to children in South Asia. Soc. Sci. Med. 1997, 44, 1685–1695. [Google Scholar] [CrossRef]
- Espo, M.; Kulmala, T.; Maleta, K.; Cullinan, T.; Salin, M.L.; Ashorn, P. Determinants of linear growth and predictors of severe stunting during infancy in rural Malawi. Acta Paediatr. 2002, 91, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Ngare, D.K.; Muttunga, J.N. Prevalence of malnutrition in Kenya. East Afr. Med. J. 1999, 76, 376–380. [Google Scholar] [PubMed]
- Wamani, H.; Åstrøm, N.; Peterson, S.; Tumwine, J.; Tylleskär, T. Boys are more stunted than girls in sub-Saharan Africa: A meta-analysis of 16 Demographic and Healthy Surveys. BMC Pediatr. 2007, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Garenne, M. Sex differences in health indicators among children in African DHS surveys. J. Biosoc. Sci. 2003, 35, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Torgerson, D.J.; Hewitt, C.E. Reporting attrition in randomised controlled trials. Br. Med. J. 2006, 332, 969–971. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.N.; Luby, S.P.; Mendoza, C. Recall errors in a weekly survey of diarrhoea in Guatemala: Determining the optimal length of recall. Epidemiol. Infect. 2009, 138, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.; Swartz, L.; Landman, M. Insiders and outsiders: Levels of collaboration in research partnerships across resource divides. Infant Ment. Health J. 2006, 27, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.; Kennedy, E. Impacts of agriculture on nutrition: Nature of the evidence and research gaps. Food Nutr. Bull. 2014, 35, 126–132. [Google Scholar] [CrossRef] [PubMed]

| Indicator | Definition | Use |
|---|---|---|
| Household Domestic Asset Index (HDAI) | A weighted sum of material and livestock assets | To characterize households’ socioeconomic status in descriptive summaries of the study population. |
| HDAI excluding cattle and chickens | A weighted sum of material and selected livestock assets (sheep, goats, donkeys, pigs) | To control for variation in socioeconomic status in models for maternal and child diets, where the influence of cattle and chicken ownership was tested separately. |
| Non-Livestock Asset Index (NLAI) | A weighted sum of material assets only | To control for variation in socioeconomic status in models for child stunting and diarrhea, where multiple livestock-associated variables were tested separately. |
| Location | Sanza Ward | Majiri Ward | Overall |
|---|---|---|---|
| Baseline data collection | May 2014 | Nov 2014 | |
| Enrolled households (n) | 229 | 274 | 503 |
| Sex of child, female (%) | 55.5 | 47.4 | 51.1 |
| Child age in months | |||
| Mean (SD) | 9.9 (6.1) a | 7.6 (4.3) a | 8.6 (5.3) |
| Range | 1.2–28.1 | 0.6–22.5 | 0.6–28.1 |
| Maternal age in years | |||
| Mean (SD) | 28.5 (7.5) a | 26.8 (7.5) a | 27.7 (7.6) |
| Range | 15–50 | 13–54 | 13–54 |
| Age unknown (%) | 9.2 b | 23.7 b | 17.1 |
| Maternal education (%) | |||
| No formal education | 22.7 b | 40.5 b | 32.4 |
| Some primary school | 68.6 b | 56.6 b | 62.0 |
| Some secondary school | 5.7 b | 1.5 b | 3.4 |
| Unspecified level | 3.1 | 1.5 | 2.2 |
| Primary language of household (%) | |||
| Kigogo | 78.2 b | 74.8 b | 76.3 |
| Kisukuma | 6.1 b | 14.6 b | 10.7 |
| Other | 4.4 | 2.6 | 3.4 |
| Unspecified | 11.4 b | 8.0 b | 9.5 |
| Parents of same language group (%) | 92.1 | 95.6 | 94.1 |
| Number of household members | |||
| Mean (SD) | 5.6 (2.0) | 5.5 (2.3) | 5.5 (2.2) |
| Range | 2–16 | 2–21 | 2–21 |
| Female-headed households (%) | 30.2 b | 16.4 b | 22.7 |
| Socioeconomic status, median (IQR) | |||
| Non-livestock and livestock assets, HDAI | 12 (5–51) | 26 (7–115) | 19 (7–76) |
| Non-livestock assets only, NLAI | 7 (3–11) | 9 (3–13) | 9 (3–12) |
| Livestock ownership (%) | |||
| Cattle | 26.7 b | 36.2 b | 31.8 |
| Sheep or goats | 27.1 b | 47.8 b | 38.3 |
| Chickens | 51.1 b | 42.1 b | 46.3 |
| Number of livestock, median (IQR) c | |||
| Cattle | 4 (2–17) a | 10 (4–20) a | 7 (4–20) |
| Sheep or goats | 14 (7–20) | 12 (5–25) | 12 (6–24) |
| Chickens | 7 (2–13) | 8 (5–13) | 8 (4–13) |
| Improved water source (%) | 2.6 | 2.2 | 4.9 |
| Improved toilet facilities (%) | 3.1 b | 0.4 b | 1.6 |
| Predictor Variables | Dietary Adequacy | DD Score b | ASF Consumption | |||
|---|---|---|---|---|---|---|
| Any | Chicken | Egg | Milk | |||
| (a) Mothers | ||||||
| Month of dietary assessment, May | <0.001 (+) | <0.001 (+) | 0.006 (+) | NS | NS | <0.001 (+) |
| Maternal age | NS | 0.067 (−) | NS | NS | 0.092 (−) | NS |
| Maternal formal education, yes | NS | NS | NS | NS | NS | NS |
| Breastfeeding, yes | NS | NS | NS | NS | NS | NS |
| Sex of household head, female | NS | 0.068 (+) | NS | NS | <0.001 (+) | 0.014 (−) |
| Number of household members | NS | NS | NS | NS | NS | NS |
| Language group, Sukuma | NS | NS | 0.032 (+) | NS | NS | <0.001 (+) |
| Household domestic asset indexc,d | 0.002 (+) | < 0.001 (+) | NS | 0.058 (+) | 0.005 (+) | NS |
| Chickens owned, yes | NS | NS | NS | NS | NS | NS |
| Chickens, above median number | 0.023 (+) e | NS | NS | NS | NS | NS |
| Chickens, number owned c | 0.032 (+) e | NS | 0.009 (+) | 0.053 (+) | NS | NS |
| Cattle owned, yes | NS | NS | NS | NS | NS | NS |
| Cattle, above median number | NS | NS | NS | NS | NS | NS |
| Cattle, number owned c | NS | NS | 0.005 (+) | NS | NS | <0.001 (+) |
| (b) Breastfed children | ||||||
| Month of dietary assessment, May | 0.002 (+) | 0.057 (+) | NS | NS | NS | 0.028 (+) |
| Child age | <0.001 (+) | <0.001 (+) | NS | NS | NS | NS |
| Sex of child, female | NS | NS | NS | NS | NS | NS |
| Maternal formal education, yes | NS | NS | NS | NS | NS | NS |
| Sex of household head, female | NS | NS | NS | NS | 0.032 (+) | NS |
| Number of household members | NS | NS | NS | NS | NS | NS |
| Language group, Sukuma | 0.046 (+) | NS | 0.014 (+) | NS | NS | 0.002 (+) |
| Household domestic asset index c,d | NS | 0.002 (+) | < 0.001 (+) | NS | <0.001 (+) | NS |
| Chickens owned, yes | NS | NS | NS | NS | NS | NS |
| Chickens, above median number | NS | 0.039 (+) | NS | NS | NS | NS |
| Chickens, number owned c | NS | NS | 0.083 (+) | 0.016 (+) | NS | NS |
| Cattle owned, yes | NS | NS | NS | NS | NS | 0.010 (+) f |
| Cattle, above median number | NS | NS | NS | NS | NS | <0.001 (+) f |
| Cattle, number owned c | <0.001 (+) | NS | NS | NS | NS | <0.001 (+) f |
| (c) Non-breastfed children | ||||||
| Month of dietary assessment, May | <0.001 (+) | <0.001 (+) | 0.003 (+) | NS | NS | <0.001 (+) |
| Child age | NS | NS | NS | NS | NS | NS |
| Sex of child, female | 0.045 (−) | 0.066 (−) | 0.014 (−) | NS | NS | NS |
| Maternal formal education, yes | NS | NS | NS | NS | NS | NS |
| Sex of household head, female | NS | NS | NS | NS | NS | NS |
| Number of household members | NS | NS | NS | 0.080 (+) | 0.059 (−) | NS |
| Language group, Sukuma | NS | NS | 0.002 (+) | NS | NS | <0.001 (+) |
| Household domestic asset index c,d | NS | NS | NS | NS | 0.023 (+) | NS |
| Chickens owned, yes | NS | NS | NS | NS | NS | NS |
| Chickens, above median number | NS | NS | NS | NS | NS | NS |
| Chickens, number owned c | NS | 0.038 (+) | NS | NS | NS | NS |
| Cattle owned, yes | NS | NS | NS | NS | NS | 0.003 (+) |
| Cattle, above median number | NS | NS | NS | NS | NS | 0.050 (+) |
| Cattle, number owned c | NS | NS | NS | NS | NS | <0.001 (+) |
| Mean HAZ (SD) | % Stunting | n | |
|---|---|---|---|
| Sanza Ward | |||
| May 2014 | −1.52 (1.13) | 36.8 | 220 |
| Nov 2014 | −1.63 (1.18) | 34.5 | 200 |
| May 2015 | −2.02 (1.14) | 49.5 | 202 |
| Nov 2015 | −1.98 (1.05) | 48.2 | 191 |
| May 2016 | −1.77 (1.05) | 39.8 | 201 |
| Majiri Ward | |||
| Nov 2014 | −1.45 (1.21) | 28.3 | 272 |
| May 2015 | −1.86 (1.05) | 41.4 | 261 |
| Nov 2015 | −1.99 (0.98) | 49.6 | 234 |
| May 2016 | −2.16 (1.00) | 53.0 | 217 |
| Predictor Variables | HAZ | Stunting | Diarrhea |
|---|---|---|---|
| Child age | <0.001 (−) | <0.001 (+) | <0.001 (−) |
| Sex of child, female | 0.022 (+) | 0.002 (−) | NS |
| Diarrhea frequency | <0.001 (−) | NS | N/A |
| Height-for-age Z-score | N/A | N/A | NS |
| Month of data collection, May c | NS | NS | NS |
| Sex of household head, female | NS | NS | NS |
| Number of household members | NS | NS | NS |
| Maternal formal education, yes | NS | NS | NS |
| Household language group, Sukuma | <0.001 (+) | 0.002 (−) | NS |
| Improved water source | NS | NS | NS |
| Improved toilet facility | NS | NS | NS |
| Household domestic asset index | |||
| Livestock and non-livestock assets d | NS | NS | NS |
| Non-livestock assets only d | NS | <0.001 (−) f | NS |
| Non-livestock assets only, quintiles | 0.009 (+) | <0.001 (−) f | NS |
| Livestock | |||
| Livestock owned, yes | NS | NS | NS |
| “Livestock ladder” e | NS | NS | NS |
| Chickens owned, yes | NS | NS | NS |
| Chickens, above median | NS | NS | NS |
| Chickens, number owned d | NS | NS | NS |
| Chickens, location of overnight housing | NS | NS | NS |
| Sheep or goats owned, yes | NS | NS | NS |
| Sheep or goats, above median | NS | NS | NS |
| Sheep or goats, number owned d | NS | NS | NS |
| Cattle owned, yes | NS | NS | 0.014 (−) |
| Cattle, above median | NS | NS | NS |
| Cattle, number owned d | NS | NS | NS |
| Children’s diet, previous 24 h | |||
| ASF consumption, yes | NS | NS | NS |
| Chicken meat consumption, yes | NS | NS | 0.059 (−) |
| Other meat or fish consumption, yes | NS | NS | NS |
| Egg consumption, yes | NS | NS | NS |
| Milk consumption, yes | NS | NS | 0.007 (+) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bruyn, J.; Thomson, P.C.; Darnton-Hill, I.; Bagnol, B.; Maulaga, W.; Alders, R.G. Does Village Chicken-Keeping Contribute to Young Children’s Diets and Growth? A Longitudinal Observational Study in Rural Tanzania. Nutrients 2018, 10, 1799. https://doi.org/10.3390/nu10111799
De Bruyn J, Thomson PC, Darnton-Hill I, Bagnol B, Maulaga W, Alders RG. Does Village Chicken-Keeping Contribute to Young Children’s Diets and Growth? A Longitudinal Observational Study in Rural Tanzania. Nutrients. 2018; 10(11):1799. https://doi.org/10.3390/nu10111799
Chicago/Turabian StyleDe Bruyn, Julia, Peter C. Thomson, Ian Darnton-Hill, Brigitte Bagnol, Wende Maulaga, and Robyn G. Alders. 2018. "Does Village Chicken-Keeping Contribute to Young Children’s Diets and Growth? A Longitudinal Observational Study in Rural Tanzania" Nutrients 10, no. 11: 1799. https://doi.org/10.3390/nu10111799
APA StyleDe Bruyn, J., Thomson, P. C., Darnton-Hill, I., Bagnol, B., Maulaga, W., & Alders, R. G. (2018). Does Village Chicken-Keeping Contribute to Young Children’s Diets and Growth? A Longitudinal Observational Study in Rural Tanzania. Nutrients, 10(11), 1799. https://doi.org/10.3390/nu10111799






