Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies
Abstract
1. Introduction
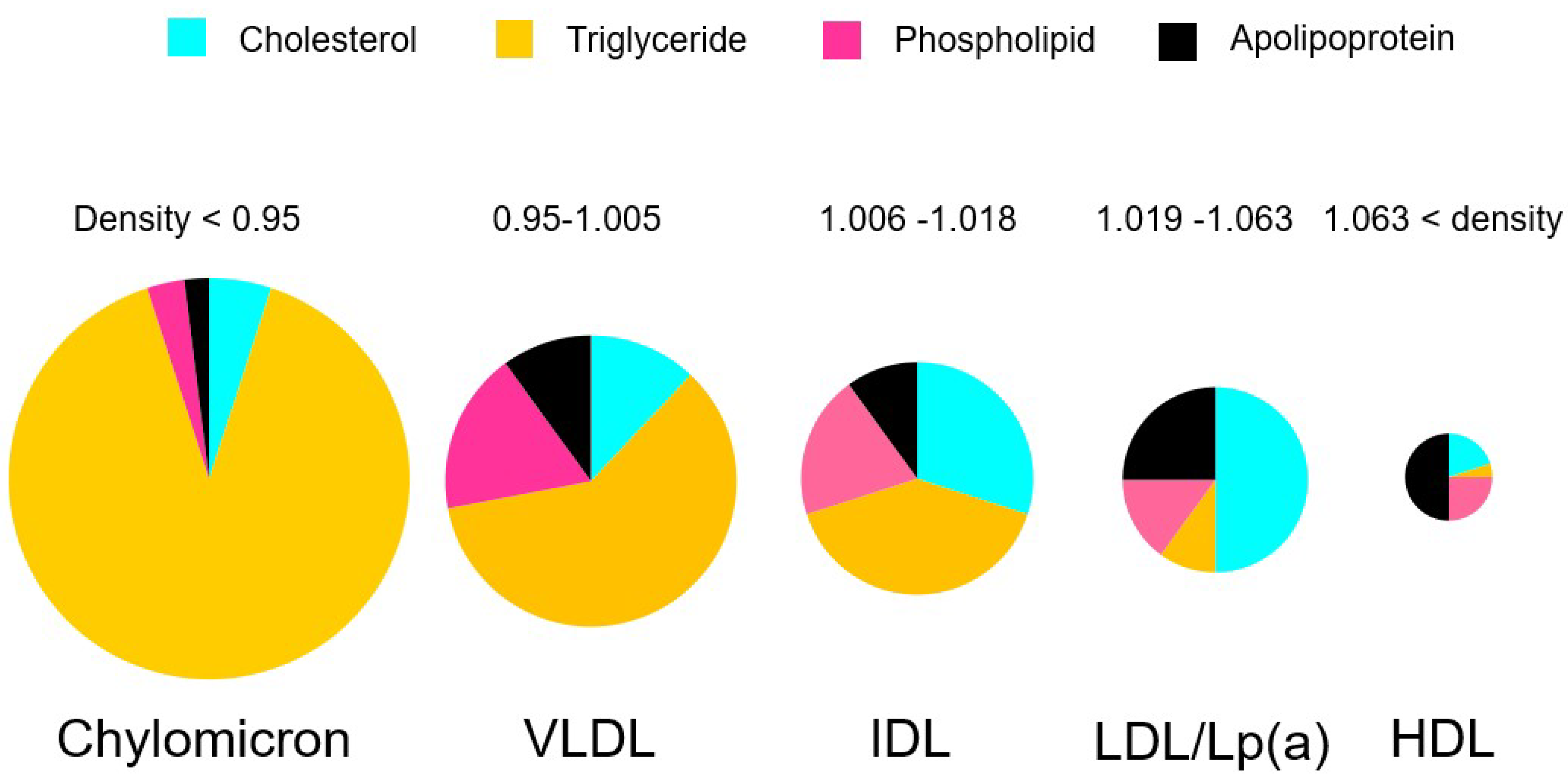
2. What Are Triglycerides?
3. Triglycerides and ASCVD
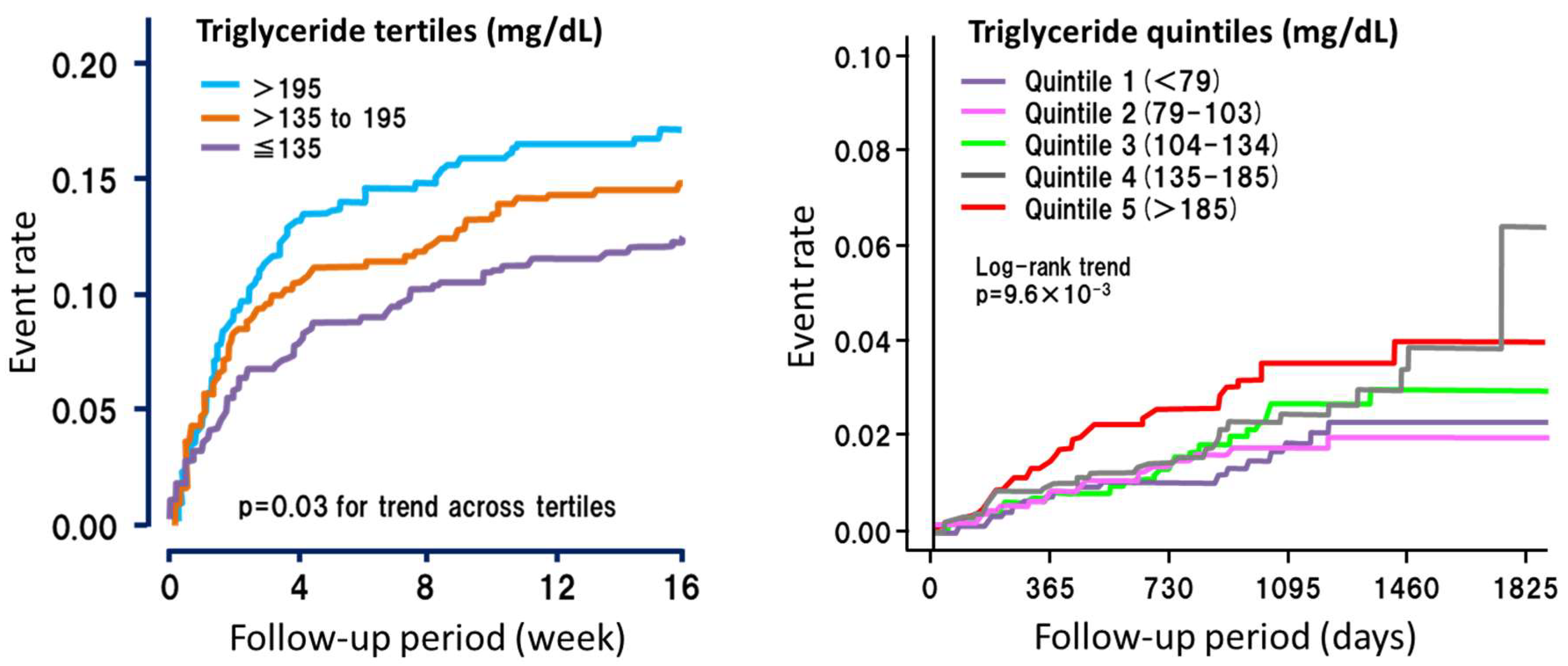
4. Lessons from Extreme Cases (Severe Hypertriglyceridemia)
5. Interventions for Hypertriglyceridemia
6. Fasting State or Post-prandial (Non-fasting) State?
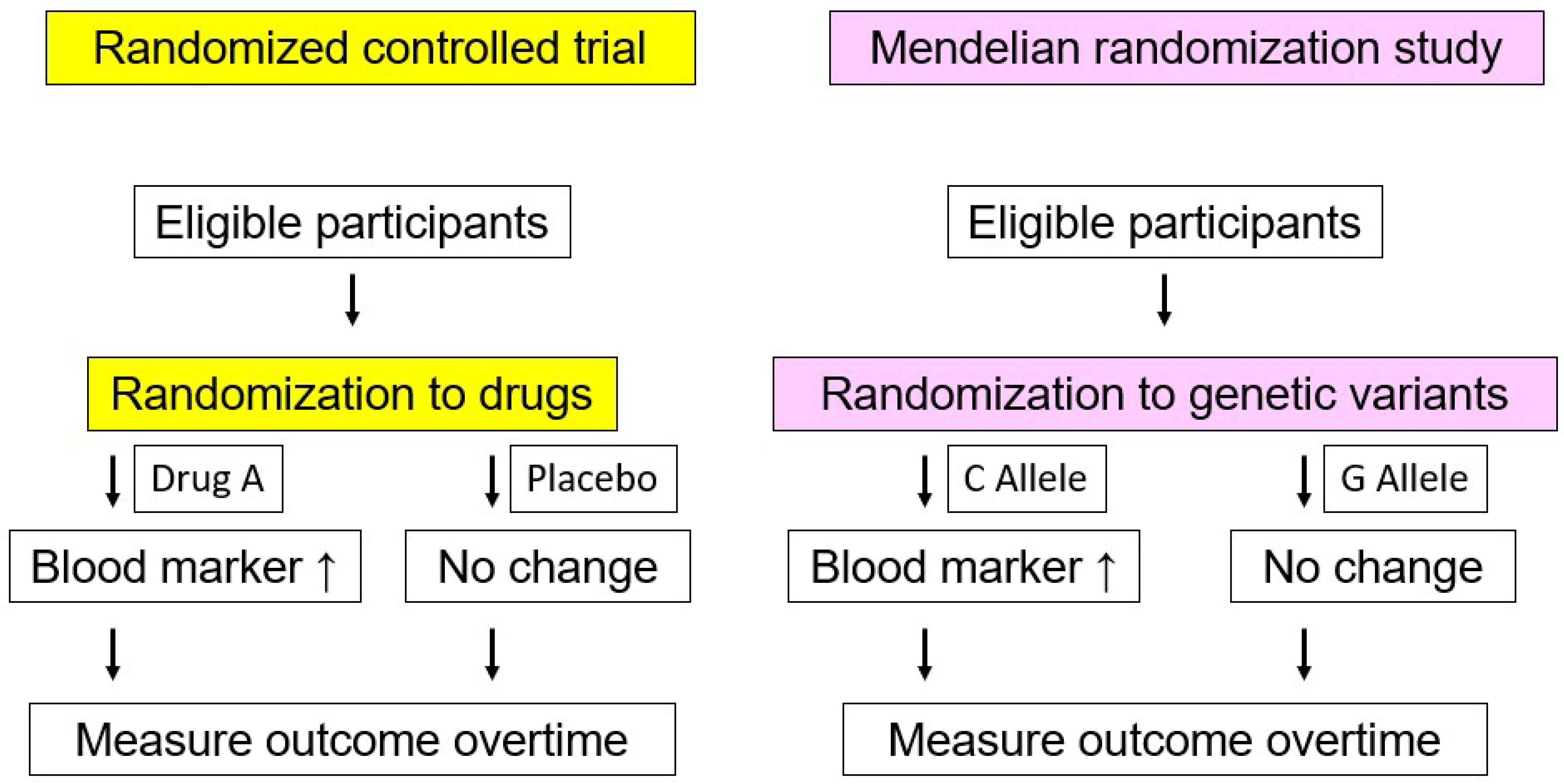
7. Residual Risk Factor for ASCVD
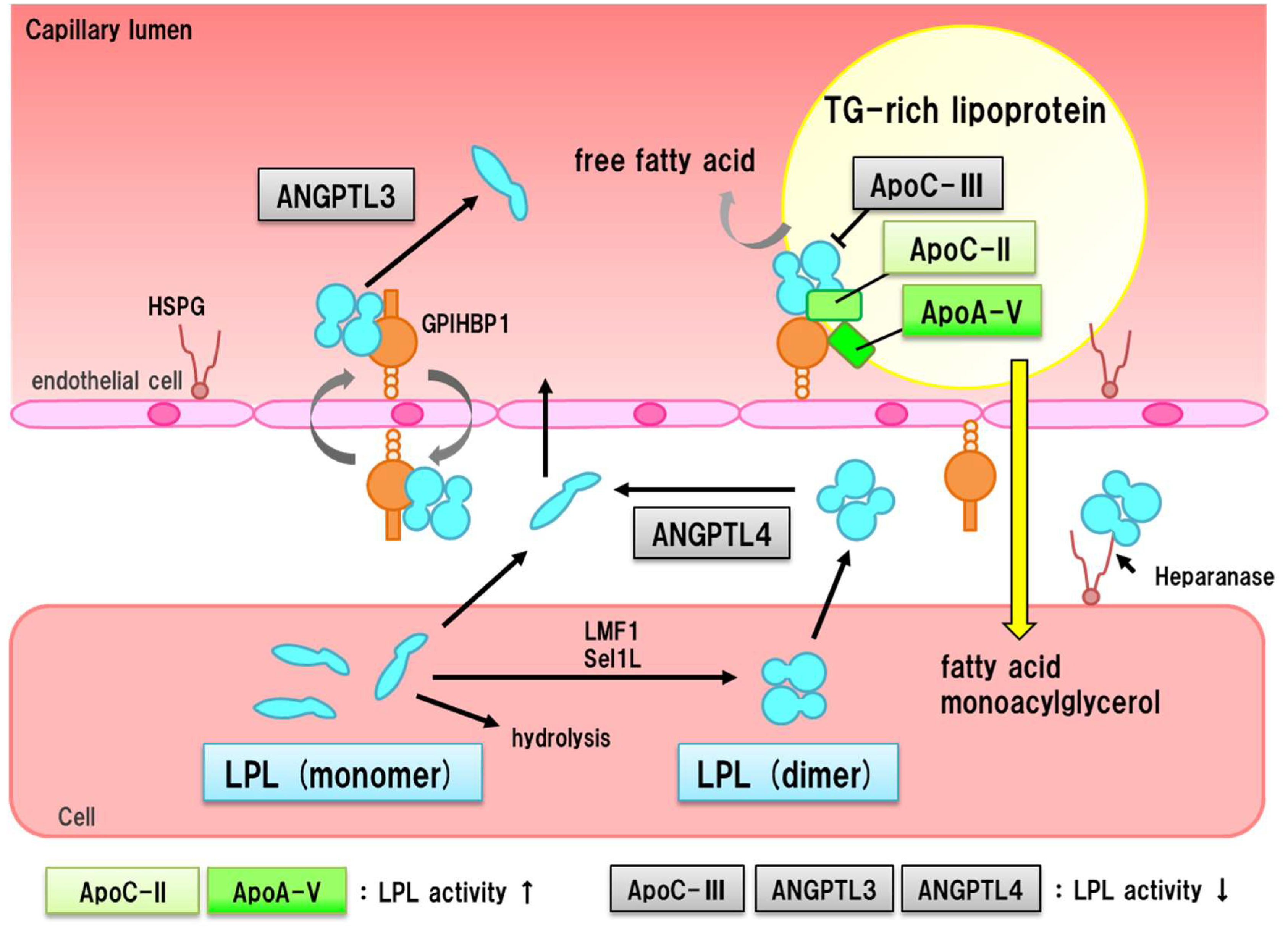
8. LPL Pathway and ASCVD
9. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Trialists, C.T. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Nordestgaard, B.G. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Nomura, A.; Yoshimura, K.; Itoh, H.; Komuro, I.; Yamagishi, M. Serum triglycerides predict first cardiovascular events in diabetic patients with hypercholesterolemia and retinopathy. Eur. J. Prev. Cardiol. 2018, 25, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: A contemporary population-based study. Eur. Heart J. 2018, 39, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar] [PubMed]
- Proctor, S.D.; Mamo, J.C. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Segrest, J.P.; Smith, K.; Griffin, F.M.; Brouillette, C.G. Lipolytic surface remnants of triglyceride-rich lipoproteins are cytotoxic to macrophages but not in the presence of high density lipoprotein. A possible mechanism of atherogenesis? J. Clin. Investig. 1989, 83, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Tomono, S.; Kawazu, S.; Kato, N.; Ono, T.; Ishii, C.; Ito, Y.; Shimizu, M.; Shimoyama, M.; Nakano, T.; Nakajima, K. Uptake of remnant like particles (RLP) in diabetic patients from mouse peritoneal macrophages. J. Atheroscler. Thromb. 1994, 1, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Nohara, A.; Sakata, K.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Remnant-like particles and coronary artery disease in familial hypercholesterolemia. Clin. Chim. Acta 2018, 482, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Yamagishi, M. Comprehensive genotyping in dyslipidemia: Mendelian dyslipidemias caused by rare variants and Mendelian randomization studies using common variants. J. Hum. Genet. 2017, 62, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Won, H.H.; Peloso, G.M.; O’Dushlaine, C.; Liu, D.; Stitziel, N.O.; Natarajan, P.; Nomura, A.; Emdin, C.A.; Gupta, N.; et al. Association of Rare and Common Variation in the lipoprotein lipase gene with coronary artery disease. JAMA 2017, 317, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.J.; Peloso, G.M.; Yu, H.; Butterworth, A.S.; Wang, X.; Mahajan, A.; Saleheen, D.; Emdin, C.; Alam, D.; Alves, A.C.; et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017, 49, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A. Genetic variations, triglycerides, and atherosclerotic disease. J. Atheroscler. Thromb. 2018, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Nakahashi, T.; Yagi, K.; Chujo, D.; Ohbatake, A.; Mori, Y.; Mori, S.; Kometani, M.; Fujii, H.; et al. Clinical characteristics of Japanese patients with severe hypertriglyceridemia. J. Clin. Lipidol. 2015, 9, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, R.; Tada, H.; Kawashiri, M.A.; Nohara, A.; Nakahashi, T.; Konno, T.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Molecular and functional characterization of familial chylomicronemia syndrome. Atherosclerosis 2018, 269, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R., 3rd; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Seafood Long-Chain n-3 polyunsaturated fatty acids and cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; et al. Helsinki Heart Study.Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Bezafibrate Infarction Prevention (BIP) Study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 2000, 102, 21–27. [Google Scholar] [CrossRef]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Nelson, D.B.; Elam, M.B.; Schaefer, E.J.; Faas, F.H.; Anderson, J.W. Diabetes, plasma insulin, and cardiovascular disease: Subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT). Arch. Intern. Med. 2002, 162, 2597–2604. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.; Forder, P.; Simes, J.; Whiting, M.; Kritharides, L.; Merrifield, A.; Donoghoe, M.; Colman, P.G.; Graham, N.; Haapamäki, H.; et al. Associations between the use of metformin, sulphonylureas, or diet alone and cardiovascular outcomes in 6005 people with type 2 diabetes in the FIELD study. Diabetes Res. Clin. Pract. 2011, 94, 284–290. [Google Scholar] [CrossRef] [PubMed]
- ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [PubMed]
- Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: The Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet 2001, 357, 905–910. [Google Scholar] [CrossRef]
- Fruchart, J.C. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor α modulator for management of atherogenic dyslipidaemia. Cardiovasc. Diabetol. 2017, 16, 124. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; K-877 Study Group. Efficacy and safety of Pemafibrate Versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: A multicenter, placebo-controlled, double-blind, randomized trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD012345. [Google Scholar] [CrossRef] [PubMed]
- TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute; Crosby, J.; Peloso, G.M.; Auer, P.L.; Crosslin, D.R.; Stitziel, N.O.; Lange, L.A.; Lu, Y.; Tang, Z.Z.; Zhang, H.; et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 2014, 371, 22–31. [Google Scholar]
- Gaudet, D.; Alexander, V.J.; Baker, B.F.; Brisson, D.; Tremblay, K.; Singleton, W.; Geary, R.S.; Hughes, S.G.; Viney, N.J.; Graham, M.J.; et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 2015, 373, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Blaha, M.J.; Elshazly, M.B.; Toth, P.P.; Kwiterovich, P.O.; Blumenthal, R.S.; Jones, S.R. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013, 310, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, J.J.; Tybjaerg-Hansen, A.; Jensen, J.S.; Nordestgaard, B.G. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA 2008, 300, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (Ascot-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar] [PubMed]
- SEARCH Study Collaborative Group; Bowman, L.; Armitage, J.; Bulbulia, R.; Parish, S.; Collins, R. Study of the effectiveness of additional reductions in cholesterol and homocysteine (SEARCH): Characteristics of a randomized trial among 12064 myocardial infarction survivors. Am. Heart J. 2007, 154, 815–823. [Google Scholar] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordonz, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Matikainen, N.; Adiels, M.; Taskinen, M.R. Postprandial hypertriglyceridemia as a coronary risk factor. Clin. Chim. Acta 2014, 431, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, MA.; Tanaka, A.; Nakano, T.; Nakajima, K.; Inoue, T.; Noguchi, T.; Nakanishi, C.; Konno, T.; Hayashi, K.; et al. Post-prandial remnant lipoprotein metabolism in autosomal recessive hypercholesterolaemia. Eur. J. Clin. Investig. 2012, 42, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Nomura, A.; Nohara, A.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Kawashiri, M.A. Post-prandial Remnant Lipoprotein Metabolism in Sitosterolemia. J. Atheroscler. Thromb. 2018. [Google Scholar] [CrossRef] [PubMed]
- Reith, C.; Armitage, J. Management of residual risk after statin therapy. Atherosclerosis 2016, 245, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Abt, M.; Bao, W.; DeMicco, D.; Kallend, D.; Miller, M.; Mundl, H.; Olsson, A.G. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 2015, 65, 2267–2275. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Okajima, F.; Miyashita, K.; Imamura, S.; Kobayashi, J.; Stanhope, K.L.; Havel, P.J.; Machida, T.; Sumino, H.; Murakami, M.; et al. The majority of lipoprotein lipase in plasma is bound to remnant lipoproteins: A new definition of remnant lipoproteins. Clin. Chim. Acta 2016, 461, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Do, R.; Stitziel, N.O.; Won, H.H.; Jørgensen, A.B.; Duga, S.; Angelica Merlini, P.; Kiezun, A.; Farrall, M.; Goel, A.; Zuk, O.; et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015, 518, 102–106. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, H.; Nohara, A.; Kawashiri, M.-a. Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies. Nutrients 2018, 10, 1789. https://doi.org/10.3390/nu10111789
Tada H, Nohara A, Kawashiri M-a. Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies. Nutrients. 2018; 10(11):1789. https://doi.org/10.3390/nu10111789
Chicago/Turabian StyleTada, Hayato, Atsushi Nohara, and Masa-aki Kawashiri. 2018. "Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies" Nutrients 10, no. 11: 1789. https://doi.org/10.3390/nu10111789
APA StyleTada, H., Nohara, A., & Kawashiri, M.-a. (2018). Serum Triglycerides and Atherosclerotic Cardiovascular Disease: Insights from Clinical and Genetic Studies. Nutrients, 10(11), 1789. https://doi.org/10.3390/nu10111789




