Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BE
2.2. Animal Experiment Design
2.3. Forelimb Grip Strength Test
2.4. Swimming Exercise Performance Test
2.5. Lactate, BUN, and GLU Levels after Acute Exercise Challenge
2.6. Clinical Biochemical Analysis after Sacrifice of the Animals
2.7. Tissue Glycogen Level Measurement and Visceral Organ Weight
2.8. Histopathological Examination
2.9. Fecal Short-Chain Fatty Acid Analysis by High-Performance Liquid Chromatography
2.10. Statistical Analysis
3. Results
3.1. Effects of BE Supplmentation on Body Weight, Skeletal Muscle Mass, Liver Weight, and Clinical Biochemistry
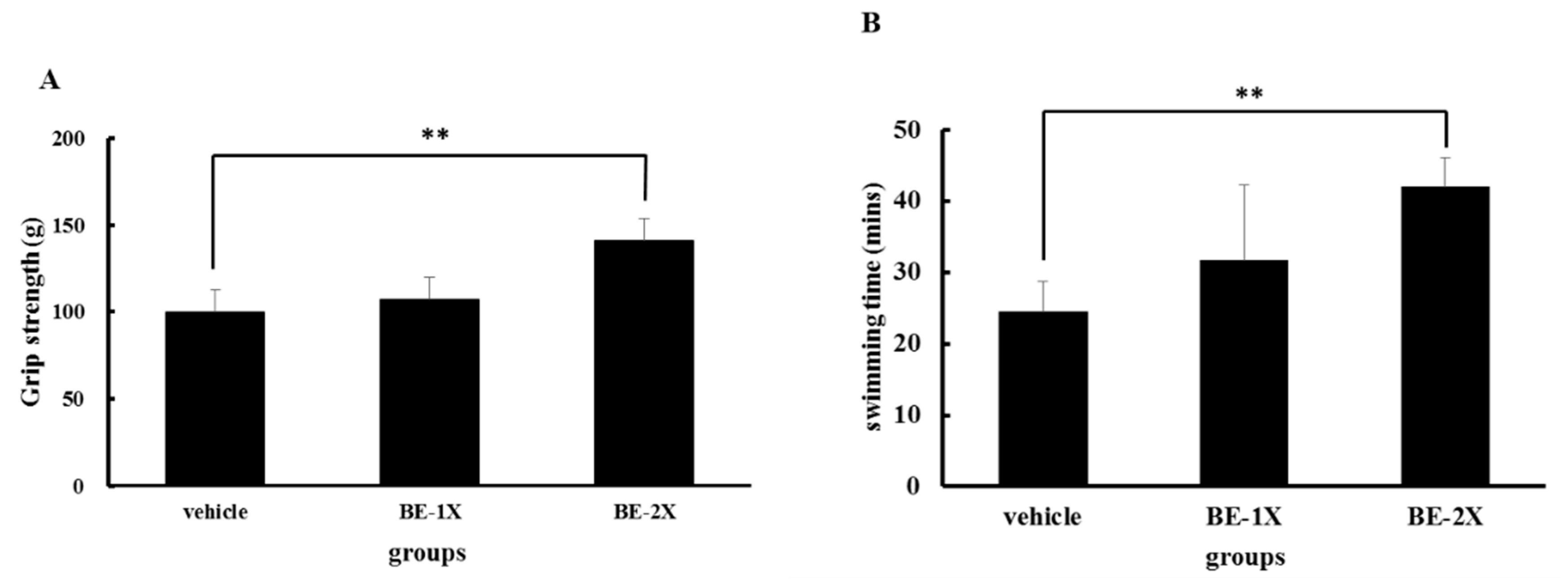
3.2. Effects of BE Supplementation on Forelimb Grip Strength and Endurance
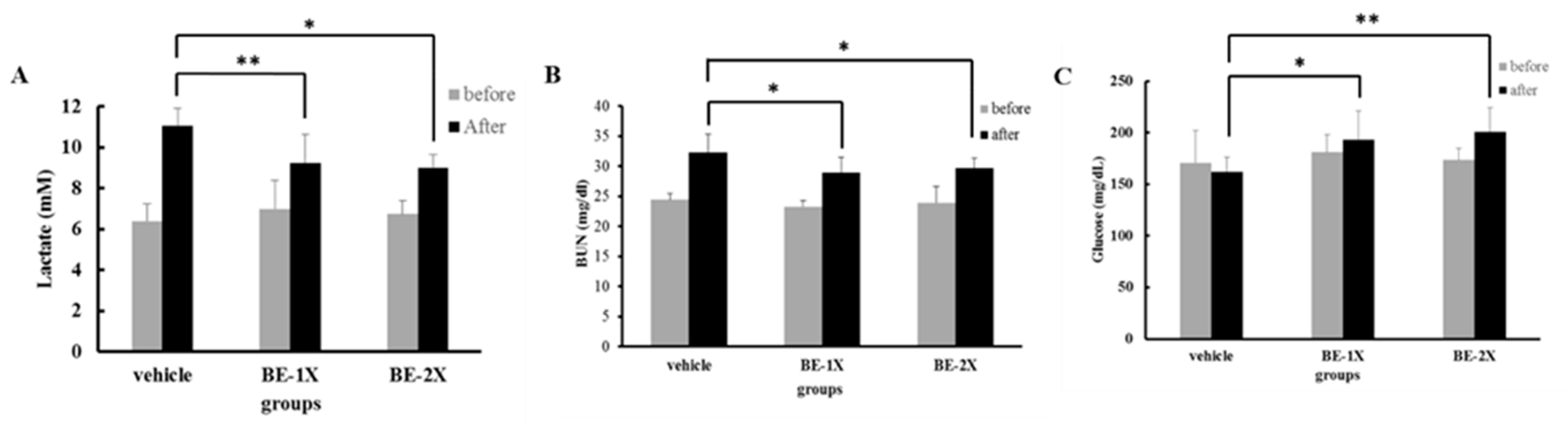
3.3. Effects of BE Supplementation on the Serum Levels of Lactate, BUN, and GLU after Acute Exercise Challenge
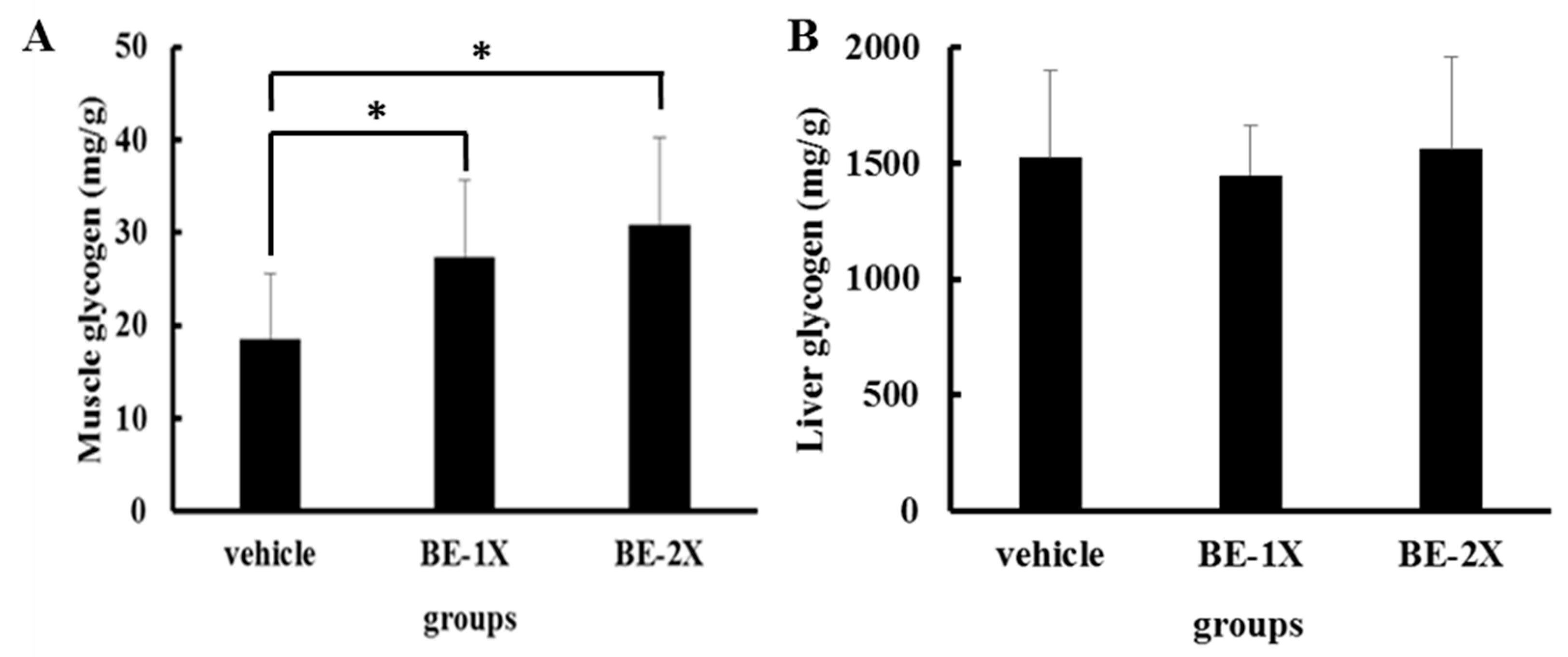
3.4. Effects of BE Supplementation on Muscular and Hepatic Glycogen Concentrations
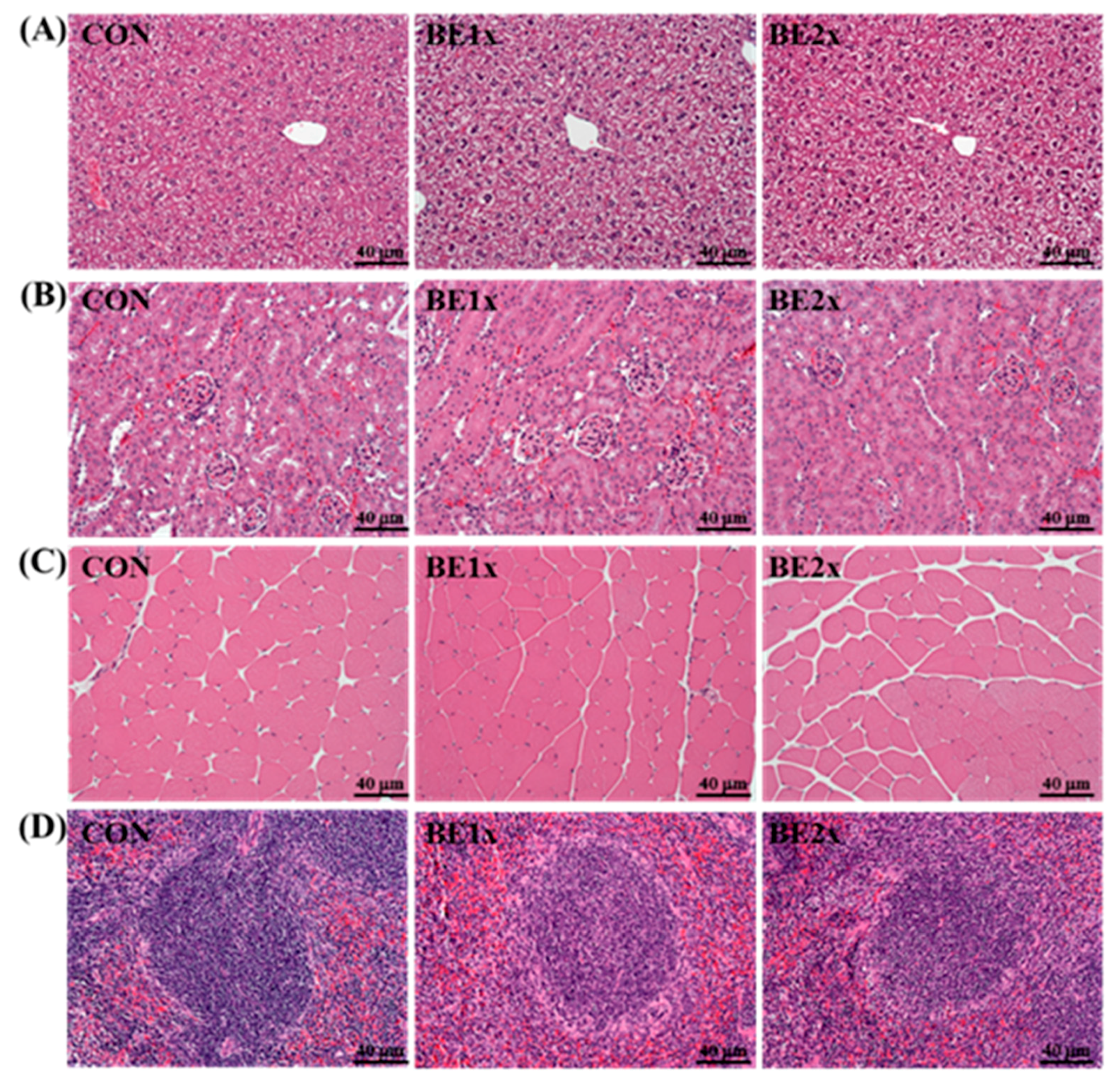
3.5. Histological Examination after BE Supplementation
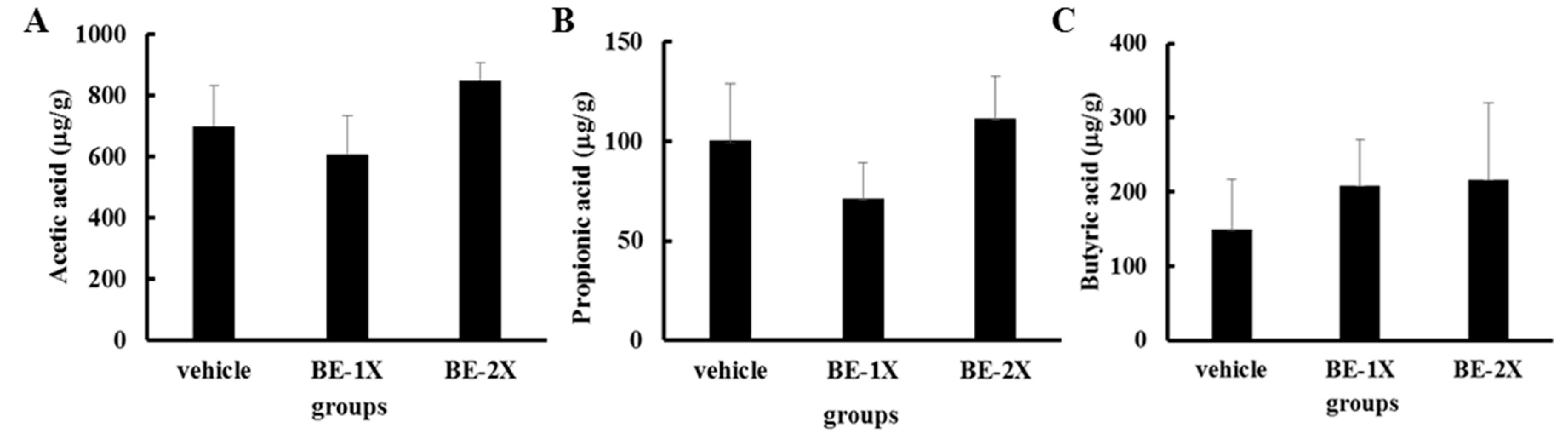
3.6. Fecal Short-Chain Fatty Acid Profiles after BE Supplementation
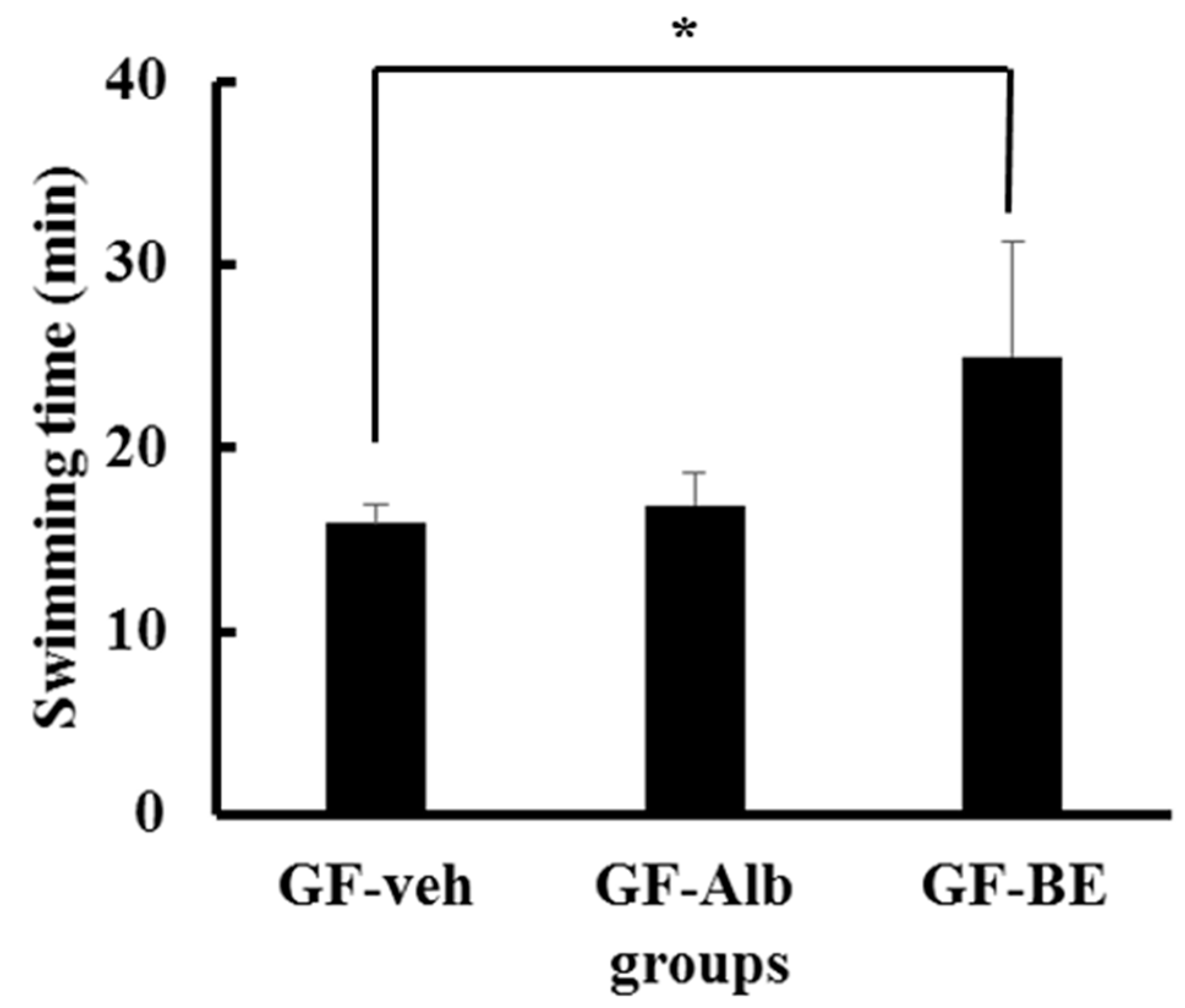
3.7. Improved Exercise Performance after BE Supplementation Is not Associated with the Gut Microbiota
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yoshihara, H.; Wakamatsu, J.-I.; Kawabata, F.; Mori, S.; Haruno, A.; Hayashi, T.; Sekiguchi, T.; Mizunoya, W.; Tatsumi, R.; Ito, T.; et al. Beef extract supplementation increases leg muscle mass and modifies skeletal muscle fiber types in rats. J. Nutr. Sci. Vitaminol. 2006, 52, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Olausson, H.; Nilsson, S.; Nookaew, I.; Khoomrung, S.; Andersson, L.; Koskela, A.; Tuukkanen, J.; Ohlsson, C.; Holmäng, A. Maternal beef and postweaning herring diets increase bone mineral density and strength in mouse offspring. Exp. Biol. Med. (Maywood) 2013, 238, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Naclerio, F.; Larumbe-Zabala, E.; Ashrafi, N.; Seijo, M.; Nielsen, B.; Allgrove, J.; Earnest, C.P. Effects of protein-carbohydrate supplementation on immunity and resistance training outcomes: A double-blind, randomized, controlled clinical trial. Eur. J. Appl. Physiol. 2017, 117, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Huang, W.-C.; Chiu, C.-C.; Chang, Y.-K.; Huang, C.-C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Yang, Y.; Moore, D.R.; Tang, J.E.; Tarnopolsky, M.A.; Phillips, S.M. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br. J. Nutr. 2012, 108, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Burd, N.A.; Breen, L.; Rerecich, T.; Yang, Y.; Hector, A.J.; Baker, S.K.; Phillips, S.M. Dose-dependent responses of myofibrillar protein synthesis with beef ingestion are enhanced with resistance exercise in middle-aged men. Appl. Physiol. Nutr. Metab. 2012, 38, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine supplementation in recovery after exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Brass, E.; Scarrow, A.; Ruff, L.; Masterson, K.; Van Lunteren, E. Carnitine delays rat skeletal muscle fatigue in vitro. J. Appl. Physiol. 1993, 75, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, Z.; Newton, M.; Sacco, P.; Nosaka, K. Effects of massage on delayed-onset muscle soreness, swelling, and recovery of muscle function. J. Athl. Train. 2005, 40, 174. [Google Scholar] [PubMed]
- Appell, H.-J.; Soares, J.; Duarte, J. Exercise, muscle damage and fatigue. Sports Med. 1992, 13, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Baker, K.M.; Kelland, R.; Lomond, J. The effect of muscle damage on strength and fatigue deficits. J. Strength Cond. Res. 2001, 15, 255–264. [Google Scholar] [PubMed]
- Wan, J.-J.; Qin, Z.; Wang, P.-Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Huang, W.-C.; Lin, J.-S.; Chen, Y.-M.; Ho, S.-T.; Huang, C.-C.; Tung, Y.-T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lin, C.-I.; Chiu, C.-C.; Lin, Y.-T.; Huang, W.-K.; Huang, H.-Y.; Huang, C.-C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, Y.H.; Kim, C.K. Biomarkers of muscle and cartilage damage and inflammation during a 200 km run. Eur. J. Appl. Physiol. 2007, 99, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, Z.; Kłapcińska, B.; Sadowska-Krępa, E.; Czuba, M.; Kempa, K.; Kimsa, E.; Gerasimuk, D. Acute metabolic responses to a 24-h ultra-marathon race in male amateur runners. Eur. J. Appl. Physiol. 2012, 112, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Lanier, A.B. Use of nonsteroidal anti-inflammatory drugs following exercise-induced muscle injury. Sports Med. 2003, 33, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Cui, C.; Sun, T.; Han, J.; Zhang, D.; Lu, C.; Zhou, J.; Cheong, L.; Li, Y. Modulation of gut microbiota by dietary supplementation with tuna oil and algae oil alleviates the effects of d-galactose-induced ageing. Appl. Microbiol. Biotechnol. 2018, 102, 2791–2801. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes Nutr. 2017, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Midtvedt, T.; Gordon, J.I. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 2002, 22, 283–307. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Chiu, C.C.; Li, Y.P.; Huang, W.C.; Te Huang, Y.; Huang, C.C.; Chuang, H.L. Effect of intestinal microbiota on exercise performance in mice. J. Strength Cond. Res. 2015, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Saint-Maurice, P.F.; Laurson, K.; Welk, G.J.; Eisenmann, J.; Gracia-Marco, L.; Artero, E.G.; Ortega, F.; Ruiz, J.R.; Moreno, L.A.; Vicente-Rodriguez, G.; et al. Grip strength cutpoints for youth based on a clinically relevant bone health outcome. Arch. Osteoporosis. 2018, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharif, F.A.-G.; Al-Jiffri, O.H.; El-Kader, S.M.A.; Ashmawy, E.M. Impact of mild versus moderate intensity aerobic walking exercise training on markers of bone metabolism and hand grip strength in moderate hemophilic A patients. Afr. Health Sci. 2014, 14, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Miura, K.; Suzuki, K.; Bannai, M. Leucine-enriched essential amino acids augment muscle glycogen content in rats seven days after eccentric contraction. Nutrients 2017, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Campos-Ferraz, P.L.; Bozza, T.; Nicastro, H.; Lancha Jr, A.H. Distinct effects of leucine or a mixture of the branched-chain amino acids (leucine, isoleucine, and valine) supplementation on resistance to fatigue, and muscle and liver-glycogen degradation, in trained rats. Nutrition 2013, 29, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic acid and exercise performance. Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Miyatake, S.; Takagi, M.; Nakamura, M.; Okeda, A.; Nakano, T.; Hirshman, M.F.; Goodyear, L.J.; Fujii, N.L. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS ONE 2012, 7, e52592. [Google Scholar] [CrossRef] [PubMed]
- Ivy, J.L. Regulation of muscle glycogen repletion, muscle protein synthesis and repair following exercise. J. Sports Sci. Med. 2004, 3, 131–138. [Google Scholar] [PubMed]
- Hausswirth, C.; Le Meur, Y. Physiological and nutritional aspects of post-exercise recovery. Sports Med. 2011, 41, 861–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rutten, N.; Besseling-van der Vaart, I.; Niers, L.; Choi, Y.; Rijkers, G.; van Hemert, S. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef. Microbes 2015, 6, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Wei, L.; Chiu, Y.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Wang, M.-F.; Huang, C.-C. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
| Nutritional Facts | Content (/100 mL of BE) |
|---|---|
| Protein | 7.7 g |
| Fat | 0 |
| Saturated fat | 0 |
| Trans fat | 0 |
| Carbohydrate | 0 |
| Sodium | 0.094 g |
| Total calories (kcal) | 30 |
| Total BCAA (mg/100 g) | |
| Valine, leucine, and isoleucine | 18.6 |
| Characteristics | Vehicle | BE-1X | BE-2X |
|---|---|---|---|
| Initial BW (g) | 25.5 ± 1.3 | 25.8 ± 1.4 | 26.1 ± 1.4 |
| Final BW (g) | 25.6 ± 1.5 | 26.4 ± 1.3 | 26.2 ± 1.7 |
| Skeletal muscle (g) | 0.32 ± 0.03 | 0.33 ± 0.02 | 0.33 ± 0.03 |
| Liver (g) | 1.32 ± 0.10 | 1.32 ± 0.08 | 1.27 ± 0.09 |
| Parameters | Vehicle | BE-1X | BE-2X |
|---|---|---|---|
| ALT (U/L) | 48.2 ± 10.7 | 51.9 ± 9.9 | 55.2 ± 14.2 |
| GLU (mg/dL) | 233.0 ± 18.1 | 212.6 ± 19.8 | 213.5 ± 19.0 |
| BUN (mg/dL) | 24.4 ± 1.1 | 23.3 ± 1.0 | 23.9 ± 2.7 |
| T-CHO (mg/dL) | 143.8 ± 7.9 | 137.7 ± 7.3 | 156.7 ± 12.1 |
| TG (mg/dL) | 241.8 ± 67.1 | 195.7 ± 42.0 | 216.5 ± 25.3 |
| Characteristics | GF-veh | GF-Alb | GF-BE |
|---|---|---|---|
| Initial BW (g) | 26.8 ± 1.0 | 26.3 ± 1.8 | 27.8 ± 1.7 |
| Final BW (g) | 27.8 ± 0.7 | 28.4 ± 1.7 | 27.9 ± 0.9 |
| Skeletal muscle (g) | 0.30 ± 0.02 | 0.30 ± 0.02 | 0.29 ± 0.01 |
| ALT (U/L) | 46.2 ± 9.7 | 49.3 ± 13.5 | 55.0 ± 0.9 |
| GLU (mg/dL) | 221.0 ± 11.8 | 201.4 ± 28.1 | 211.9 ± 36.7 |
| BUN (mg/dL) | 23.9 ± 1.1 | 21.3 ± 3.0 | 22.9 ± 2.0 |
| T-CHO (mg/dL) | 193.8 ± 16.7 | 195.3 ± 22.1 | 195.9 ± 8.9 |
| TG (mg/dL) | 121.0 ± 25.1 | 99.2 ± 17.0 | 108.1 ± 13.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-H.; Chiu, C.-C.; Wang, Y.-C.; Chen, T.-H.; Chen, Y.-H.; Lee, Y.-P.; Hung, S.-W.; Wu, C.-P.; Chuang, H.-L. Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota. Nutrients 2018, 10, 1740. https://doi.org/10.3390/nu10111740
Hsu T-H, Chiu C-C, Wang Y-C, Chen T-H, Chen Y-H, Lee Y-P, Hung S-W, Wu C-P, Chuang H-L. Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota. Nutrients. 2018; 10(11):1740. https://doi.org/10.3390/nu10111740
Chicago/Turabian StyleHsu, Tsung-Hsien, Chien-Chao Chiu, Yu-Chih Wang, Ter-Hsin Chen, Yi-Hsun Chen, Yen-Peng Lee, Shao-Wen Hung, Chean-Ping Wu, and Hsiao-Li Chuang. 2018. "Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota" Nutrients 10, no. 11: 1740. https://doi.org/10.3390/nu10111740
APA StyleHsu, T.-H., Chiu, C.-C., Wang, Y.-C., Chen, T.-H., Chen, Y.-H., Lee, Y.-P., Hung, S.-W., Wu, C.-P., & Chuang, H.-L. (2018). Supplementation with Beef Extract Improves Exercise Performance and Reduces Post-Exercise Fatigue Independent of Gut Microbiota. Nutrients, 10(11), 1740. https://doi.org/10.3390/nu10111740






