Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation of Polyphenols from Aronia Berries
2.4. Evaluation of Anti-Adipogenic Activities in 3T3-L1 Cells
2.4.1. Cell Culture
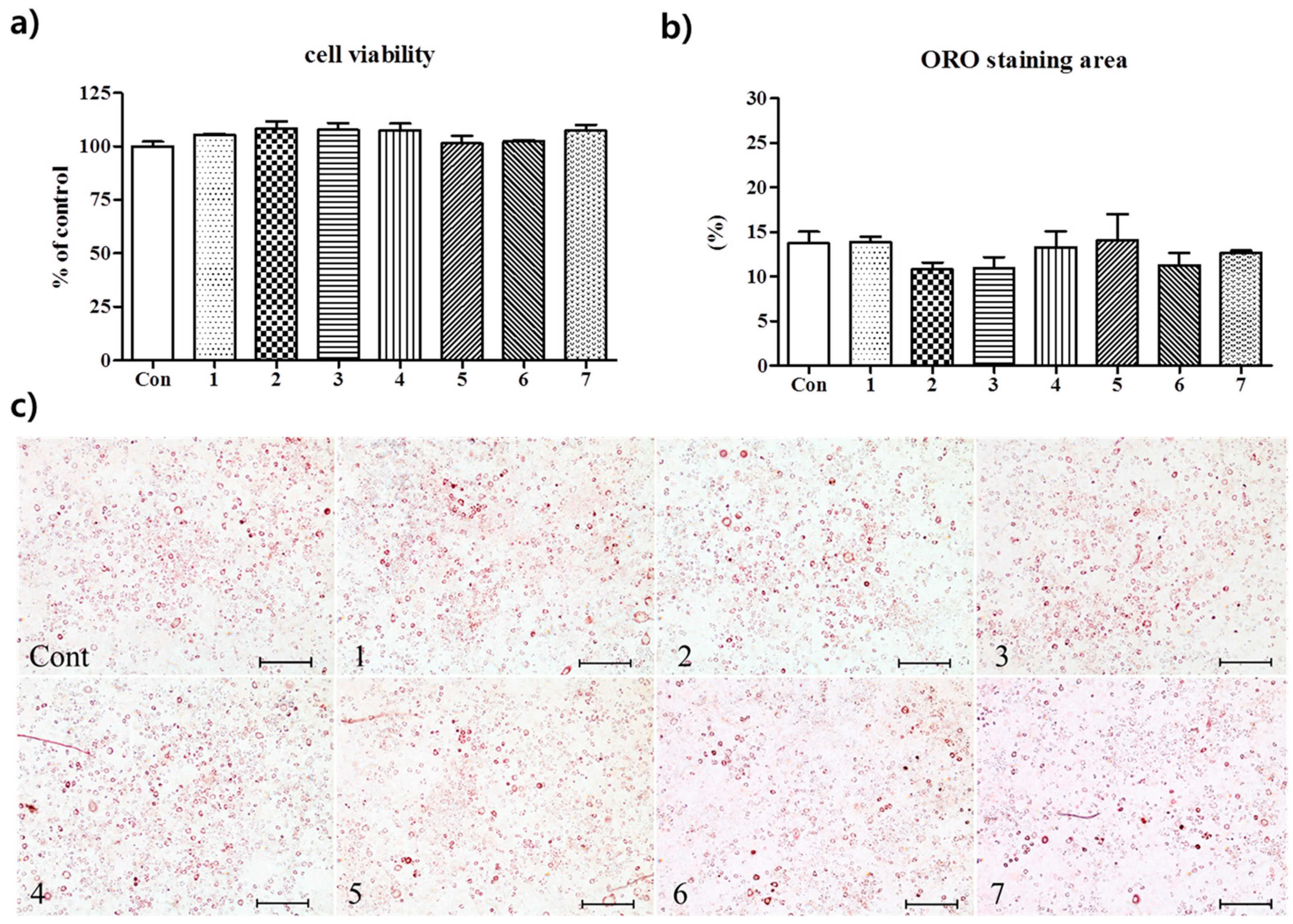
2.4.2. Oil Red O Staining
2.4.3. Measurement of Cell Proliferation
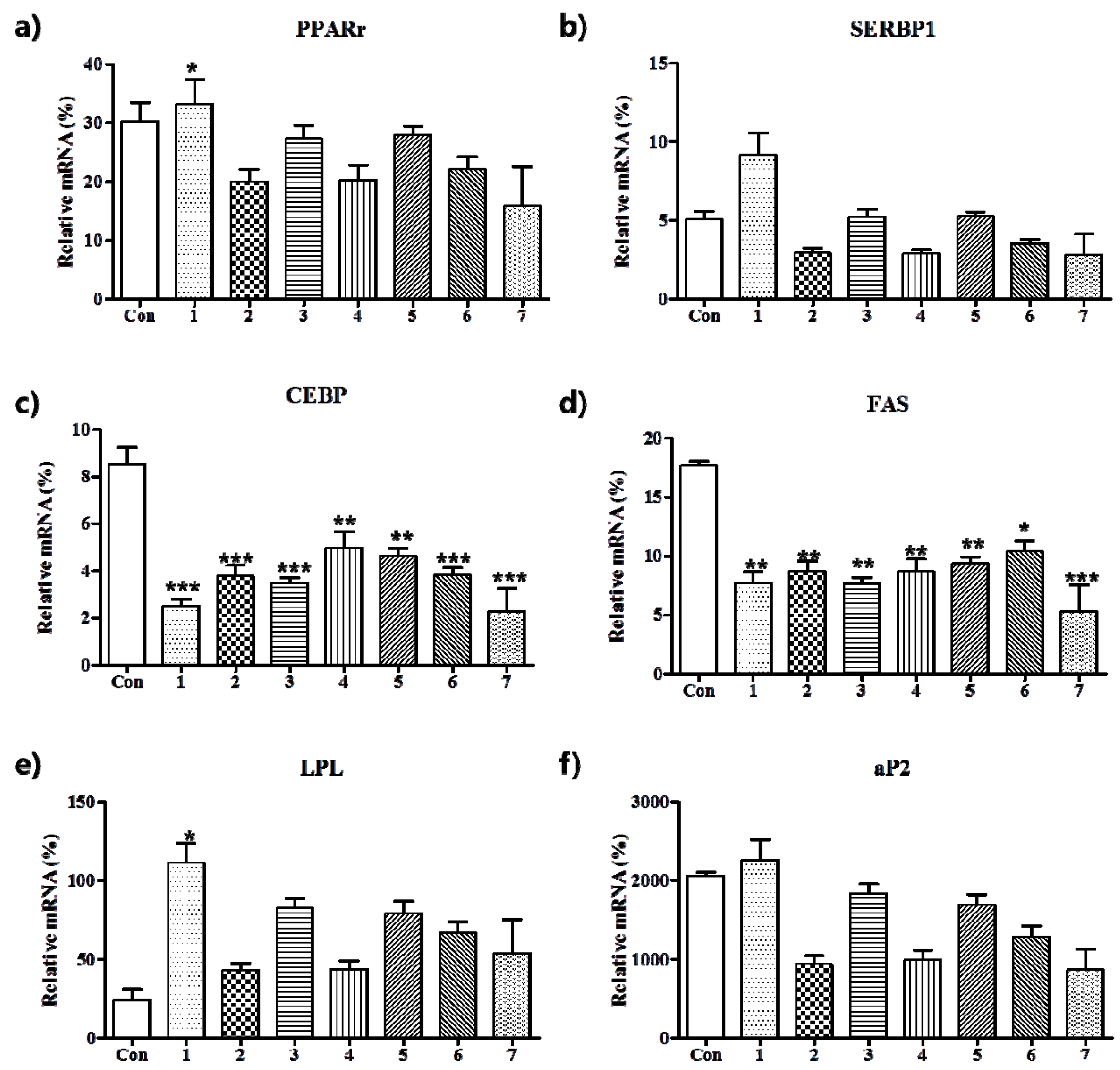
2.4.4. Real-Time RT-PCR
2.5. Evaluation of Anti-Obesity Effects in HFD-Induced Obese Mice
2.5.1. Sample Preparations
2.5.2. Animals and Diets
2.5.3. OGTT and IPITT Tests
2.5.4. Body and Adipose Tissue Weights Food Consumption and Biochemical Analysis of Serum Samples
2.6. Statistical Analysis
3. Results and Discussion
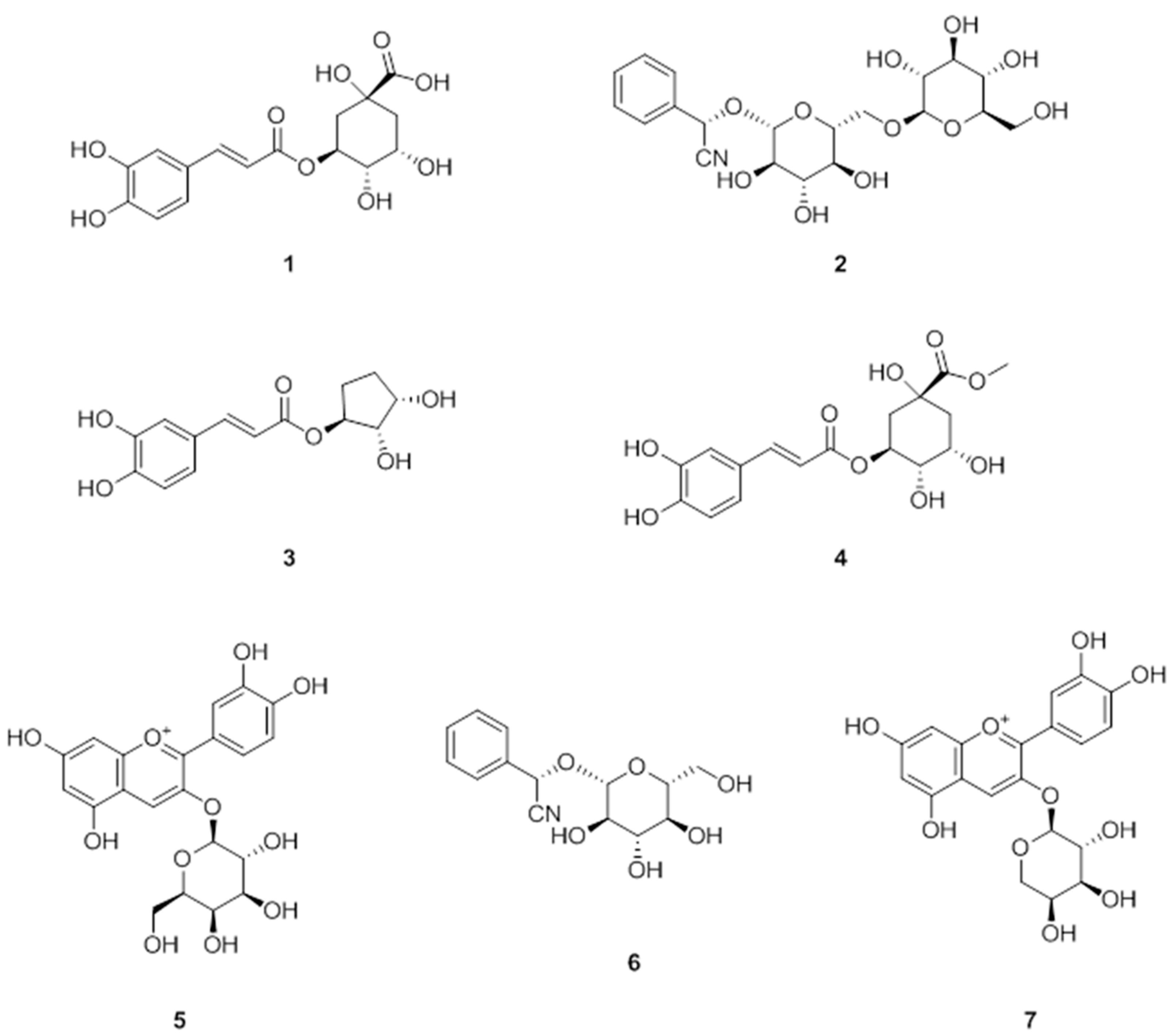
3.1. Isolation of Polyphenols from A. melanocarpa
3.2. Anti-Adipogenic Effects of the Seven Polyphenols on 3t3-L1 Preadipocytes
3.3. Anti-Obesity Effects of A. melanocarpa Extract in HFD-Induced Obese Mice
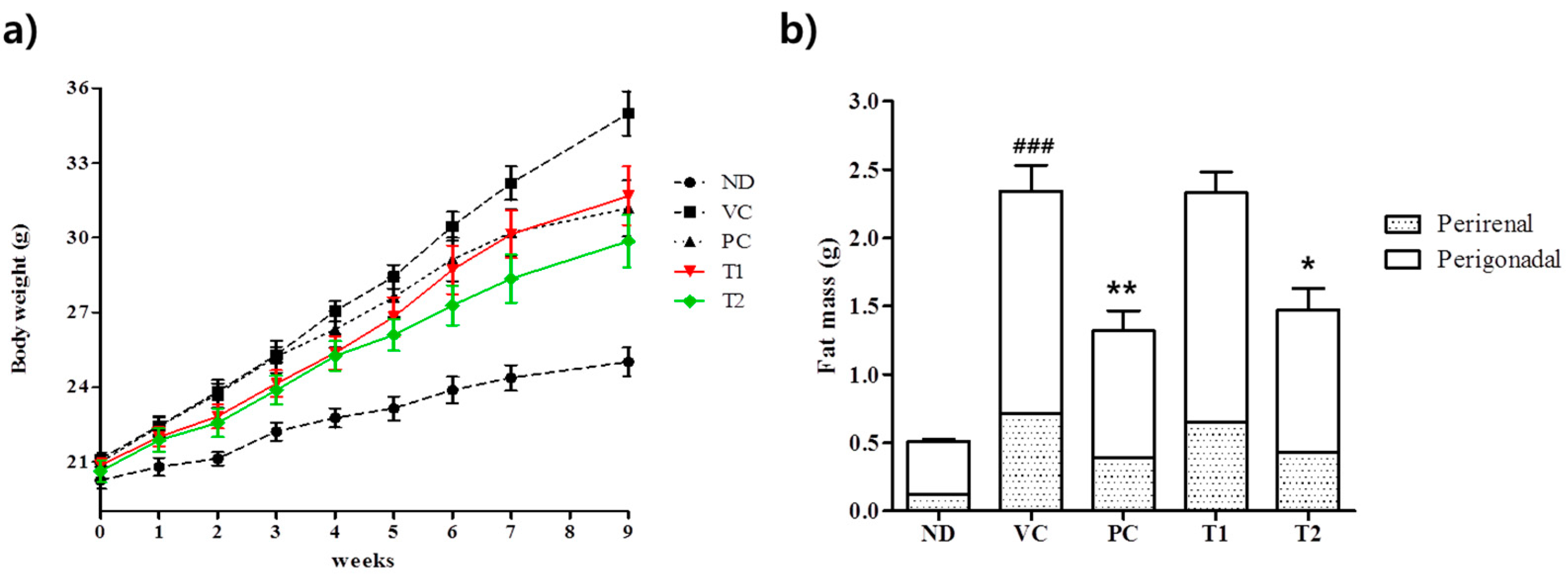
3.3.1. Effects of A. melanocarpa Extract on Body Weight Gains and Adipose Tissue Weights in HFD-Induced Obese Mice
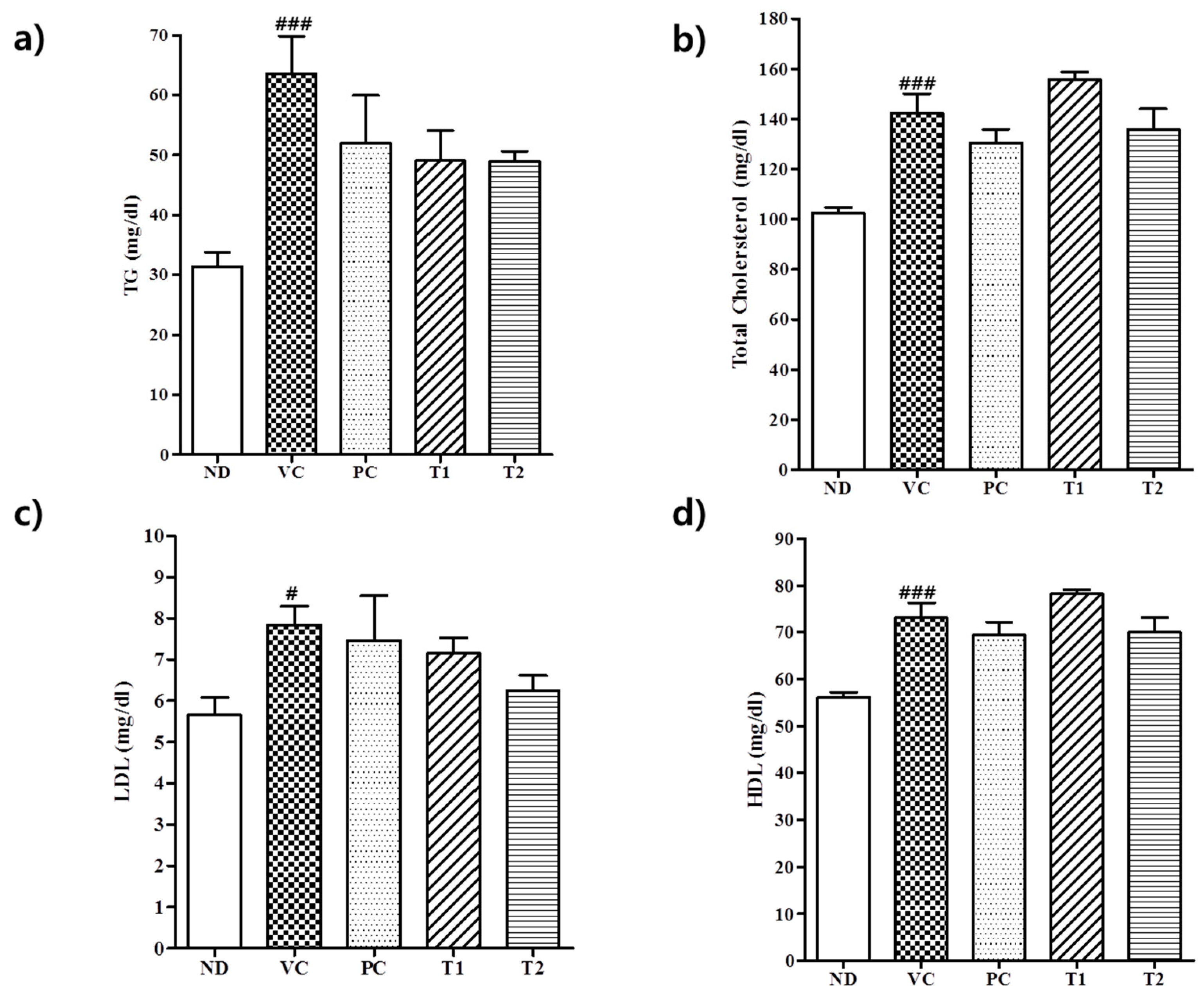
3.3.2. Effect of A. melanocarpa Extract on Serum Lipid Levels in HFD Mice
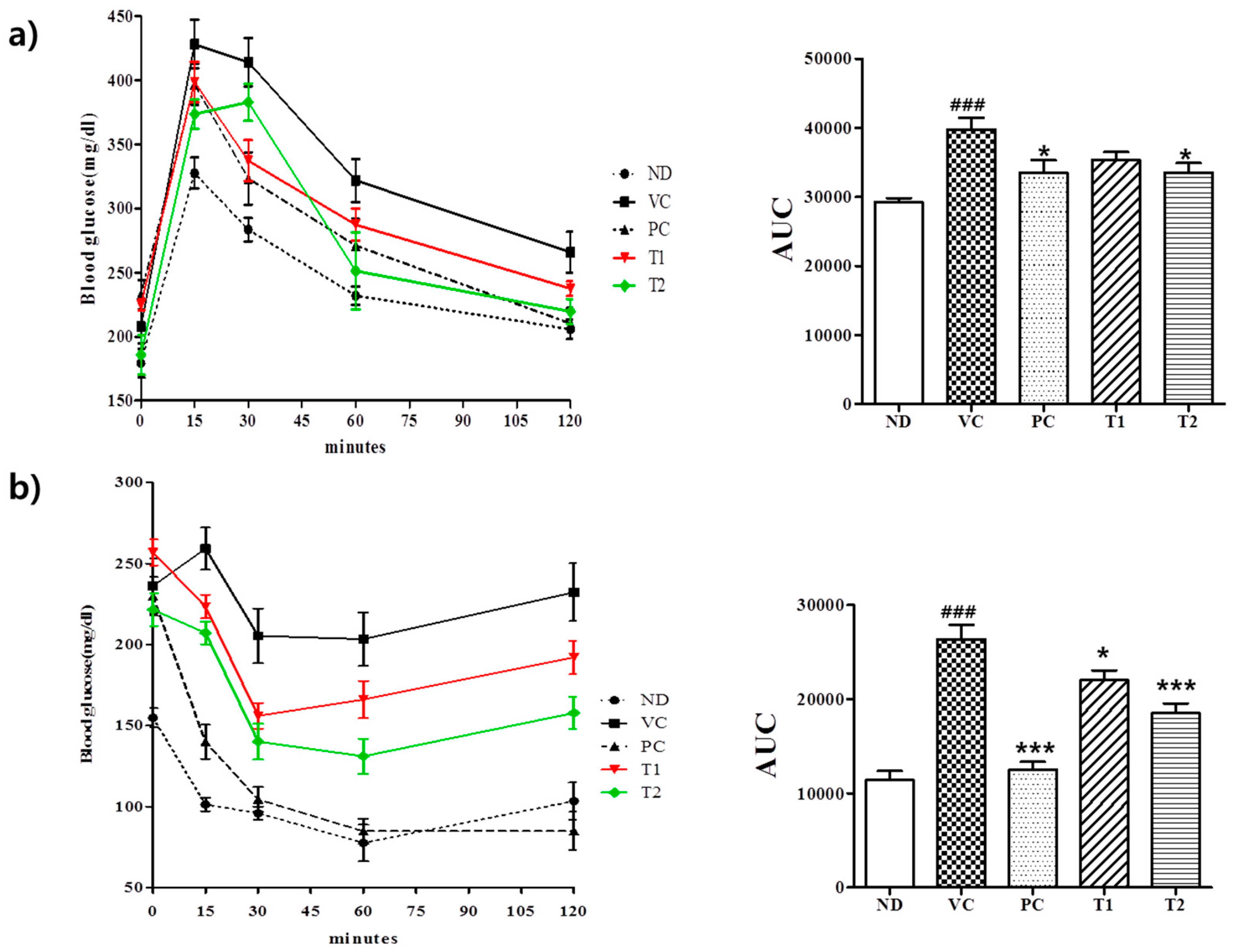
3.3.3. Effects of A. melanocarpa Extract on HFD-Induced Glucose Intolerance and Insulin Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PPARγ | peroxisome proliferator-activated receptor γ |
| C/EBPα | CCAAT/enhancer binding protein α |
| SREBP1c | Sterol regulatory element binding protein 1c |
| FAS | Fatty acid synthase |
| aP2 | Adipocyte fatty-acid–binding protein |
| TG | Triglyceride |
| TC | Total cholesterol |
| LDLC | Low-density lipoprotein cholesterol |
| HDLC | High-density lipoprotein cholesterol |
| OGTT | Oral glucose tolerance test |
| IPITT | Intraperitoneal insulin tolerance test |
References
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Pozza, C.; Isidori, A.M. What’s behind the obesity epidemic. In Imaging in Bariatric Surgery; Springer: Cham, Switzerland, 2018; pp. 1–8. [Google Scholar]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Moustaid-Moussa, N. Symposium: Adipocyte function, differentiation and metabolism. J. Nutr. 2000, 130, 3110S–3115S. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or hyperplasia: Dynamics of adipose tissue growth. PLOS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Signalling role of adipose tissue: Adipokines and inflammation in obesity. Biochem. Soc. Trans. 2005, 33, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2014, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The Major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.V.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2006, 48, 11–17. [Google Scholar]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compost. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food. 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Borisova, P.; Galunska, B.; Krasnaliev, I.; Belcheva, A. Hepatoprotective effect of the natural fruit juice from Aronia melanocarpa on carbon tetrachloride-induced acute liver damage in rats. Exp. Toxicol. Pathol. 2004, 56, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłużniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Vinué, Á.; González-Navarro, H. Glucose and insulin tolerance tests in the mouse. Methods Mol. Biol. 2015, 1339, 247–254. [Google Scholar] [PubMed]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.H.; Hellström, J.; Törrönen, A.R. Phenolic acids in berries, fruits, and beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. Sublines of mouse 3T3 cells that accumulate lipid. Cell 1974, 1, 113–116. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Kim, J.B.; Sarraf, P.; Wright, M.; Mueller, E.; Lowell, B.B.; Spiegelman, B.M. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Investig. 1998, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Chung, S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food. Chem. 2011, 59, 3666–3673. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Rahman, M.M.; Williams, P.J.; Fernandes, G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J. Nutr. Biochem. 2010, 21, 1162–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.; Cabezas, M. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Relation of high TG–low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: An 8-year follow-up in the Copenhagen male study. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Farese, R.V., Jr. Inhibition of triglyceride synthesis as a treatment strategy for obesity: Lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Paccaud, F.; Schiuter-Fasmeyer, V.; Wietlisbach, V.; Bovet, P. Dyslipidemia and abdominal obesity: An assessment in three general populations. J. Clin. Epid. 2000, 53, 393–400. [Google Scholar] [CrossRef]
- Pratley, R.E.; Weyer, C.; Bogardus, C. Metabolic abnormalities in the development of noninsulin-dependent diabetes mellitus. In Diabetes Mellitus, 2nd ed.; LeRoith, D., Taylor, S.I., Olefsky, J.M., Eds.; Lippincot-Raven Publishers: Philadelphia, PA, USA, 2000; pp. 548–557. [Google Scholar]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Vettor, R.; Milan, G.; Rossato, M.; Federspil, G. Adipocytokines and insulin resistance. Aliment. Pharmacol. Ther. 2005, 22, 3–10. [Google Scholar] [CrossRef] [PubMed]
| Week | ND | VC | PC | T1 | T2 |
|---|---|---|---|---|---|
| gram per animal | |||||
| 1 | 2.35 | 2.20 | 2.10 | 2.10 | 2.20 |
| 2 | 2.00 | 2.25 | 2.55 | 2.15 | 2.20 |
| 3 | 1.95 | 2.30 | 2.20 | 2.20 | 2.20 |
| 4 | 2.40 | 2.40 | 2.40 | 2.25 | 2.05 |
| 5 | 2.95 | 2.50 | 2.90 | 2.30 | 2.15 |
| 6 | 2.45 | 2.70 | 2.40 | 2.60 | 2.45 |
| 7 | 2.65 | 2.50 | 2.15 | 2.25 | 2.45 |
| 8 | 2.55 | 2.65 | 2.35 | 2.10 | 2.30 |
| Average | 2.42 | 2.44 | 2.38 | 2.25 | 2.25 |
| S.D. | 0.33 | 0.18 | 0.26 | 0.16 | 0.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.-H.; Jegal, J.; Kim, Y.N.; Heo, J.-D.; Rho, J.-R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. https://doi.org/10.3390/nu10111734
Kim N-H, Jegal J, Kim YN, Heo J-D, Rho J-R, Yang MH, Jeong EJ. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients. 2018; 10(11):1734. https://doi.org/10.3390/nu10111734
Chicago/Turabian StyleKim, Na-Hyun, Jonghwan Jegal, Yun Na Kim, Jeong-Doo Heo, Jung-Rae Rho, Min Hye Yang, and Eun Ju Jeong. 2018. "Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice" Nutrients 10, no. 11: 1734. https://doi.org/10.3390/nu10111734
APA StyleKim, N.-H., Jegal, J., Kim, Y. N., Heo, J.-D., Rho, J.-R., Yang, M. H., & Jeong, E. J. (2018). Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients, 10(11), 1734. https://doi.org/10.3390/nu10111734






