Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
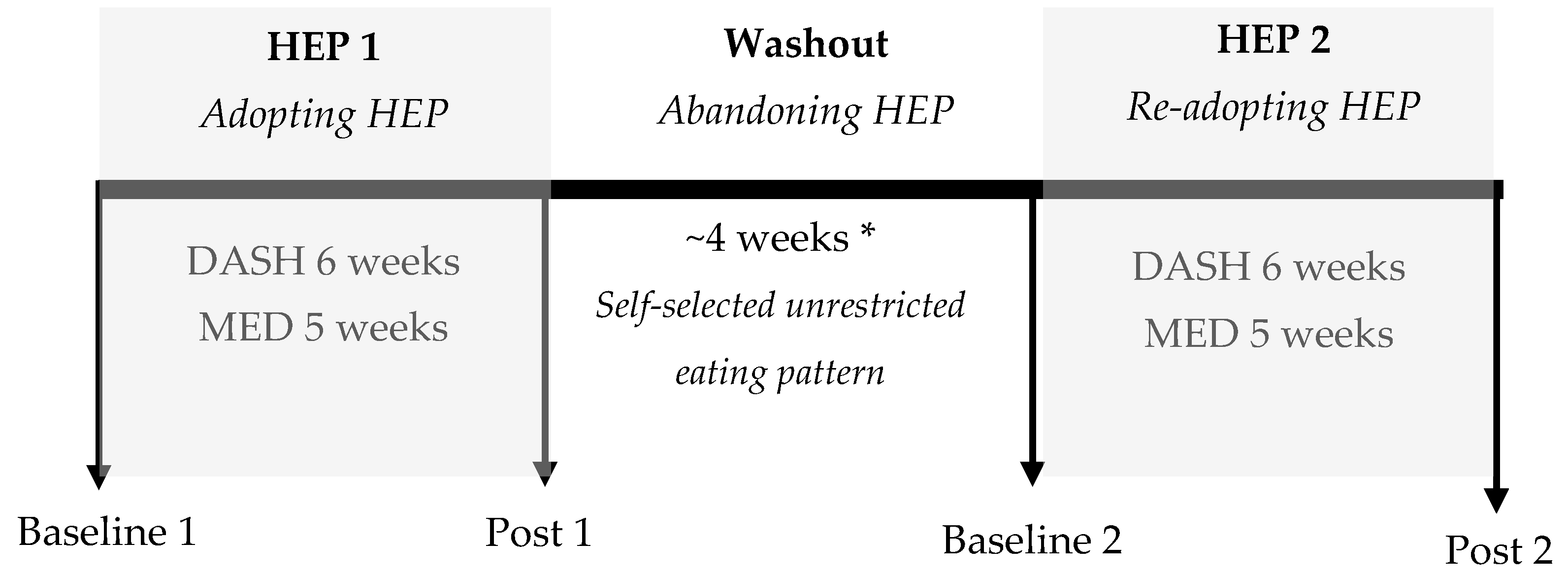
2.1. Experimental Design
2.2. Ethics
2.3. Participant Inclusion Criteria
2.4. Decsription of Prescribed HEPs
2.5. Assessment of Baseline and Prescribed Eating Patterns
2.6. Outcomes Measured
2.7. Statistics
3. Results
3.1. Participants
3.2. Dietary Intakes
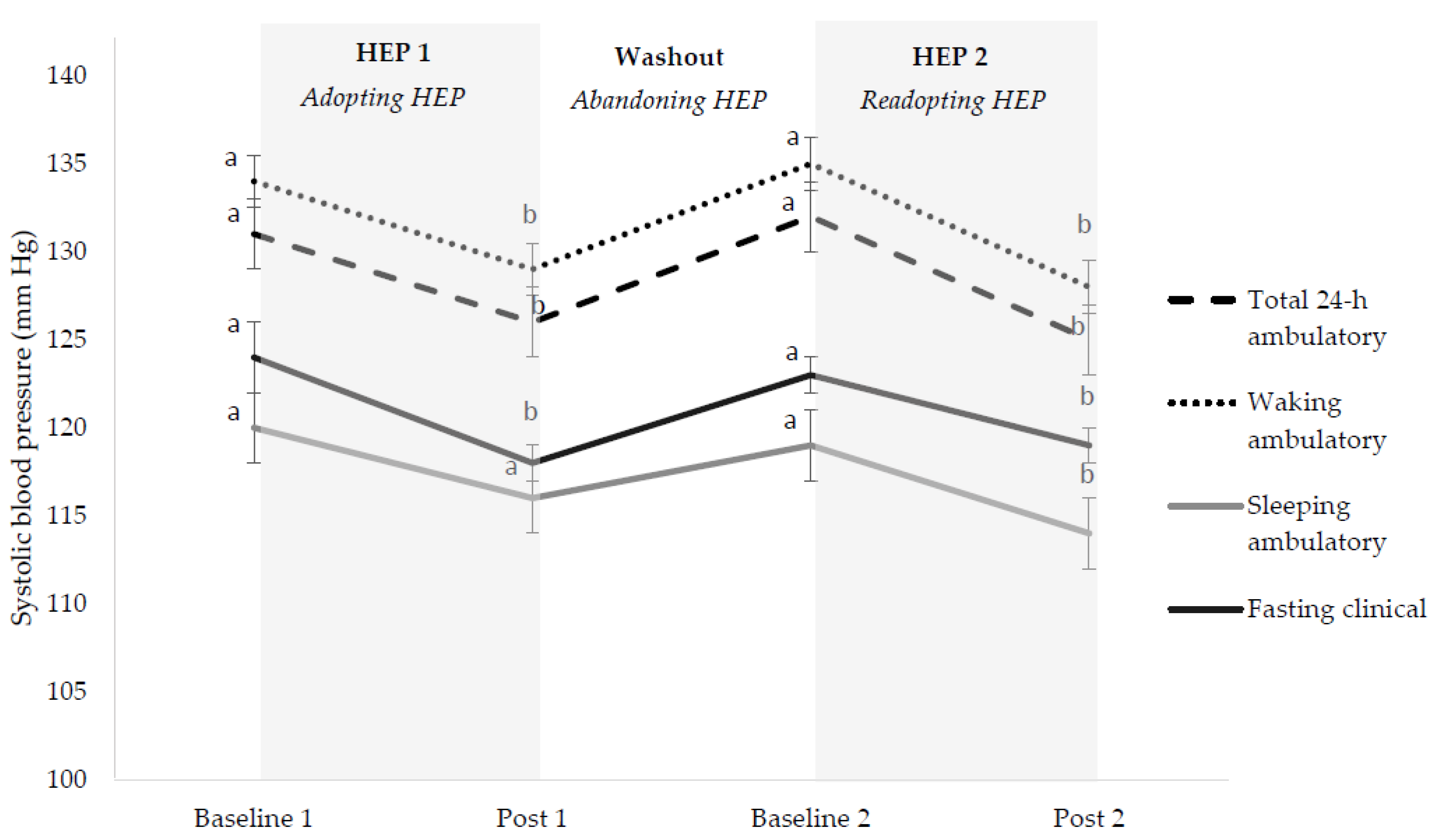
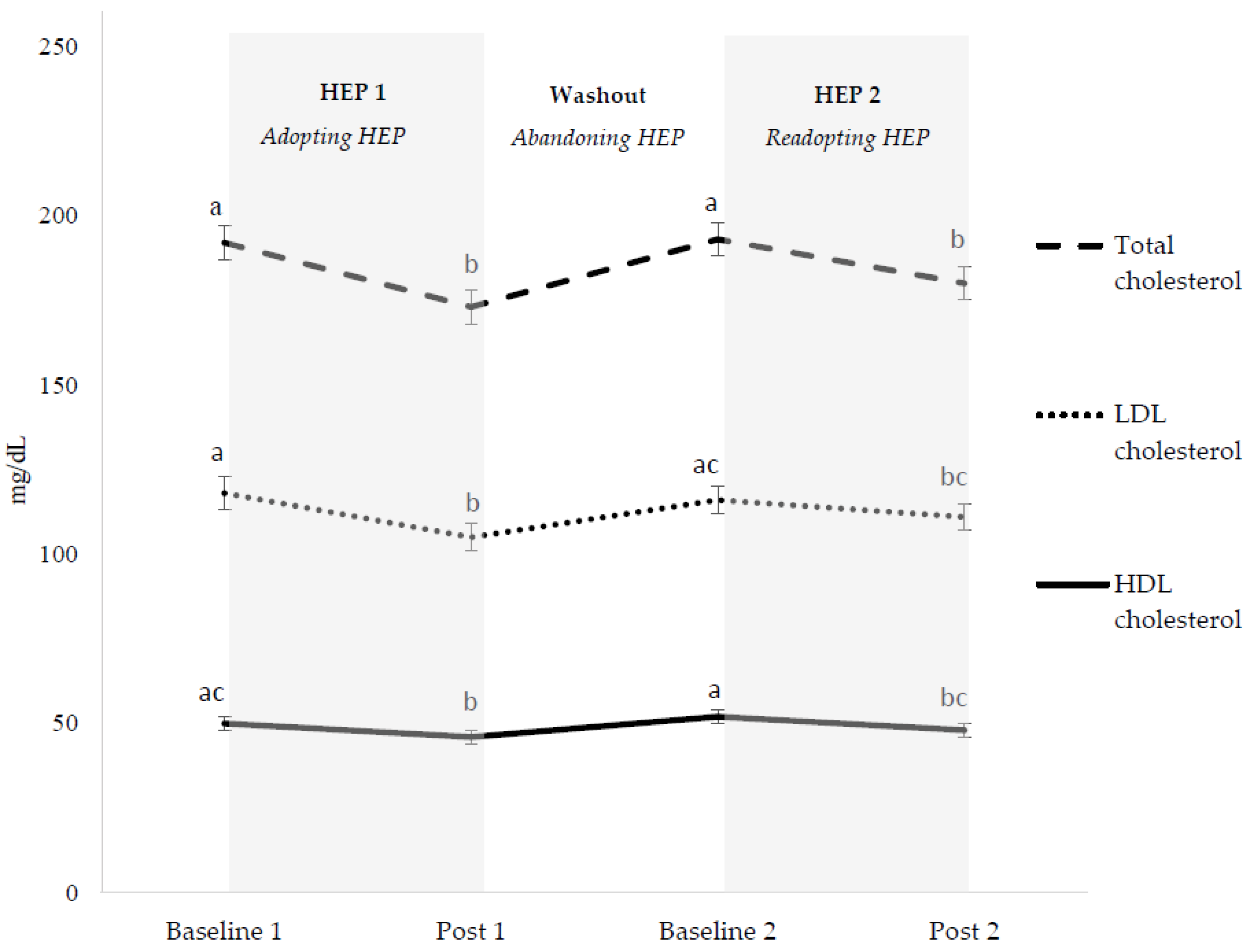
3.3. Cardiovascular Disease Risk Factors
3.3.1. Overall HEP Adoption
3.3.2. Initial HEP Adoption (HEP 1)
3.3.3. HEP Abandonment (Washout)
3.3.4. HEP Readoption (HEP 2)
3.3.5. HEP Cycling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. Department of Agriculture. 2010–2015 Dietary Guidelines for Americans, 2012. Available online: https://health.gov/dietaryguidelines/dga2010/dietaryguidelines2010.pdf (accessed on 10 June 2018).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th Edition, Chapter 1: Key elements of healthy eating patterns. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 10 June 2018).
- Van Horn, L.; Carson, J.A.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: A scientific statement from the american heart association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef] [PubMed]
- Vogt, T.M.; Appel, L.J.; Obarzanek, E.; Moore, T.J.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Cutler, J.A.; Windhauser, M.M.; et al. Dietary approaches to stop hypertension: Rationale, design, and methods. DASH Collaborative Research Group. J. Am. Diet. Assoc. 1999, 99, S12–S18. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Esposito, K.; Giugliano, D.; Panagiotakos, D. The effect of Mediterranean diet on the development of type 2 diabetes mellitus: A meta-analysis of 10 prospective studies and 13,6846 subjects. Metabolism 2014, 63, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health an updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 53,4906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th Edition, Introduction, Figure I-1 Adherence of the U.S. Population Ages 2 Years and Older to the 2010 Dietary Guidelines, as Measured by Average Total Healthy Eating Index-2010 (HEI-2010) Scores, 2015. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 10 June 2018).
- Moore, L.V.; Thompson, F.E. Adults meeting fruit and vegetable intake recommendations—United States, 2013. MMWR Morb. Mortal Wkly. Rep. 2015, 64, 709–713. [Google Scholar] [PubMed]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Palacios, O.M.; Lindner, E.; Nieman, K.M.; Bell, M.; Sorce, J. Replacement of refined starches and added sugars with egg protein and unsaturated fats increases insulin sensitivity and lowers triglycerides in overweight or obese adults with elevated triglycerides. J. Nutr. 2017, 147, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Lawless, A.L.; Kelley, K.M.; Kaden, V.N.; Geiger, C.J.; Palacios, O.M.; Dicklin, M.R. Corn oil intake favorably impacts lipoprotein cholesterol, apolipoprotein and lipoprotein particle levels compared with extra-virgin olive oil. Eur. J. Clin. Nutr. 2017, 71, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Roussell, M.A.; Hill, A.M.; Gaugler, T.L.; West, S.G.; Ulbrecht, J.S.; Vanden Heuvel, J.P.; Gillies, P.J.; Kris-Etherton, P.M. Effects of a DASH-like diet containing lean beef on vascular health. J. Hum. Hypertens. 2014, 28, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Roussell, M.A.; Hill, A.M.; Gaugler, T.L.; West, S.G.; Vanden Heuvel, J.P.; Alaupovic, P.; Gillies, P.J.; Kris-Etherton, P.M. Beef in an optimal lean diet study: Effects on lipids, lipoproteins, and apolipoproteins. Am. J. Clin. Nutr. 2012, 95, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.; Smith, D.L.; Muhammad, J., Jr.; Casazza, K. Impact of weight cycling on risk of morbidity and mortality. Obes. Rev. 2014, 15, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Montani, J.P.; Schutz, Y.; Dulloo, A.G. Dieting and weight cycling as risk factors for cardiometabolic diseases: Who is really at risk? Obes. Rev. 2015, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Strohacker, K.; Carpenter, K.C.; McFarlin, B.K. Consequences of weight cycling: An increase in disease risk? Int. J. Exerc. Sci. 2009, 2, 191–201. [Google Scholar] [PubMed]
- Sayer, R.D.; Wright, A.J.; Chen, N.; Campbell, W.W. Dietary approaches to stop hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. Am. J. Clin. Nutr. 2015, 102, 302–308. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.E.; Paddon-Jones, D.; Wright, A.J.; Campbell, W.W. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018, 108, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US), Panel on Macronutrients, and Institute of Medicine (US), Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Dixon, L.B.; Subar, A.F.; Peters, U.; Weissfeld, J.L.; Bresalier, R.S.; Risch, A.; Schatzkin, A.; Hayes, R.B. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J. Nutr. 2007, 137, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2010–2015 Dietary Guidelines for Americans, 2005. Available online: https://health.gov/dietaryguidelines/dga2005/document/default.htm (accessed on 10 June 2018).
- Sullivan, G.M.; Feinn, R. Using effect size-or why the p value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Obarzanek, E.; Sacks, F.M.; Vollmer, W.M.; Bray, G.A.; Miller, E.R.; 3rd Lin, P.H.; Karanja, N.M.; Most-Windhauser, M.M.; Moore, T.J.; Swain, J.F.; et al. Effects on blood lipids of a blood pressure-lowering diet: The Dietary Approaches to Stop Hypertension (DASH) trial. Am. J. Clin. Nutr. 2001, 74, 80–89. [Google Scholar] [PubMed]
- Hinderliter, A.L.; Babyak, M.A.; Sherwood, A.; Blumenthal, J.A. The DASH diet and insulin sensitivity. Curr. Hypertens. Rep. 2011, 13, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Zhang, J.; Steck, S.E.; Fung, T.T.; Hazlett, L.J.; Han, K.; Ko, S.H.; Merchant, A.T. Obesity mediates the association between mediterranean diet consumption and insulin resistance and inflammation in US adults. J. Nutr. 2017, 147, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Rothman, A.J.; Nicastro, H.L.; Czajkowski, S.M.; Agurs-Collins, T.; Rice, E.L.; Courcoulas, A.P.; Ryan, D.H.; Bessesen, D.H.; Loria, C.M. The accumulating data to Optimally Predict Obesity Treatment (ADOPT) core measures project: Rationale and approach. Obesity 2018, 26, S6–S15. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.E.; Masters, K.S.; Peters, J.C.; Bryan, A.D.; Grigsby, J.; Hooker, S.A.; Wyatt, H.R.; Hill, J.O. Harnessing centred identity transformation to reduce executive function burden for maintenance of health behaviour change: The Maintain IT model. Health Psychol. Rev. 2018, 14, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Saelens, B.E.; Arteaga, S.S.; Berrigan, D.; Ballard, R.M.; Gorin, A.A.; Powell-Wiley, T.M.; Pratt, C.; Reedy, J.; Zenk, S.N. Accumulating data to Optimally Predict Obesity Treatment (ADOPT) core measures: environmental domain. Obesity 2018, 26, S35–S44. [Google Scholar] [CrossRef] [PubMed]
- Kajioka, T.; Tsuzuku, S.; Shimokata, H.; Sato, Y. Effects of intentional weight cycling on non-obese young women. Metabolism 2002, 51, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Montani, J.P.; Viecelli, A.K.; Prevot, A.; Dulloo, A.G. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: The “repeated overshoot” theory. Int. J. Obes. (Lond) 2006, 30, S58–S66. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.W.; Wong, M.C.; Wang, H.H.; Liu, K.Q.; Lee, C.L.; Yan, B.P.; Yu, C.M.; Griffiths, S.M. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: A systematic review. PLoS ONE 2013, 8, e78412. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Kunzova, S.; Medina-Inojosa, J.R.; Agodi, A.; Barchitta, M.; Homolka, M.; Klacova, N.; Bauerova, H.; Sochor, O.; Lopez-Jimenez, F.; Vinciguerra, M. Association between eating time interval and frequency with ideal cardiovascular health: Results from a random sample Czech urban population. Nutr. Metab. Cardiovascular Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
| DASH Study | MED Study | |
|---|---|---|
| Fruits (servings/day b) | 7 | 4 |
| Vegetables (servings/day c) | 8 | 6 |
| Dark green vegetables | 1 | 1 |
| Red and orange vegetables | 2 | 1 |
| Legumes | 1 | 1 |
| Starchy vegetables | 2 | 1 |
| Other vegetables | 2 | 3 |
| Whole grains (servings/day d) | 5 | 4 |
| Protein-rich foods (g/week e) | ||
| Red meat | 422 | 336 |
| Poultry | 299 | 266 |
| Seafood | 185 | 336 |
| Whole eggs | 0 | 3 |
| Nuts, seeds, soy f | 80 | 588 |
| Dairy (servings/day g) | 4 | 3 |
| Olive oil (tsp/week h) | n/a | 55 |
| Corresponding diet index score | 7 out of 9 total points i | 13 out of 14 total points j |
| Outcome | DASH(n = 19) | MED(n = 41) | All(n = 60) |
|---|---|---|---|
| Age (years) | 61 ± 2 | 46 ± 2 | 52 ± 2 |
| BMI (kg/m2) | 31.0 ± 1.4 | 30.5 ± 0.6 | 30.6 ± 0.6 |
| Fasting systolic BP (mm Hg) | 130 ± 2 | 118 ± 2 | 121 ± 2 |
| Fasting diastolic BP (mm Hg) | 85 ± 2 | 80 ± 1 | 82 ± 1 |
| Total cholesterol (mg/dL) | 199 ± 9 | 192 ± 5 | 195 ± 4 |
| Low-density lipoprotein (LDL) cholesterol (mg/dL) | 120 ± 8 | 119 ± 4 | 120 ± 4 |
| High-density lipoprotein (HDL) cholesterol (mg/dL) | 54 ± 3 | 49 ± 2 | 51 ± 2 |
| Total cholesterol:HDL cholesterol | 3.78 ± 0.20 | 4.18 ± 0.19 | 4.05 ± 0.15 |
| Triglycerides (mg/dL) | 121 ± 12 | 118 ± 10 | 119 ± 7 |
| Glucose (mg/dL) | 93 ± 2 | 99 ± 1 | 97 ± 1 |
| Insulin (µIU/mL) | 12.6 ± 2.1 | 12.4 ± 1.2 | 12.5 ± 1.1 |
| HOMA-IR | 3.005 ± 0.573 | 3.076 ± 0.324 | 3.053 ± 0.281 |
| Body mass (kg) | 86.4 ± 3.7 | 87.5 ± 2.6 | 87.2 ± 2.1 |
| Body fat (%) | 41.7 ± 2.1 | 38.9 ± 1.5 | 39.8 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, L.E.; Li, J.; Sayer, R.D.; Hennessy, J.E.; Campbell, W.W. Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials. Nutrients 2018, 10, 1725. https://doi.org/10.3390/nu10111725
O’Connor LE, Li J, Sayer RD, Hennessy JE, Campbell WW. Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials. Nutrients. 2018; 10(11):1725. https://doi.org/10.3390/nu10111725
Chicago/Turabian StyleO’Connor, Lauren E., Jia Li, R. Drew Sayer, Jane E. Hennessy, and Wayne W. Campbell. 2018. "Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials" Nutrients 10, no. 11: 1725. https://doi.org/10.3390/nu10111725
APA StyleO’Connor, L. E., Li, J., Sayer, R. D., Hennessy, J. E., & Campbell, W. W. (2018). Short-Term Effects of Healthy Eating Pattern Cycling on Cardiovascular Disease Risk Factors: Pooled Results from Two Randomized Controlled Trials. Nutrients, 10(11), 1725. https://doi.org/10.3390/nu10111725





