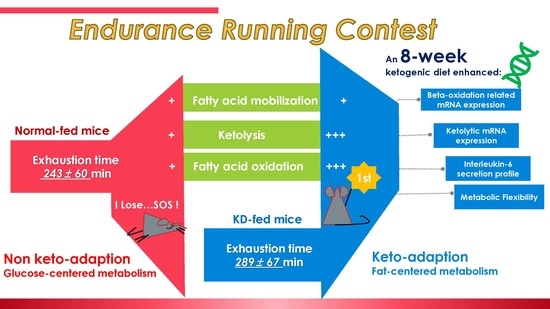
An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Maintenance and Diets
2.2. Endurance Capacity Test Protocol
2.3. Real-Time PCR
2.4. ELISA Procedure and Glycerol Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. A Review of Endurance Capacity Test and Plasma Non-Esterified Fatty Acids and β-Hydroxybutyrate Concentration
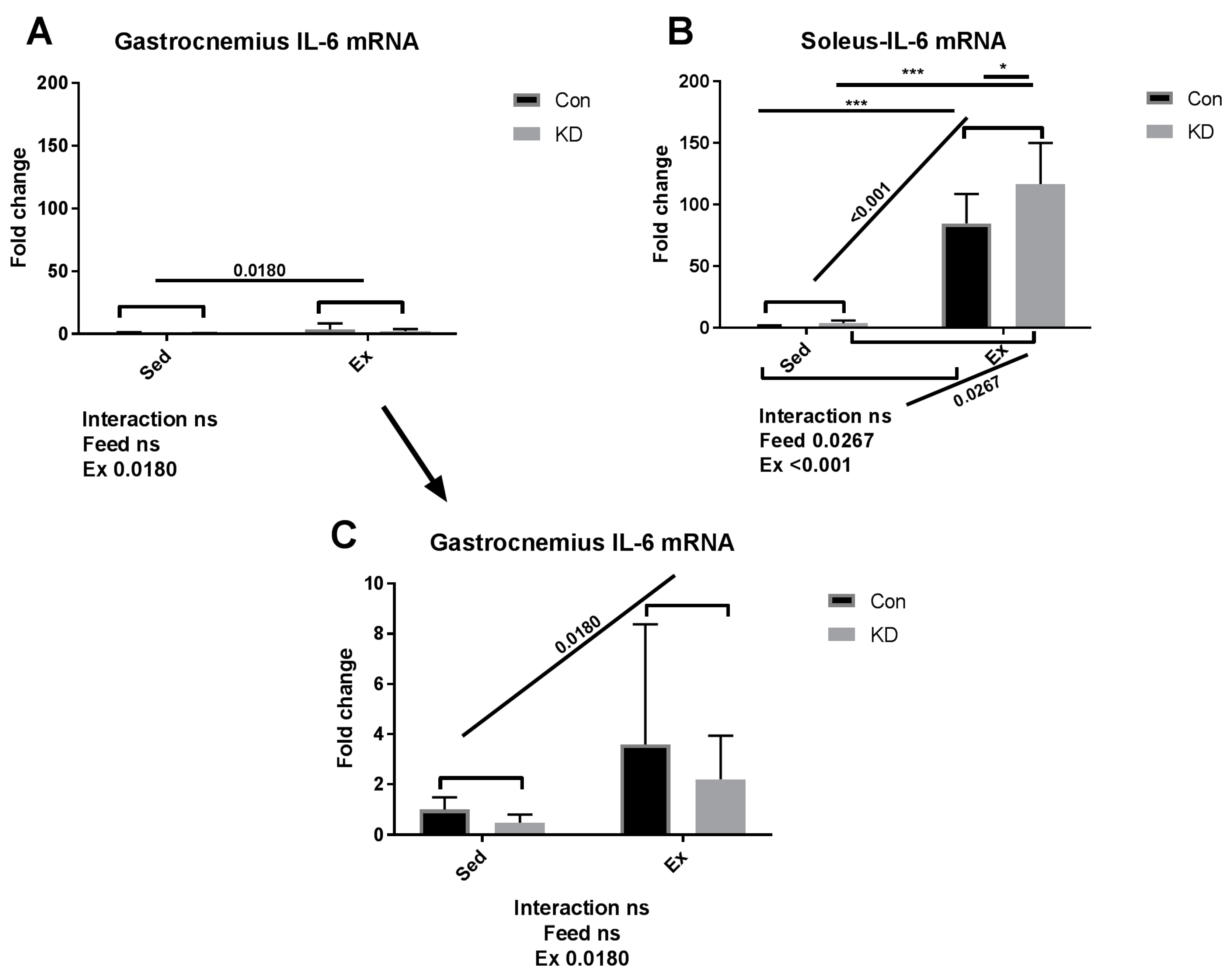
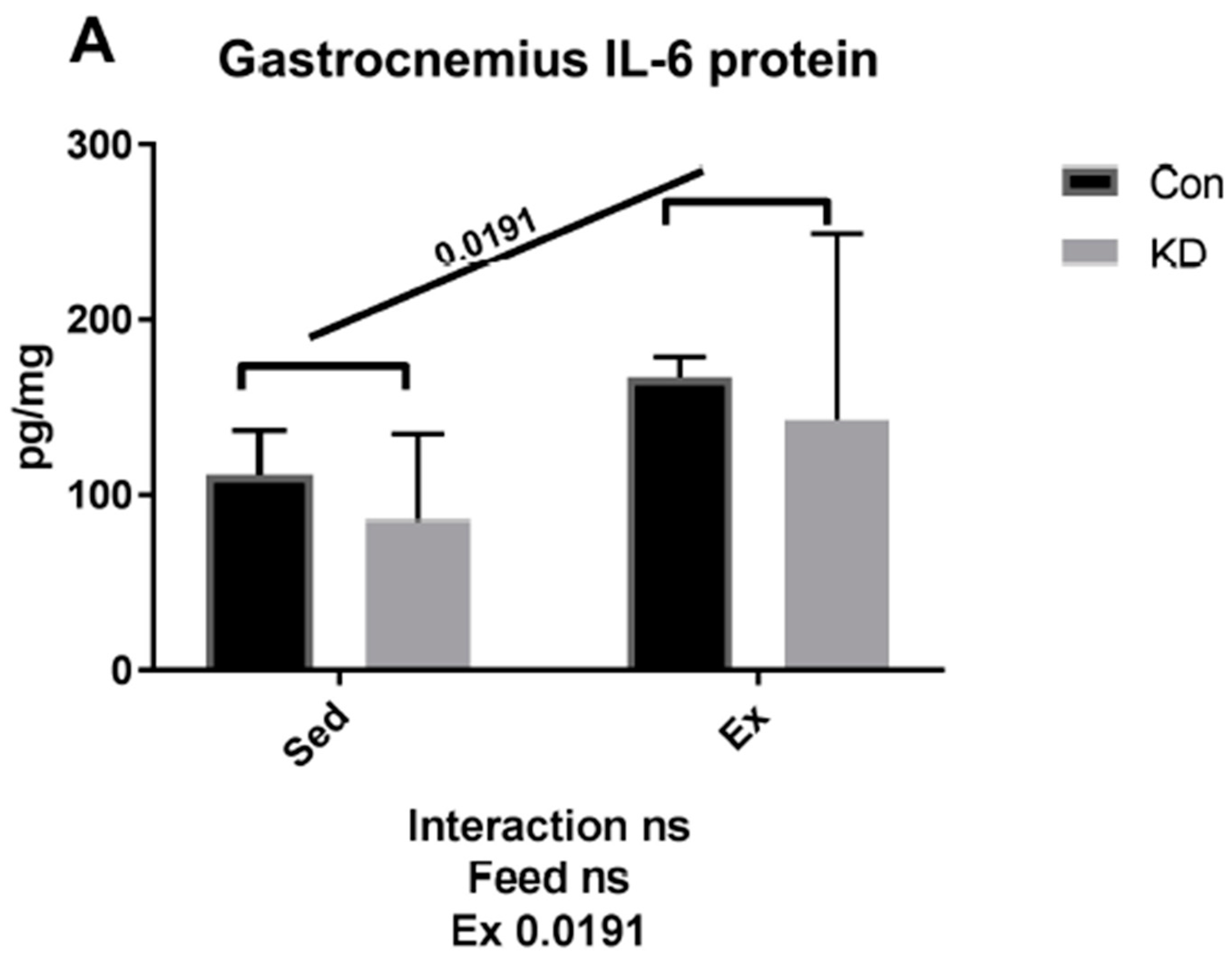
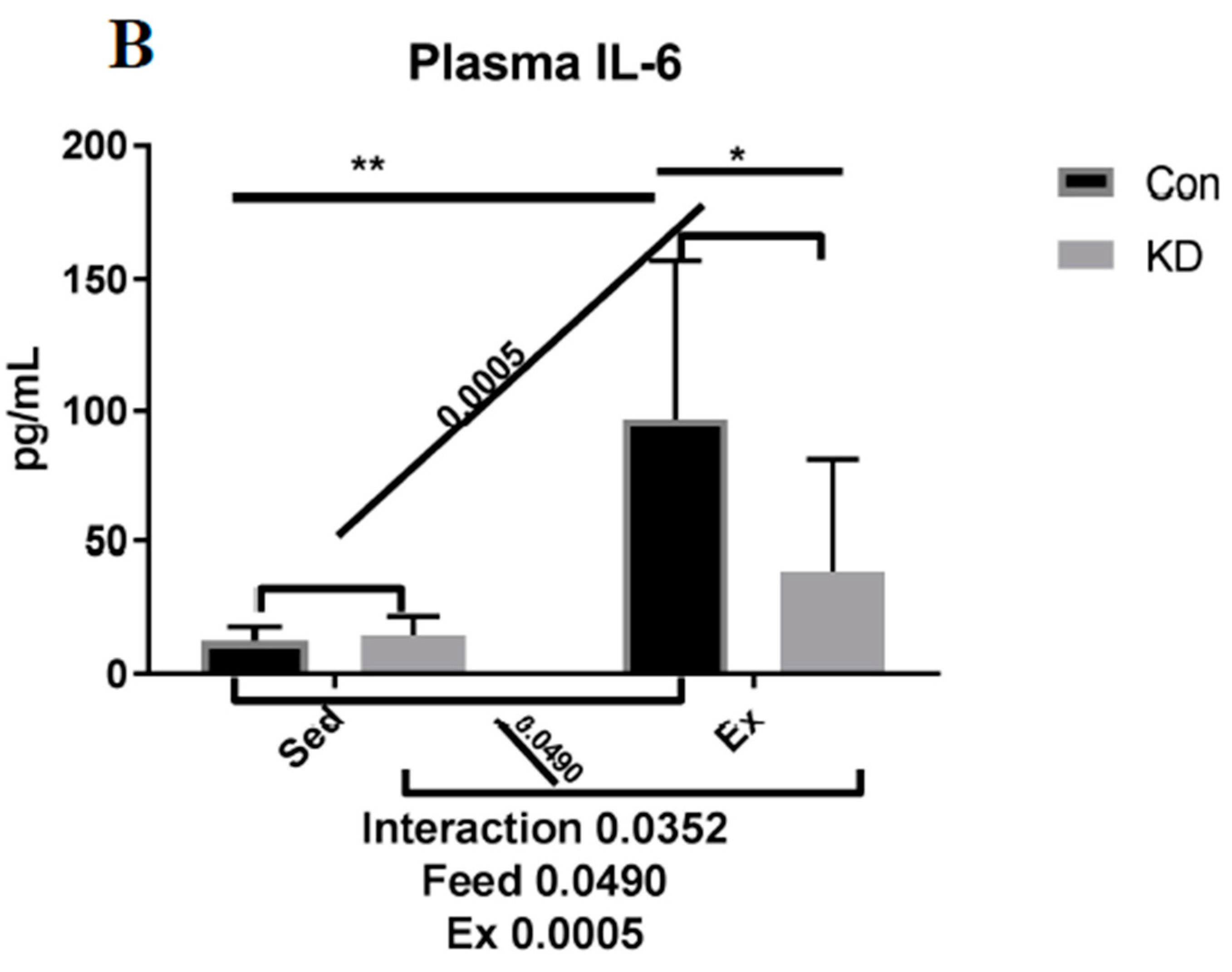
3.2. Plasma IL-6 Concentration and Exercise-Induced IL-6 mRNA Alternation in Both Slow- and Fast-Twitch Muscles
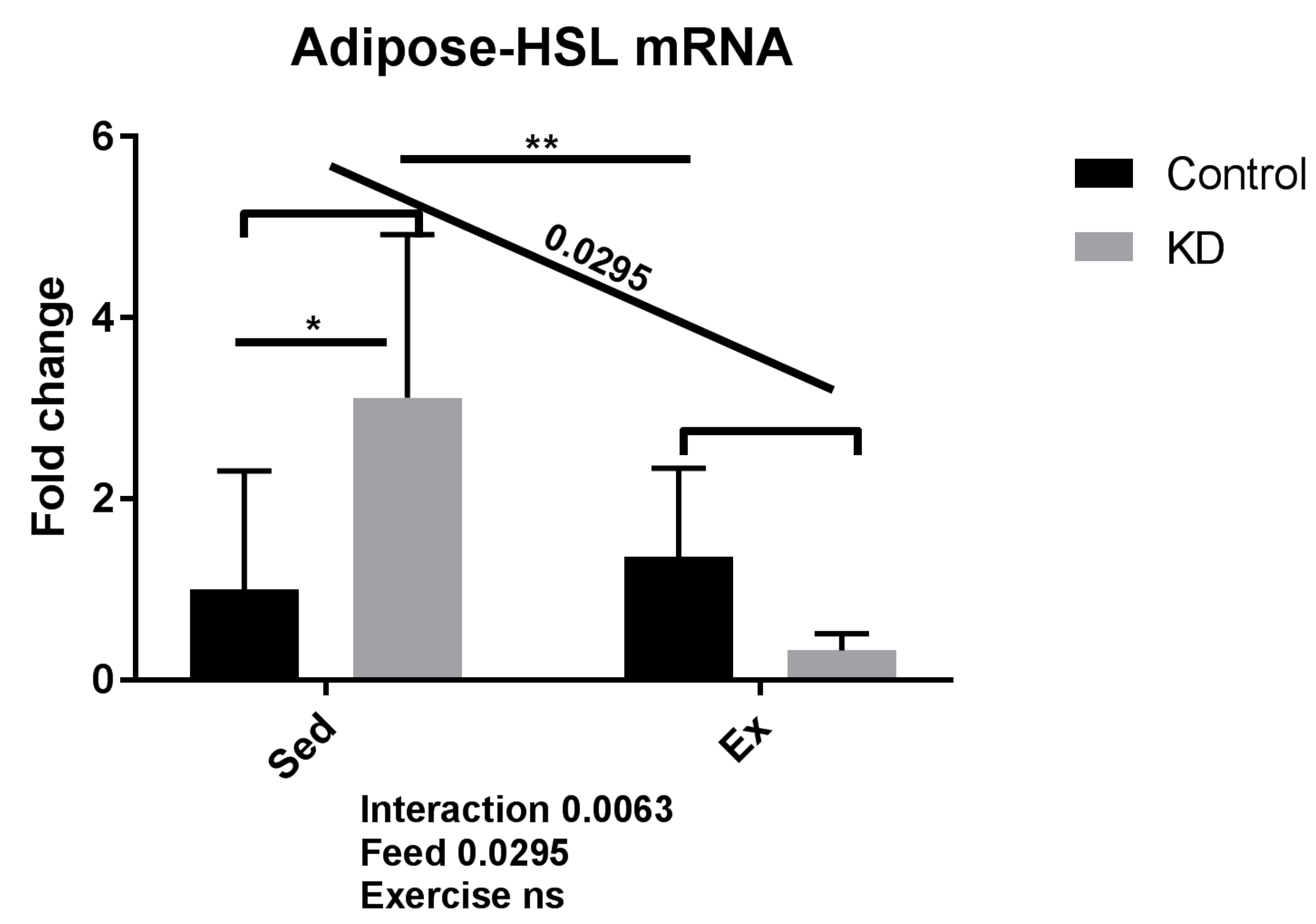
3.3. Fatty Acid Mobilation-Related Gene Expression after Exhaustive Exercise under Endogenous Ketosis in Epididymal Adipose Tissue
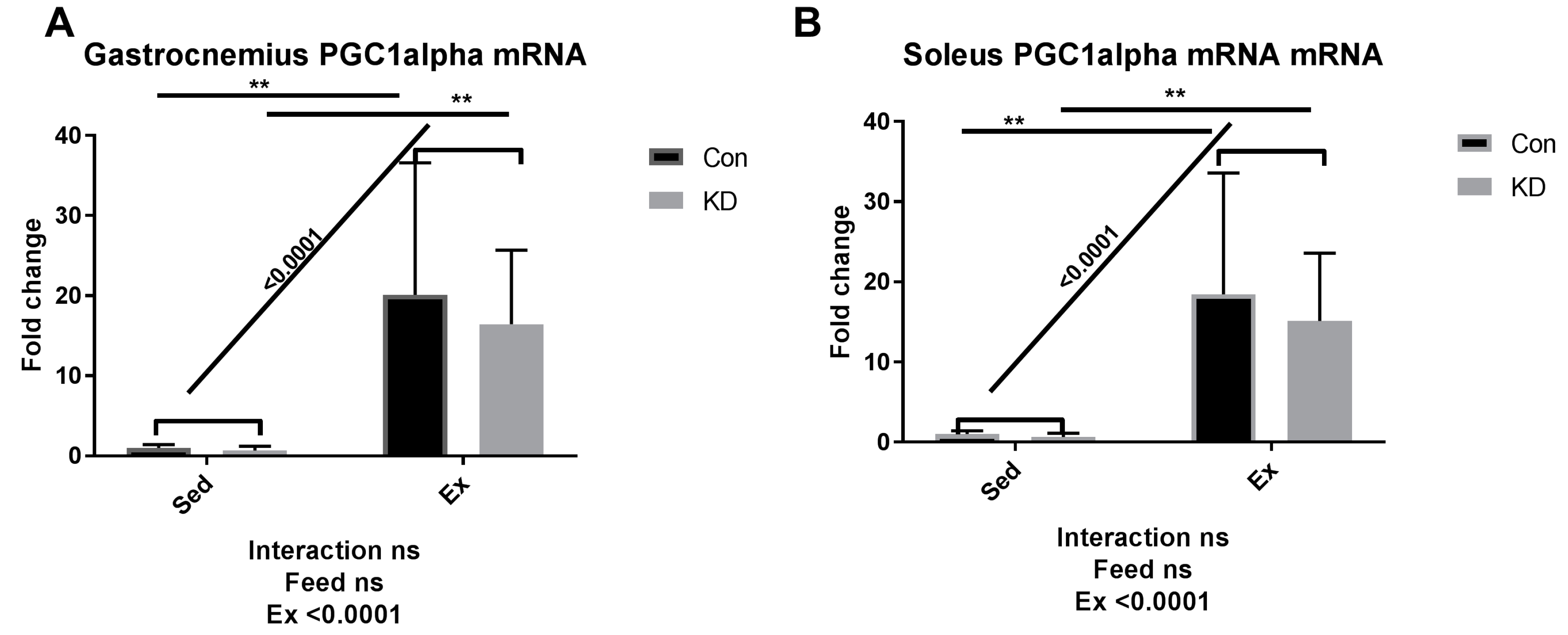
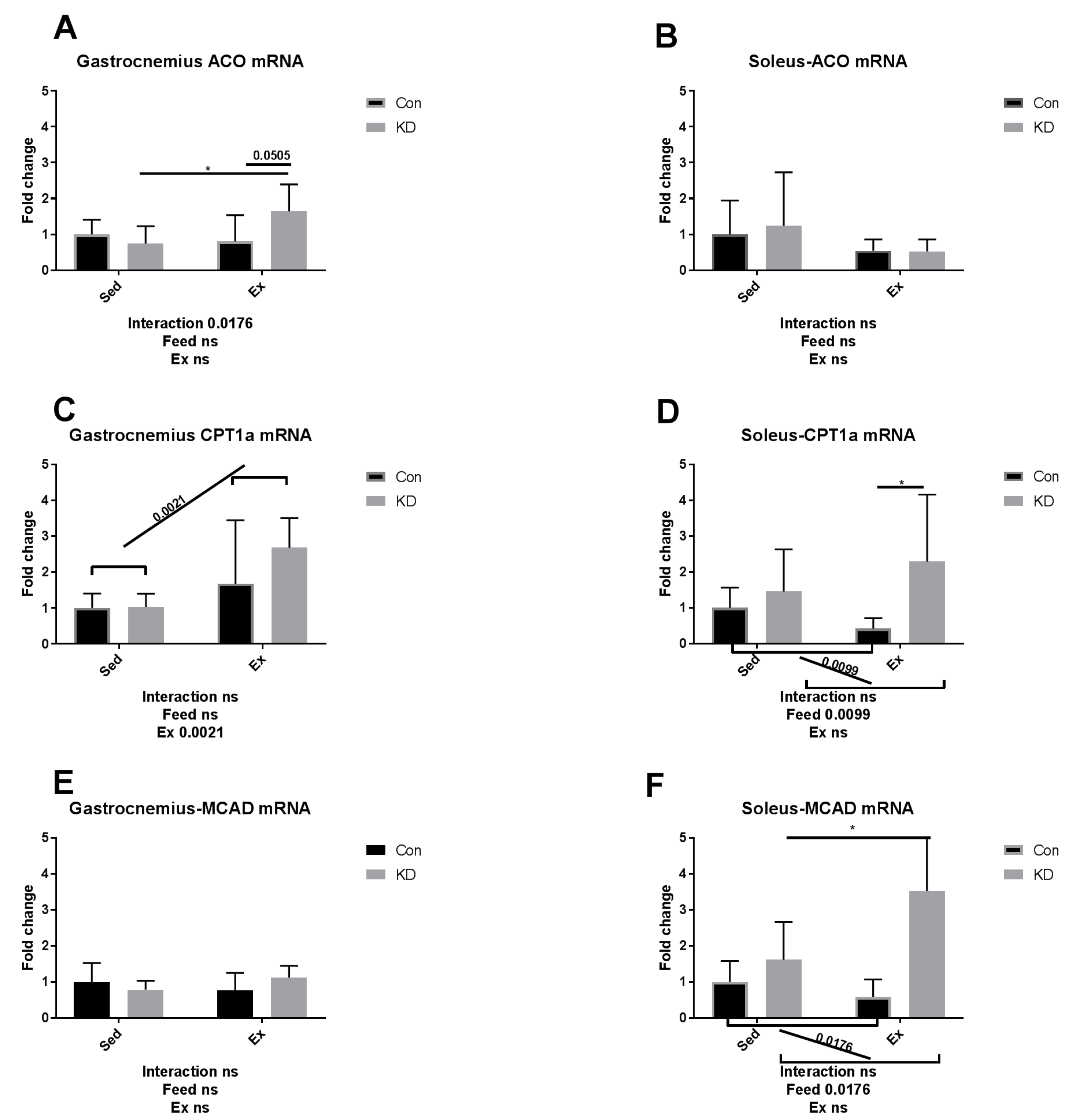
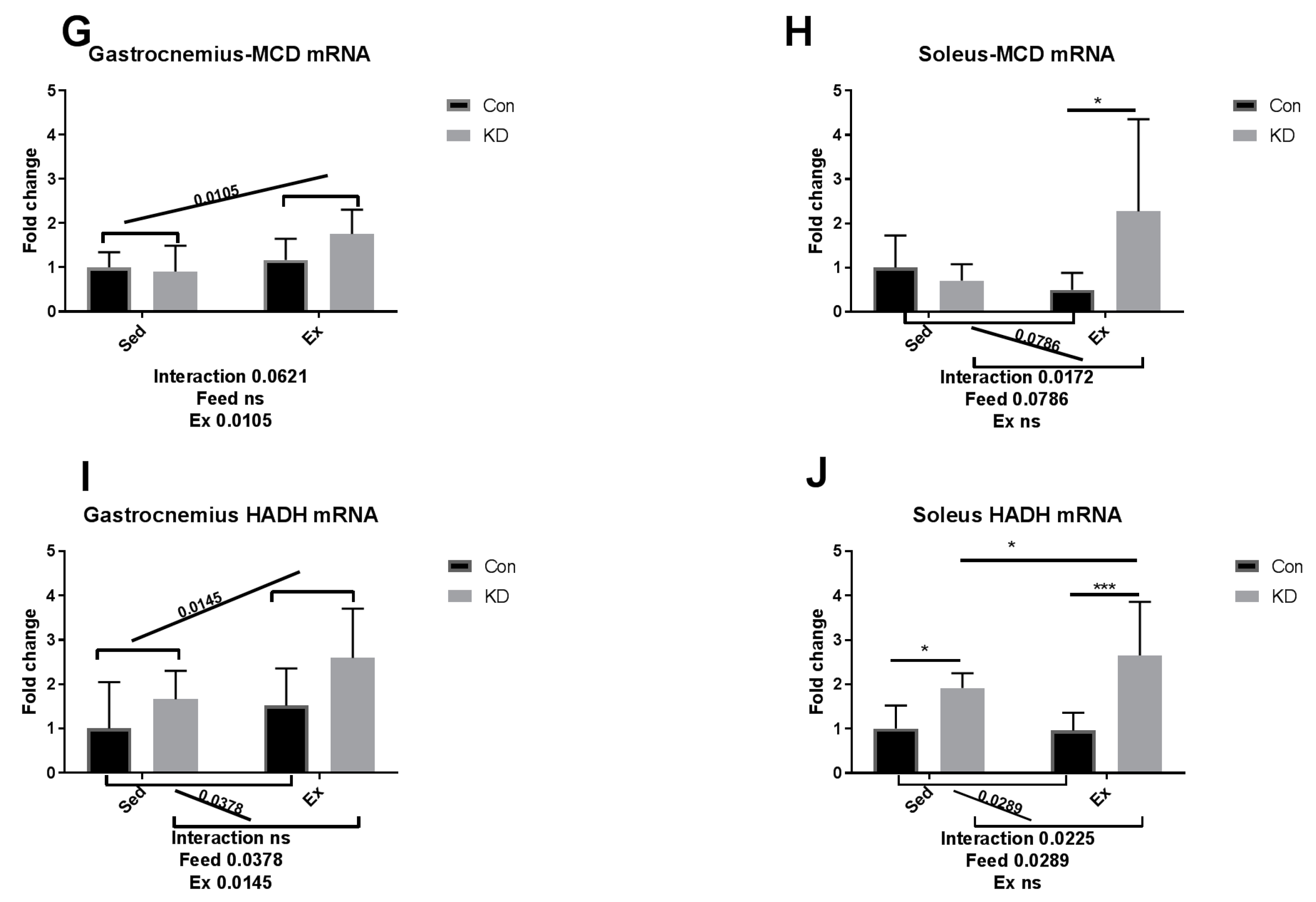
3.4. Ketolytic Gene Expression after Exhaustive Exercise under Conditions of Endogenous Ketosis in Both Slow- and Fast-Twitch Muscles
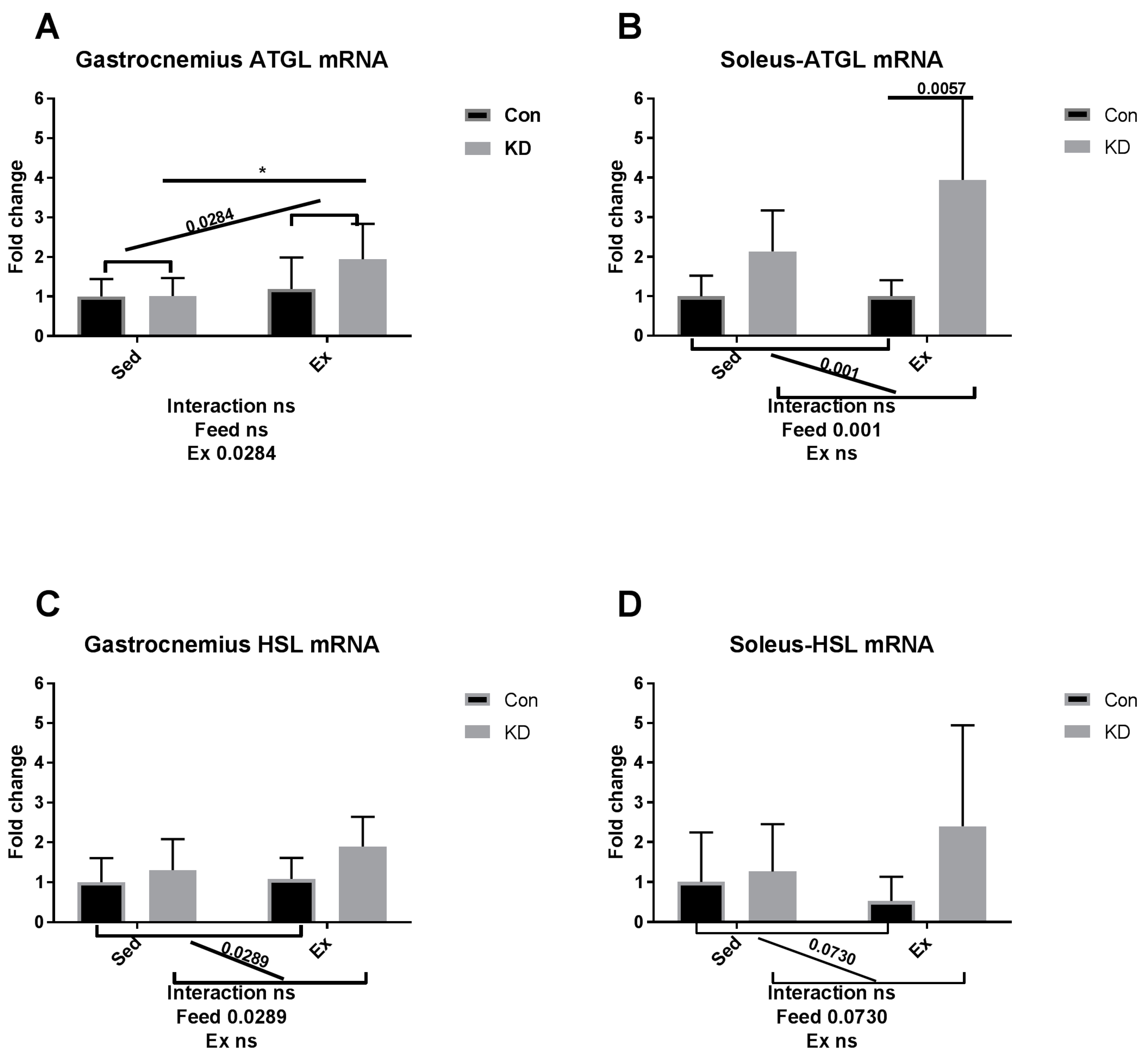
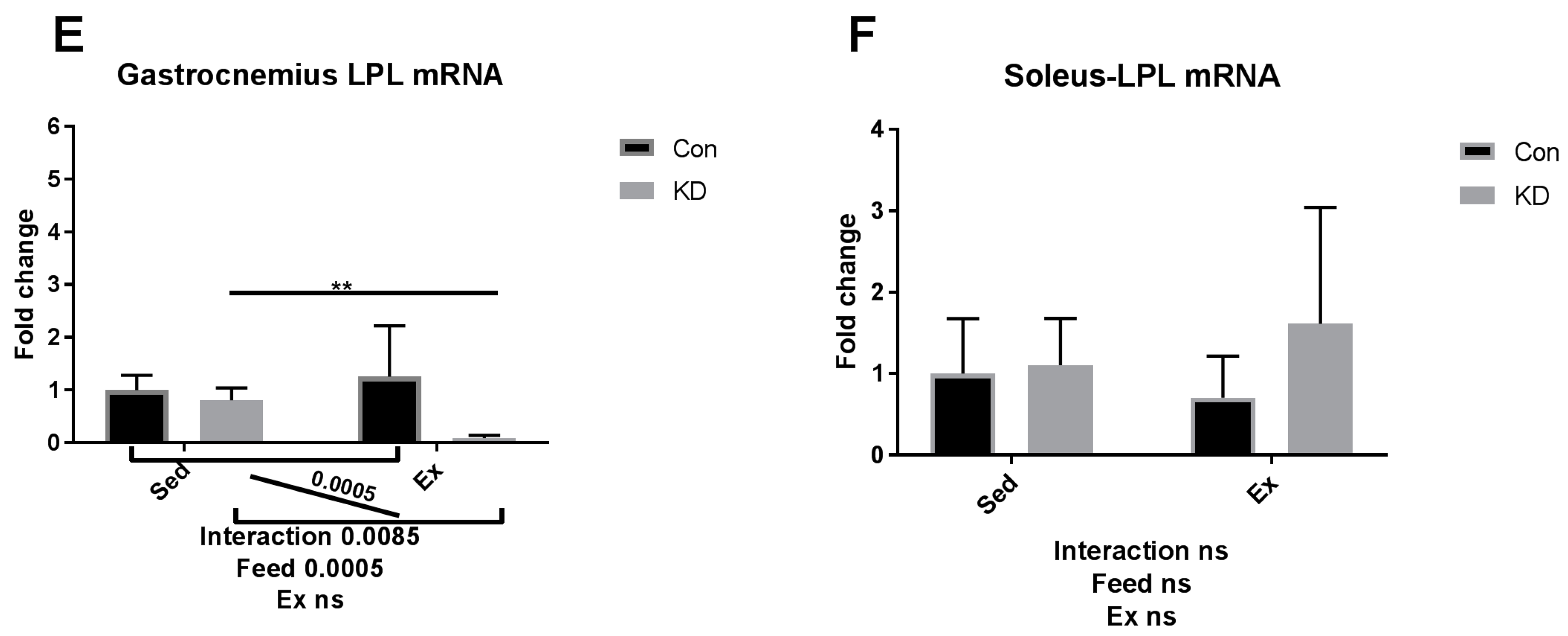
3.5. Lipolysis- and Fatty Acid Oxidation-Related Gene Expression after Exhaustive Exercise during Endogenous Ketosis in Both Slow- and Fast-Twitch Muscles
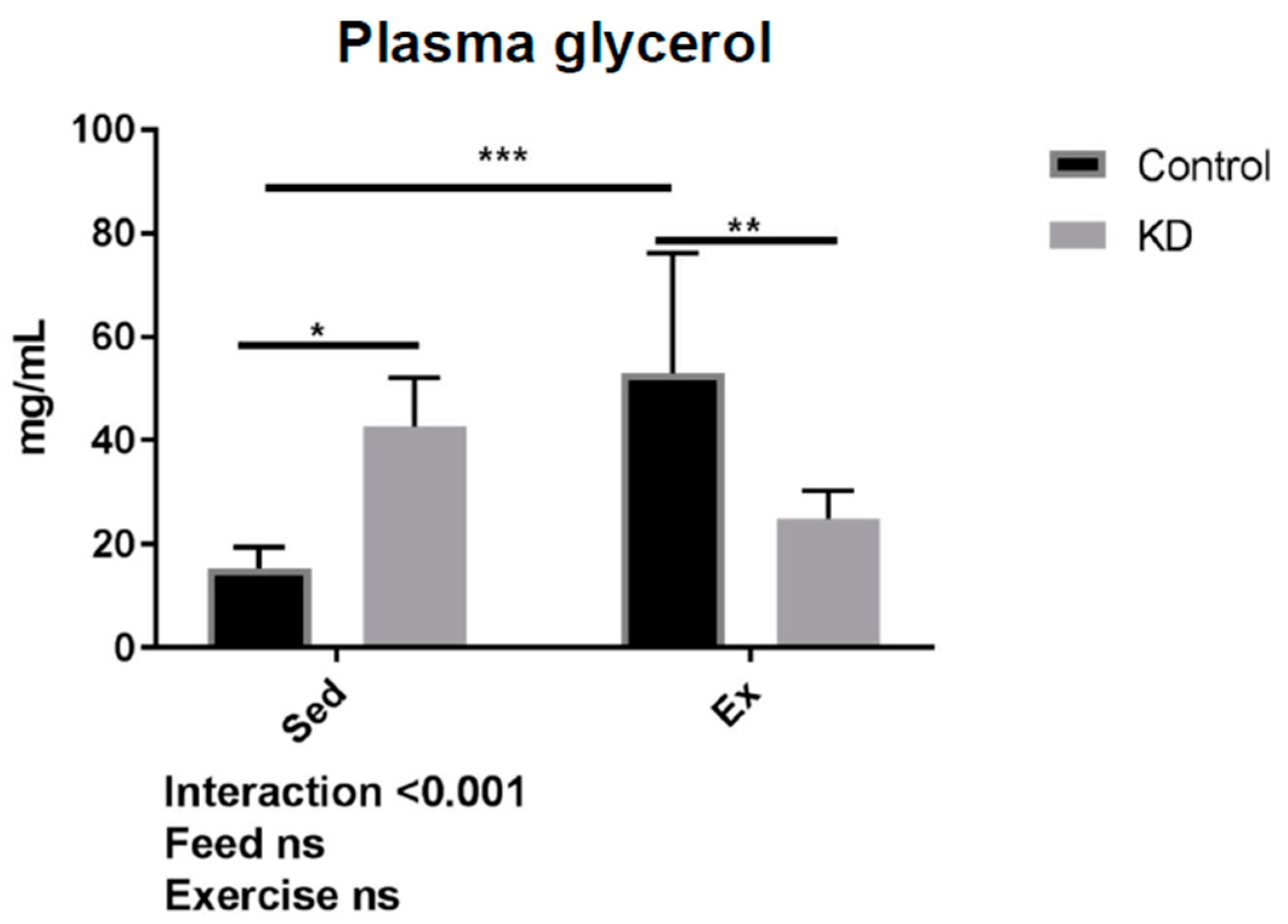
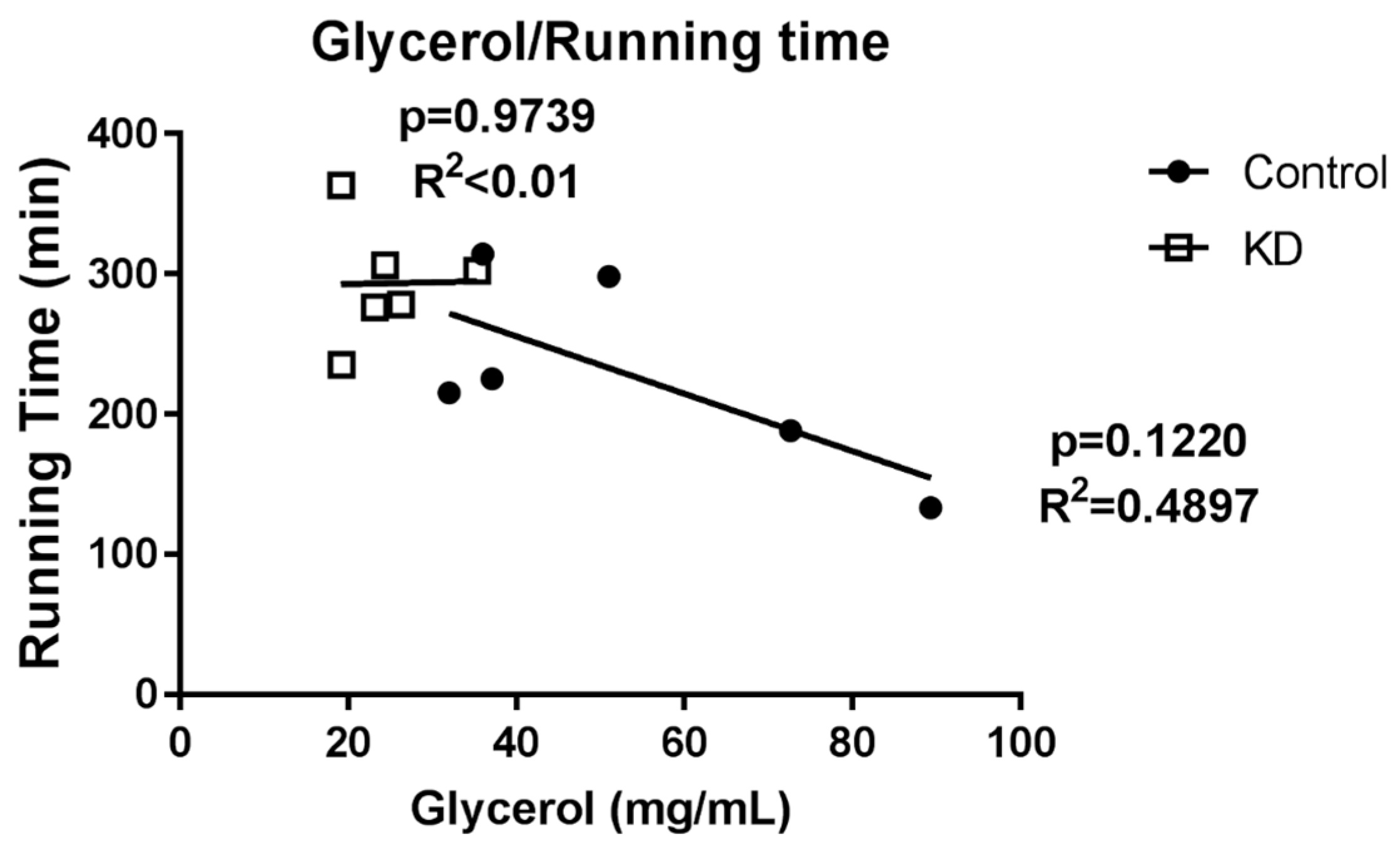
3.6. Glycerol Profile after Exhaustive Exercise during Endogenous Ketosis
3.7. A Comprehensive Summary of this Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol. Metab. 2003, 14, 386–392. [Google Scholar] [CrossRef]
- Volek, J.S.; Noakes, T.; Phinney, S.D. Rethinking fat as a fuel for endurance exercise. Eur. J. Sport Sci. 2015, 15, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, H.S.; Cho, S.Y. The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of taekwondo athletes. J. Exerc. Rehabil. 2014, 10, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.J. Body composition of elite American athletes. Am. J. Sports Med. 1983, 11, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Flatt, J.P.; Jéquier, E. Failure of dietary fat intake to promote fat oxidation: A factor favoring the development of obesity. Am. J. Clin. Nutr. 1989, 50, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Optimizing fat oxidation through exercise and diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Raguso, C.A.; Gastaldelli, A.; Sidossis, L.S.; Yeckel, C.W. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism 2000, 49, 122–128. [Google Scholar] [CrossRef]
- Watt, M.J.; Heigenhauser, G.J.F.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-week ketogenic low carbohydrate, high fat diet enhanced exhaustive exercise capacity in mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.V.; Speechly, D.P.; Dennis, S.C.; Noakes, T.D. Enhanced endurance in trained cyclists during moderate intensity exercise following 2 weeks adaptation to a high fat diet. Eur. J. Appl. Physiol. 1994, 69, 287–293. [Google Scholar] [CrossRef]
- Hancock, C.R.; Han, D.-H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Higashida, K.; Kawamura, T.; Higuchi, M. Alternate-day high-fat diet induces an increase in mitochondrial enzyme activities and protein content in rat skeletal muscle. Nutrients 2016, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Sparks, L.M.; Xie, H.; Koza, R.A.; Mynatt, R.; Hulver, M.W.; Bray, G.A.; Smith, S.R. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 2005, 54, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Smith, D.; Burke, L.M.; Angus, D.J.; Tunstall, R.J.; Cox, G.R.; Bonen, A.; Hawley, J.A.; Hargreaves, M. A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am. J. Clin. Nutr. 2003, 77, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, C.; Vistisen, B.; Kiens, B. Intramuscular triacylglycerol in energy metabolism during exercise in humans. Exerc. Sport Sci. Rev. 2005, 33, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Badin, P.-M.; Vila, I.K.; Louche, K.; Mairal, A.; Marques, M.-A.; Bourlier, V.; Tavernier, G.; Langin, D.; Moro, C. High-fat diet-mediated lipotoxicity and insulin resistance is related to impaired lipase expression in mouse skeletal muscle. Endocrinology 2013, 154, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Shay, C.; Goodpaster, B.H. Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J. Appl. Physiol. 2008, 105, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O.; Booth, F.W. Biochemical adaptations to endurance exercise in muscle. Annu. Rev. Physiol. 1976, 38, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.B.; Barnard, R.J.; Edgerton, V.R.; Gillespie, C.A.; Stempel, K.E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry 1972, 11, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.T. Chemical energetics of slow- and fast-twitch muscles of the mouse. J. Gen. Physiol. 1982, 79, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J. Appl. Physiol. 1999, 87, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur. J. Appl. Physiol. 2000, 81, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Päth, G.; Bornstein, S.R.; Gurniak, M.; Chrousos, G.P.; Scherbaum, W.A.; Hauner, H. Human breast adipocytes express interleukin-6 (IL-6) and its receptor system: Increased IL-6 production by β-adrenergic activation and effects of IL-6 on adipocyte function. J. Clin. Endocrinol. Metabol. 2001, 86, 2281–2288. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Potential application of ketogenic diet to metabolic status and exercise performance: A review. EC Nutr. 2018, 13, 496–499. [Google Scholar]
- Huang, Q.; Ma, S.; Tominaga, T.; Suzuki, K.; Liu, C. An 8-Week, Low carbohydrate, high fat, ketogenic diet enhanced exhaustive exercise capacity in mice Part 2: Effect on fatigue recovery, post-exercise biomarkers and anti-oxidation capacity. Nutrients 2018, 10, 1339. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Wallenius, K.; Ahrén, B.; Rudling, M.; Carlsten, H.; Dickson, S.L.; Ohlsson, C.; Jansson, J.O. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Goldberg, E.B.; Makarova, K.S.; Lin, L.; Brown, W.J.; Jackson, C.L. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006, 7, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 2010, 298, C961–C971. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Sitnick, M.T.; Schoiswohl, G.; Wills, R.C.; Basantani, M.K.; Cai, L.; Pulinilkunnil, T.; Kershaw, E.E. Adipose triglyceride lipase deletion from adipocytes, but not skeletal myocytes, impairs acute exercise performance in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E879–E890. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.A.M. Disorders of ketogenesis and ketolysis. In Inborn Metabolic Diseases; Saudubray, J.-M., Baumgartner, M.R., Walter, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 215–221. ISBN 978-3-662-49769-2. [Google Scholar]
- Svensson, K.; Albert, V.; Cardel, B.; Salatino, S.; Handschin, C. Skeletal muscle PGC-1α modulates systemic ketone body homeostasis and ameliorates diabetic hyperketonemia in mice. FASEB 2016, 30, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.S.; Clark, J.A.; Shepherd, S.O. HSL and ATGL: The movers and shakers of muscle lipolysis. J. Physiol. 2013, 591, 6137–6138. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Wanders, R.J.A. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Schoiswohl, G.; Schweiger, M.; Schreiber, R.; Gorkiewicz, G.; Preiss-Landl, K.; Taschler, U.; Zierler, K.A.; Radner, F.P.W.; Eichmann, T.O.; Kienesberger, P.C.; et al. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J. Lipid Res. 2010, 51, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lin, C.-I.; Chiu, C.-C.; Lin, Y.-T.; Huang, W.-K.; Huang, H.-Y.; Huang, C.-C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Tsai, Y.-H.; Tsai, T.-Y.; Chiu, Y.-S.; Wei, L.; Chen, W.-C.; Huang, C.-C. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 2014, 7, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition position stand: Creatine supplementation and exercise. J. Int. Soc. Sports Nutr. 2007, 4. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Haramizu, S.; Shimotoyodome, A.; Nagasawa, A.; Tokimitsu, I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, 8. [Google Scholar] [CrossRef] [PubMed]
- Oz, H. Chronic inflammatory diseases and green tea polyphenols. Nutrients 2017, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Cialdella-Kam, L.; Ghosh, S.; Meaney, M.; Knab, A.; Shanely, R.; Nieman, D. Quercetin and green tea extract supplementation downregulates genes related to tissue inflammatory responses to a 12-week high fat-diet in mice. Nutrients 2017, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single Dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Saito, H.; Sumi, K.; Sakamoto, Y.; Tachi, Y.; Iida, K. Short-term and long-term ketogenic diet therapy and the addition of exercise have differential impacts on metabolic gene expression in the mouse energy-consuming organs heart and skeletal muscle. Nutr. Res. 2018, 60, 77–86. [Google Scholar] [CrossRef]
- Patlar, S.; Yalçin, H.; Boyali, E. The effect of glycerol supplements on aerobic and anaerobic performance of athletes and sedentary subjects. J. Hum. Kinet. 2012, 34, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients 2018, 10, 1696. https://doi.org/10.3390/nu10111696
Ma S, Huang Q, Tominaga T, Liu C, Suzuki K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients. 2018; 10(11):1696. https://doi.org/10.3390/nu10111696
Chicago/Turabian StyleMa, Sihui, Qingyi Huang, Takaki Tominaga, Chunhong Liu, and Katsuhiko Suzuki. 2018. "An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice" Nutrients 10, no. 11: 1696. https://doi.org/10.3390/nu10111696
APA StyleMa, S., Huang, Q., Tominaga, T., Liu, C., & Suzuki, K. (2018). An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients, 10(11), 1696. https://doi.org/10.3390/nu10111696