Complementary Feeding Practices for South Asian Young Children Living in High-Income Countries: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Participants: Children aged 0–2 years of SA descent living in high-income countries according to World Bank definitions [19].
- Outcomes: Adequacy of CF (based on minimum dietary diversity and meal frequency), timing of introduction of CF and barriers/promoters to incorporating WHO-recommended CFP
- Language: Studies published in English, or with translation available
- Year: Published from 1990–2018
2.2. WHO IYCF Definitions of Adequate Complementary Feeding
- (1)
- Grains, roots and tubers
- (2)
- Legumes and nuts
- (3)
- Dairy products (e.g., milk, yoghurt, cheese)
- (4)
- Flesh foods (e.g., meat, fish, poultry, and liver/organ meats)
- (5)
- Eggs
- (6)
- Vitamin A rich fruits and vegetables
- (7)
- Other fruits and vegetables
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection and Data Extraction
2.6. Result Synthesis
- (1)
- Cohort study; An observational study in which a group of patients are followed over time. These may be prospective or retrospective.
- (2)
- Cross sectional study; An observational study that examines the relationship between health-related characteristics and other variables of interest in a defined population at one particular time.
- (3)
- Mixed methods; A study that combines both quantitative and qualitative methodology.
2.7. Quality Assurance
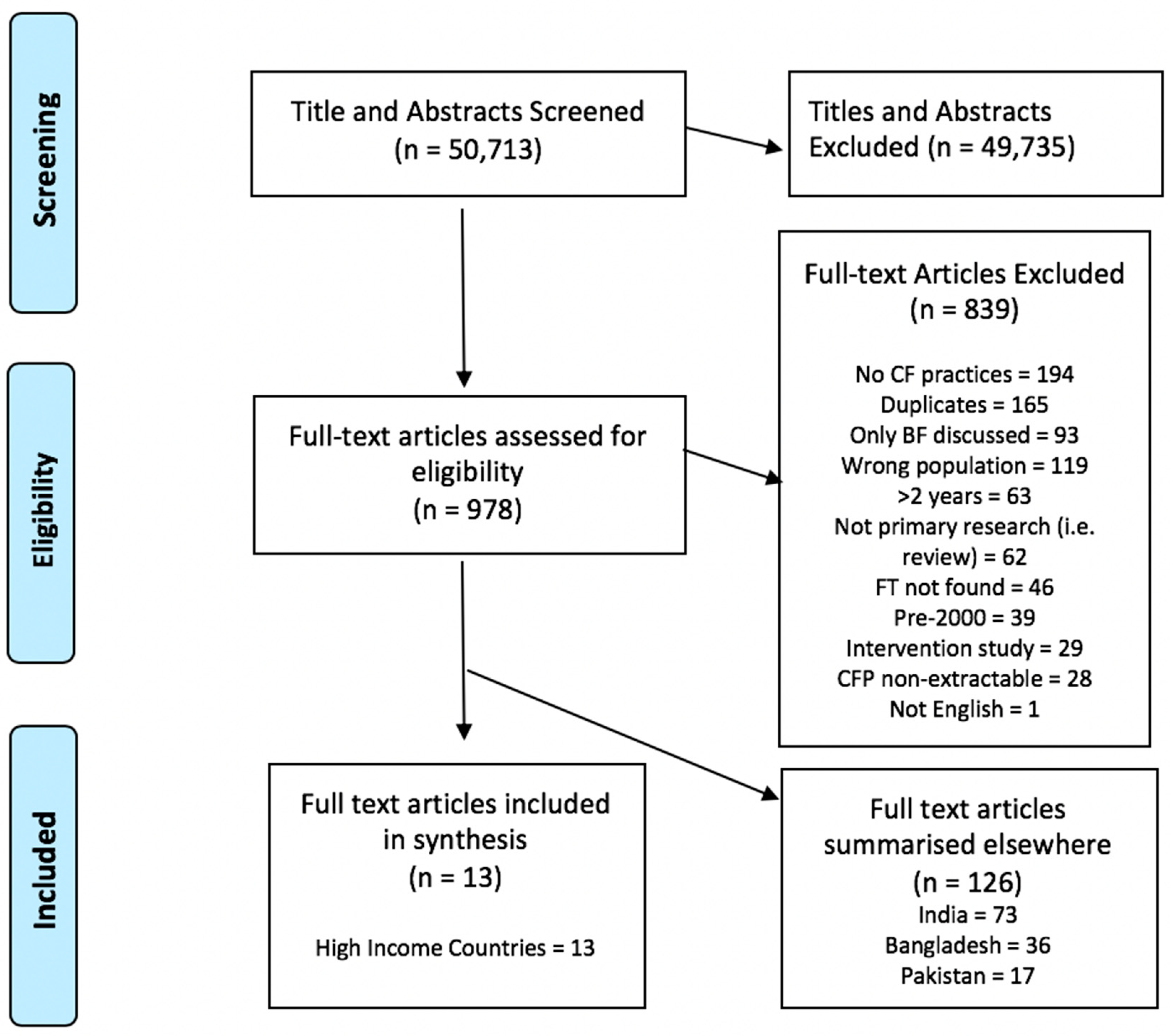
3. Results
3.1. Study and Participant Characteristics
3.2. Adequacy of Complementary Feeding
3.3. Dietary Diversity
3.4. Frequency and Timing
3.4.1. Meal Frequency
3.4.2. Timing of Introducing Complementary Feeding
3.5. Sources of Advice
3.6. Barriers and Promoters
3.6.1. Factors Associated with CFP
3.6.2. Promoters
Education
Understanding of Guidelines
Healthcare Professionals
Provision of Information
3.6.3. Barriers
Familial Pressure
Knowledge
Income
Interactions and Advice from Healthcare Professionals
4. Discussion
4.1. Comparison with SA Countries of Origin
4.2. Comparison with Non-SA Residents of High-Income Countries
4.3. Implication of Key Findings
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organisation WHO—Appropriate Complementary Feeding. Available online: http://www.who.int/elena/titles/complementary_feeding/en/ (accessed on 1 October 2016).
- Programming Guide on Infant and Young Child Feeding. Available online: https://www.unicef.org/nutrition/files/Final_IYCF_programming_guide_2011.pdf (accessed on 1 June 2018).
- Moore, A.P.; Milligan, P.; Goff, L.M. An online survey of knowledge of the weaning guidelines, advice from health visitors and other factors that influence weaning timing in UK mothers. Matern. Child Nutr. 2014, 10, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.; Theurich, M. Complementary feeding and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Xiong, G.; Chao, T.; Jin, Q.; Liu, R.; Hao, L.; Wei, S.; Yang, N.; Yang, X. Introduction of complementary feeding before 4 months of age increases the risk of childhood overweight or obesity: A meta-analysis of prospective cohort studies. Nutr. Res. 2016, 36, 759–770. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Prevalence of Obesity among Children and Adolescents, BMI>+2 Standard Deviation above the Median, Crude: Estimates by World Bank Income Group; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Obesity and Ethnicity. Available online: https://khub.net/documents/31798783/32039025/Obesity+and+ethnicity/834368ce-e47a-4ec6-b71c-7e4789bc7d19 (accessed on 1 June 2018).
- Gujral, U.P.; Pradeepa, R.; Weber, M.B.; Narayan, K.M.V.; Mohan, V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013, 1281, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Faris, P.; McNeil, D.A.; Patterson, S.; Potestio, M.L.; Thawer, S.; McLaren, L. Ethnic disparities in children’s oral health: Findings from a population-based survey of grade 1 and 2 schoolchildren in Alberta, Canada. BMC Oral Health 2018, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Morris, A.J.; Davies, J. The oral health of South Asian five-year-old children in deprived areas of Dudley compared with White children of equal deprivation and fluoridation status. Community Dent. Health 2000, 17, 243–245. [Google Scholar] [PubMed]
- Lennox, A.; Sommerville, J.; Ong, K.; Henderson, H.; Allen, R. Diet and Nutrition Survey of Infants and Young Children, 2011; Department of Health: London, UK, 2013. [Google Scholar]
- Brown, K.H.; Creed-Kanashiro, H.; Dewey, K.G. Optimal complementary feeding practices to prevent childhood malnutrition in developing countries. Food Nutr. Bull. 1995, 16, 320–339. [Google Scholar] [CrossRef]
- Taqi, I. Global breastfeeding advocacy initiative. Breastfeed. Med. 2014, 9, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, N.; Mazumder, S.; Bahl, R.; Martines, J.; Black, R.E.; Bhan, M.K. An Educational Intervention to Promote Appropriate Complementary Feeding Practices and Physical Growth in Infants and Young Children in Rural Haryana, India. J. Nutr. 2004, 134, 2342–2348. [Google Scholar] [PubMed]
- Imdad, A.; Yakoob, M.Y.; Bhutta, Z.A. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 2011, 11, S25. [Google Scholar] [CrossRef] [PubMed]
- Manikam, L.; Prasad, A.; Dharmaratnam, A.; Moen, C.; Robinson, A.; Light, A.; Ahmed, S.; Lingam, R.; Lakhanpaul, M. Systematic review of infant and young child complementary feeding practices in South Asian families: The India perspective. Public Health Nutr. 2017, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Manikam, L.; Robinson, A.; Kuah, J.Y.; Vaidya, H.J.; Alexander, E.C.; Miller, G.W.; Singh, K.K.; Dawe, V.; Ahmed, S.; Lingam, R.; et al. A systematic review of complementary feeding practices in South Asian infants and young children: The Bangladesh perspective. BMC Nutr. 2017, 3, 56. [Google Scholar] [CrossRef]
- Manikam, L.; Sharmila, A.; Dharmaratnam, A.; Alexander, E.; Kuah, J.; Prasad, A.; Ahmed, S.; Lingam, R.; Lakhanpaul, M. Systematic review of infant and young child complementary feeding practices in South Asian families: The Pakistan perspective. Public Health Nutr. 2018, 21, 655–668. [Google Scholar] [CrossRef]
- The World Bank High Income. Available online: https://data.worldbank.org/income-level/high-income (accessed on 1 June 2018).
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; Molgaard, C. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- WHO. Indicators for Assessing Infant and Young Child Feeding Practices: Part 1-Definitions: Conclusions of A Consensus Meeting Held 6–8 November 2007; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Government of Canada. Infant Feeding Joint Working Group Nutrition for Healthy Term Infants: Recommendations from Birth to Six Months; Government of Canada: Ottawa, ON, Canada, 2015. [Google Scholar]
- Department of Health. The Baby Friendly Initiative Introducing Solid Foods: Giving Your Baby A Better Start in Life; Department of Health: London, UK, 2015. [Google Scholar]
- University of York. Critical Appraisal Skills Programme; University of York: Toronto, ON, Canada, 2013. [Google Scholar]
- Dixon-Woods, M.; Agarwal, S.; Jones, D.; Young, B.; Sutton, A. Synthesising qualitative and quantitative evidence: A review of possible methods. J. Health Serv. Res. Policy 2005, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Fauser, B.C.J.M. Human Reproduction Update; Oxford University Press: Oxford, UK, 2005; pp. 103–104. [Google Scholar]
- Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.178.3100&rep=rep1&type=pdf (accessed on 1 June 2018).
- Gough, D. Weight of Evidence: A framework for the appraisal of the quality and relevance of evidence. Res. Pap. Educ. 2007, 22, 213–228. [Google Scholar] [CrossRef]
- Condon, L.; Ingram, J.; Hamid, N.; Hussein, A. Cultural influences on breastfeeding and weaning. Community Pract. 2003, 76, 344–349. [Google Scholar]
- Duggan, M.B.; Harbottle, L.; Noble, C. The weaning diet of healthy Asian children living in Sheffield. 1. The level and composition of the diet in children from 4 to 40 months of age. J. Hum. Nutr. Diet. 1992, 5, 189–200. [Google Scholar] [CrossRef]
- Dykes, J.; Watt, R.G.; Nazroo, J. Socio-economic and ethnic influences on infant feeding practices related to oral health. Community Dent. Health 2002, 19, 137–143. [Google Scholar] [PubMed]
- Griffiths, L.J.; Tate, A.R.; Dezateux, C. Do early infant feeding practices vary by maternal ethnic group? Public Health Nutr. 2007, 10, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Carruth, B.R.; Skinner, J. Infant feeding practices of Anglo American and Asian Indian American mothers. J. Am. Coll. Nutr. 1999, 18, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.P.; Nanthagopan, K.; Hammond, G.; Milligan, P.; Goff, L.M. Influence of weaning timing advice and associated weaning behaviours in a survey of black and minority ethnic groups in the UK. Public Health Nutr. 2013, 17, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Sahota, P.; Gatenby, L.A.; Greenwood, D.C.; Bryant, M.; Robinson, S.; Wright, J. Ethnic differences in dietary intake at age 12 and 18 months: The Born in Bradford 1000 Study. Public Health Nutr. 2016, 19, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Santorelli, G.; Fairley, L.; Petherick, E.S.; Cabieses, B.; Sahota, P. Ethnic differences in infant feeding practices and their relationship with BMI at 3 years of age—Results from the Born in Bradford birth cohort study. Br. J. Nutr. 2014, 111, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, T. Infant feeding practices of Pakistani mothers in England and Pakistan. J. Hum. Nutr. Diet. 2002, 15, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Stearns, J.C.; Zulyniak, M.A.; de Souza, R.J.; Campbell, N.C.; Fontes, M.; Shaikh, M.; Sears, M.R.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; et al. Ethnic and diet-related differences in the healthy infant microbiome. Genome Med. 2017, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Avery, V. Infant Feeding in Asian Families; Palgrave Macmillan: London, UK, 1997. [Google Scholar]
- Toh, J.; Yip, G.; Han, W.; Fok, D.; Low, Y.-L.; Lee, Y.; Rebello, S.; Saw, S.-M.; Kwek, K.; Godfrey, K.; et al. Infant Feeding Practices in a Multi-Ethnic Asian Cohort: The GUSTO Study. Nutrients 2016, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Sahota, P. An enquiry into the attitudes of Muslim Asian mothers regarding infant feeding practices and dental health. J. Hum. Nutr. Diet. 1990, 3, 393–401. [Google Scholar] [CrossRef]
- Office of National Statistics. 2011 Census: Key Statistics and Quick Statistics for Local Authorities in the United Kingdom; Office of National Statistics: Newport, UK, 2013. [Google Scholar]
- Singapore Residents by Age Group, Ethnic Group and Gender, End June, Annual. Available online: https://data.gov.sg/dataset/resident-population-by-ethnicity-gender-and-age-group (accessed on 7 September 2018).
- United States Census Bureau. The Asian Population 2010; United States Census Bureau: Suitland, MA, USA, 2012. [Google Scholar]
- Statistics Canada Immigration and Ethnocultural Diversity in Canada. Available online: https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-010-x/99-010-x2011001-eng.cfm (accessed on 7 September 2018).
- Australian Bureau of Statistics. 2016 Census QuickStats; Australian Bureau of Statistics: Canberra, Australia, 2016. [Google Scholar]
- McAndrew, F.; Thompson, J.; Fellows, L.; Large, A.; Speed, M.; Renfrew, M.J. Infant Feeding Survey 2010; Health and Social Care Information Centre: Leeds, UK, 2012. [Google Scholar]
- Department of Health. Infant Feeding Recommendation; Department of Health: London, UK, 2003. [Google Scholar]
- Barrera, C.M.; Hamner, H.C.; Perrine, C.G.; Scanlon, K.S. Timing of Introduction of Complementary Foods to US Infants, National Health and Nutrition Examination Survey 2009–2014. J. Acad. Nutr. Diet. 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Kapoor, D.; Singh, K.; Narayan, K.M.V.; Ali, M.K.; Kadir, M.M.; Mohan, V.; Tandon, N.; Prabhakaran, D. Vegetarianism and cardiometabolic disease risk factors: Differences between South Asian and US adults. Nutrition 2016, 32, 975–984. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Underwood, A. Ethnic influences on weaning diet in the UK. Proc. Nutr. Soc. 1997, 56, 121–130. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vitamin A Supplementation in Infants 1–5 Months of Age; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Imdad, A.; Yakoob, M.Y.; Sudfeld, C.; Haider, B.A.; Black, R.E.; Bhutta, Z.A. Impact of vitamin A supplementation on infant and childhood mortality. BMC Public Health 2011, 11, S20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Maternal and Child Nutrition: Public Health Guideline [PH11]; National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Department of Health. Healthy Child Programme: Pregnancy and the First Five Years of Life; Department of Health: London, UK, 2009. [Google Scholar]
- National Health & Medical Research Council. Infant feeding guidelines. Iowa Med. 2013, 75, 1–26. [Google Scholar]
- American Academy of Pediatrics Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [CrossRef] [PubMed]
- Rote, S. Traditional and modern Asian weaning patterns in Britain. Br. J. Community Heal. Nurs. 1996, 1, 81–85. [Google Scholar] [CrossRef]
- Pearce, J.; Taylor, M.A.; Langley-Evans, S.C. Timing of the introduction of complementary feeding and risk of childhood obesity: A systematic review. Int. J. Obes. 2013, 37, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.M.; Marriott, L.D.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Inskip, H.M.; Baird, J.; Law, C.M.; Godfrey, K.M.; Cooper, C. Variations in infant feeding practice are associated with body composition in childhood: A prospective cohort study. J. Clin. Endocrinol. Metab. 2009, 94, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Bégin, F.; Aguayo, V. First foods: Why improving young children’s diets matter. Matern. Child Nutr. 2017, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health. Supplementary Evidence Review on the Effectiveness of Public Health Interventions to Improve the Nutrition of Infants/Children Aged 6 Months to 5 Years; NCC-WCH: London, UK, 2007. [Google Scholar]
- Ilett, S.; Freeman, A. Improving the diet of toddlers of Pakistani origin: A study of intensive dietary health education. J. Fam. Health Care 2004, 14, 16–19. [Google Scholar] [PubMed]
- Smith, S.; Randhawa, A. Extending the role of the linkworker in weaning support. Community Pract. 2004, 77, 146–149. [Google Scholar]
- Dewey, K. Guiding Principles for Complementary Feeding of the Breastfed (PAHO and WHO); Pan American Health Organization: Washington, DC, USA, 2001; pp. 18–25. [Google Scholar]
- UNICEF. A Global Meeting to Accelerate Progress on Complementary Feeding in Young Children; UNICEF: New York, NY, USA, 2016. [Google Scholar]
- Cunningham, K.; Ruel, M.; Ferguson, E.; Uauy, R. Women’s empowerment and child nutritional status in South Asia: A synthesis of the literature. Matern. Child Nutr. 2015, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
| Author | Weight of Evidence A | Weight of Evidence B | Weight of Evidence C | Weight of Evidence D |
|---|---|---|---|---|
| Quality of Methodology: The Accuracy, Coherency and Transparency of Evidence. | Relevance of Methodology: The Appropriateness of the Methodology for Answering the Review Question. | Relevance of Evidence to the Review Question: The Relevance of the Focus of the Evidence for Answering the Review Question. | Overall Weight of Evidence: Overall Assessment of the Extent to which the Study Provides Evidence to Answer the Review Question. | |
| Condon et al. (2003) [29] | High | Medium | Medium | Medium |
| Duggan et al. (1992) [30] | High | Medium | Medium | Medium |
| Dykes et al. (2002) [31] | High | Medium | Low | Medium |
| Griffiths et al. (2007) [32] | High | High | Medium | High |
| Kannan et al. (1999) [33] | Medium | Medium | High | Medium |
| Moore et al. (2013) [34] | High | Medium | Medium | Medium |
| Sahota et al. (2015) [35] | High | High | High | High |
| Santorelli et al. (2014) [36] | Medium | Medium | Medium | Medium |
| Sarwar et al. (2002) [37] | High | High | High | High |
| Stearns et al. (2017) [38] | High | Medium | Medium | Medium |
| Thomas and Avery (1997) [39] | Medium | High | High | High |
| Toh et al. (2016) [40] | High | High | High | High |
| Williams and Sahota (1990) [41] | Medium | Low | Medium | Medium |
| Author | Study Title | Study Type | Location | Population | Sample Size | Diversity | Timing | Frequency | Advice | Factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Studies from the United Kingdom | ||||||||||
| Condon et al. (2003) [29] | Cultural influences on breastfeeding and weaning | Cross-sectional | Bristol, UK | Natural mothers, singleton birth, who had breastfed in the last year from Pakistani, Bangladeshi, Somali and Afro-Caribbean background. | 75 (26 in focus group (17 SA), 49 in phone survey (13 SA) | Egg custard and tinned baby food were mentioned by a Bangladeshi focus group. | At 12 weeks complementary feeding (CF) had been commenced by 29% of black and 7% white mothers, 0% of Asians. By 16 weeks; 43% of Asians, 89% black and 90% white mothers. | / | Healthcare professionals, family members, religious texts, and British custom were mentioned. | Weaning began at four months as it was believed this was in accordance with healthcare professional (HCP) advice. |
| Duggan et al. (1992) [30] | The weaning diet of healthy Asian children living in Sheffield. 1. The level and composition of the diet in children from 4 to 40 months of age | Cross-sectional | Sheffield, UK | Healthy Asian weanlings aged 4–40 months and living in Sheffield | 120 Asian children (72% born in Pakistan, 18% in Bangladesh, 10% in Britain) | Meat, fish, commercial baby foods, and fruit juices were described as being amongst foods for CF. | 74% commenced weaning before six months of age. | / | / | / |
| Dykes et al. (2002) [31] | Socio-economic and ethnic influences on infant feeding practices related to oral health | Cohort | UK | Families with babies of Bangladeshi, Indian, Pakistani or White origin. Secondary analysis of Thomas and Avery [39] | 2382 families (764 Indian, 593 Pakistani, 477 Bangladeshi, 548 White) | Described addition of sugar and sugary foods to the bottle at nine months, including sugar, honey, rusks, chocolate powder, and biscuits; 19.8% of Bangladeshi mothers did so, compared to 10.7% (Pakistani), 6.6% (Indian), 6.9% (White). | / | / | / | Education–Pakistani mothers who were in education up to 18 years were significantly less likely to supplement drinks with sugary foods (11.8% vs. 5.3%, p < 0.05 in those who left <18). Low income families were more likely to supplement with sugary foods. |
| Griffiths et al. (2007) [32] | Do early infant feeding practices vary by maternal ethnic group? | Cohort | England, UK | Natural mothers of singleton infants across England | 18,150 (11,286 in England, of which 452 Indian, 857 Pakistani, 249 Bangladeshi) | / | Indian, Pakistani and Bangladeshi mothers were less likely to introduce solids early at <4 months (17%, 12%, 14% respectively) compared to 37% of white mothers. Figure 1 illustrates that close to 100% of mothers had introduced solids by six months. | / | / | / |
| Moore et al. (2013) [34] | Influence of weaning timing advice and associated weaning behaviours in a survey of black and minority ethnic groups in the UK | Cross-sectional | London, UK | Parents/carers recruited from London boroughs with a high percentage BME population | 349 (120 South Asian, 107 Black African, 54 Black Caribbean, 64 Black mixed-race) | / | 100% of SA parents had commenced weaning at 29 weeks, 87% by 26 weeks, 37% by 22 weeks. The mean weaning age for SAs was 23 weeks. The mean for Black Caribbean was 21.1 weeks and was 20.9 for Black African. | / | 76% received advice from a health visitor. Other sources named as the most influential source included mother/grandmother, the internet, GP, friends, internet, and books. | Health visitor advice was associated with a later weaning age. Having a good understanding of the Department of Health weaning guidelines was associated with later weaning (p < 0.001). |
| Sahota et al. (2015) [35] | Ethnic differences in dietary intake at age 12 and 18 months: the Born in Bradford 1000 Study | Cohort | Bradford, UK | Children aged 12–18 months | 1259 (473 White British, 613 Pakistani, 89 Other South Asian, 84 Other) | At 12 months, Pakistani infants consumed more commercial sweet baby meals per week (Odds Ratio (OR) 1.90), more chips/roast potatoes (OR 2.79), more sugar-sweetened drinks (OR 1.68), more fruit (OR 2.20), more pure fruit juice (OR 1.87), less processed meat products (OR 0.11), less commercial savoury baby meals (OR 0.59) than White British infants. | / | / | / | / |
| Santorelli et al. (2014) [36] | Ethnic differences in infant feeding practices and their relationship with BMI at 3 years of age –results from the born in Bradford birth cohort study | Cohort | Bradford, UK | Children from birth to three years of age | 1326 (507 White British, 646 Pakistani, 91 Other SA, 82 Other) | Various food groups were assessed; including non-sweetened solid foods, sweetened solid foods, sweetened and non-sweetened drinks. Sweetened foods were more frequently used as first CF by Pakistani mothers (RR 1.17) compared to White British mothers. | Pakistani (RR 0.88) and Other South Asian mothers (RR 0.82) were less likely to start CF early (<17 weeks) than White British groups; 21% of Pakistani mothers did so compared to White British (37%). Pakistani and Other South Asian mothers commenced CF at a mean of 20–22 weeks. | / | / | / |
| Sarwar (2002) [37] | Infant feeding practices of Pakistani mothers in England and Pakistan | Cross-sectional | Nottingham (UK) and Mian Channu (Pakistan) | Mothers of weaning aged children aged 3–12 months | 90 (45 in England and 45 in Pakistan) | Pakistani mothers in England most commonly use rice as a first food (55%), followed by sweet convenience food (40%), cereal (33%), eggs (26%), savoury convenience (19%), fruit (12%), vegetables and meat (7% each). At the time of the study sweet convenience food and vegetables (45% each) were most commonly eaten. | 40% of Pakistani mothers vs. 49% UK mothers commenced weaning between three and four months; 26% of mothers in Pakistani and 15% in UK did not start to wean until after seven months. | / | Family and friends, in-laws, health professionals. | Familial pressure was present with sometimes conflicting advice, some mothers had lack of confidence in advice given by HCPs. Mothers were given booklets in English and Urdu in Nottingham and audio tapes were desired. |
| Thomas and Avery (1997) [39] | Infant feeding in Asian families | Cohort | England, UK | Families with babies of Bangladeshi, Indian, Pakistani or White origin. | 2382 families (764 Indian, 593 Pakistani, 477 Bangladeshi, 548 White) | Foods used for CF were described from all groups although Vitamin A-rich fruits and vegetables were not separately investigated. Foods used included Rusk, rice cereal, bread, pasta, rice, meat dishes, vegetables, egg or dairy, fresh fruit, desserts, sweets, chocolate, beef, poultry, fish, vegetables, potatoes, yoghurt. The most common food on day before nine months interview for each group was dessert (50% Bangladeshi), fruit (48% Pakistani), non-rice cereal (63% Indian), non-rice cereal (82% white). | At nine months, 100% of Bangladeshi/Pakistani/Indian mothers had introduced CF. At six weeks, it was 1% for all groups; at three months, it was 72%, 73%, and 70% respectively; at six months, 99%, 98%, and 99% respectively. | Among Bangladeshi, Pakistani and Indian mothers, at three months, 8%, 7%, and 6% were giving three meals a day; at six months, 59%, 61%, and 75%; at nine months, 83%, 85%, and 93%; at 12 months, 97%, 98%, and 99%; at 15 months, 100%. | Listed advice providers included baby food company, health clinic, health visitor, hospital, doctor’s surgery, family and friends, mother in law, books/magazines/leaflets, public services. | The survey examined beliefs on CF and lack of knowledge was evident in a high proportion of cases. |
| Williams and Sahota (1990) [41] | An enquiry into the attitudes of Muslim Asian mothers regarding infant feeding practices and dental health | Cross-sectional | Leeds, UK | First generation Muslim Asian mothers; Half of the mothers originated from the Sylhet Region of Bangladesh, and half from Mirpur in Pakistan. | 100 Muslim Asian mothers (50 Bangladeshi origin, 50 Pakistani origin) | Listed drinks at three months included Ribena, orange juice, water, juices, delrosa (rosehip syrup), Gripe water. Energy sources considered suitable included ‘fish, meat, eggs, soup, vegetables, butter, honey’ Apples and oranges were most frequently mentioned daily fruits. | By three months, various non-milk drinks were being given; 15% described giving extra sweeteners, such as honey and rusks. | Midwives, health visitors, television, friends, mother in law, extended family. | Barriers included inadequate knowledge and incorrect advice from a health visitor; a promoter was advice from a doctor. | |
| Studies from the United States | ||||||||||
| Kannan et al. (1999) [33] | Infant feeding practices of Anglo American and Asian Indian American mothers | Cohort | USA | Mothers of Anglo-American and Asian-Indian American children | 50 mothers (25 Anglo-American, 25 Asian-Indian American) | Foods included cereal, juice, fruits, vegetables, meat; rice and banana kheer, potato podimas, dhal laddu, rice khitchri, idli, chappati, bengal gram sundal. Asian-Indian mothers most frequently used iron-fortified rice cereal in the first six months. | Mean age of introduction by Asian-Indian American vs. Anglo-American mothers of various foods: Cereal (3.1 vs. 4.2 months), fruit (3.1 vs. 4.6), juice (2.3 vs. 3.6), vegetables (3.6 vs. 5.3), meat (6.3 vs. 7.2). | Sources were family network, HCPs, paediatricians, literature, and grandmothers. For Asian-Indian mothers grandmothers were primary source for first six months. | ||
| Studies from Canada | ||||||||||
| Stearns et al. (2017) [38] | Ethnic and diet-related differences in the healthy infant microbiome | Cohort | Brampton and Peel region of Ontario, Canada | Mother-child pairs from the South Asian Birth Cohort (START-Canada). | 182 South Asian and 173 White Caucasian mother-child pairs | 88.33% of SA infants commenced feeding with solids at 3–6 months of age, followed by 9.44% at 6–9 months, and 1.11% each at 0–3 months or 9–12 months. | ||||
| Studies from Singapore | ||||||||||
| Toh et al. (2016) [40] | Infant Feeding Practices in a Multi-Ethnic Asian Cohort: The GUSTO Study | Cohort | Singapore | Indian-origin infant-mother dyads recruited from the Singapore National University Hospital and KK Women’s and Children’s Hospital. | 842 mother-infant dyads (510 Chinese, 194 Malay, 138 Indian) | For Indian infants, first food was most commonly rice cereal (42.0%) followed by non-rice cereal (16.7%), rice porridge (13.0%), fruit puree (11.6%), vegetable puree (5.8%), rice (4.3%), baby biscuit (2.2%), others (3.6%), not answered (0.7%). Indian infants were significantly (p < 0.001) more likely to have oil added to their foods than Chinese and Malay counterparts at 9 and 12 months of age. | The majority of Indian infants received their first foods at 24–31 weeks (58.7%) followed by 16–23 weeks (34.1%), ≤15 weeks (4.3%), and ≥32 weeks (2.9%). The time of 24–31 weeks was also most common for Chinese infants (63.7%) and Malay infants (46.4%). | Main food decision maker was mother (78.3%), grandparent (13.0%). Others (8.7%) comprised of father, secondary caregiver, shared responsibility, and not reported. | ||
| Food Type | Study Reference |
|---|---|
| Grains, roots and tubers | 5 studies-[33,35,37,39,40] |
| Legumes and nuts | 2 studies-[33,39] |
| Flesh foods (e.g., meat, fish, poultry and liver/organ meats) | 6 studies-[30,33,35,37,39,41] |
| Dairy products (e.g., milk, yogurt, cheese) | 6 studies-[29,33,35,37,39,41] |
| Eggs | 3 studies-[37,39,41] |
| Vitamin A-rich fruit and vegetables | Not specified |
| Other fruit and vegetables | 6 studies-[33,35,37,39,40,41] |
| Family Level | |||
| Promoters | Study Reference | Barriers | Study Reference |
| Education | 1 study-[31] | Familial pressure | 2 studies-[37,41] |
| Understanding of guidelines | 1 study-[34] | Incorrect knowledge | 2 studies-[39,41] |
| Low income | 1 study-[31] | ||
| Organisational Level | |||
| Promoters | Study Reference | Barriers | Study Reference |
| Healthcare professionals | 3 studies-[34,37,41] | Interactions and advice from healthcare professionals | 3 studies-[29,37,41] |
| Provision of information | 1 study-[37] | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikam, L.; Lingam, R.; Lever, I.; Alexander, E.C.; Amadi, C.; Milner, Y.; Shafi, T.; Stephenson, L.; Ahmed, S.; Lakhanpaul, M. Complementary Feeding Practices for South Asian Young Children Living in High-Income Countries: A Systematic Review. Nutrients 2018, 10, 1676. https://doi.org/10.3390/nu10111676
Manikam L, Lingam R, Lever I, Alexander EC, Amadi C, Milner Y, Shafi T, Stephenson L, Ahmed S, Lakhanpaul M. Complementary Feeding Practices for South Asian Young Children Living in High-Income Countries: A Systematic Review. Nutrients. 2018; 10(11):1676. https://doi.org/10.3390/nu10111676
Chicago/Turabian StyleManikam, Logan, Raghu Lingam, Isabel Lever, Emma C. Alexander, Chidi Amadi, Yasmin Milner, Taimur Shafi, Lucy Stephenson, Sonia Ahmed, and Monica Lakhanpaul. 2018. "Complementary Feeding Practices for South Asian Young Children Living in High-Income Countries: A Systematic Review" Nutrients 10, no. 11: 1676. https://doi.org/10.3390/nu10111676
APA StyleManikam, L., Lingam, R., Lever, I., Alexander, E. C., Amadi, C., Milner, Y., Shafi, T., Stephenson, L., Ahmed, S., & Lakhanpaul, M. (2018). Complementary Feeding Practices for South Asian Young Children Living in High-Income Countries: A Systematic Review. Nutrients, 10(11), 1676. https://doi.org/10.3390/nu10111676





