Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Probiotic Supplements and Allocation
2.3. Anthropometric Measurement
2.4. Functional Parameters
2.5. Biochemical Parameters
2.6. In Vitro Assay
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barton, M. Endothelin in the microvasculature. In W: Microvascular Research: Biology and Pathology; Shepro, D., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2006; pp. 5–12. [Google Scholar]
- Sagach, V.; Bondarenko, A.; Bazilyuk, O.; Kotsuruba, A. Endothelial dysfunction: Possible mechanisms and ways of correction. Exp. Clin. Cardiol. 2006, 11, 107–110. [Google Scholar] [PubMed]
- Sivitz, W.I.; Wayson, S.M.; Bayless, M.L.; Sinkey, C.A.; Haynes, W.G. Obesity impairs vascular relaxation in human subjects: Hyperglycemia exaggerates adrenergic vasoconstriction arterial dysfunction in obesity and diabetes. J. Diabetes Complicat. 2007, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Mundy, A.L.; Haas, E.; Bhattacharya, I.; Widmer, C.C.; Kretz, M.; Hofmann-Lehmann, R.; Minotti, R.; Barton, M. Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: Implications for diet-induced obesity. Cardiovasc. Res. 2007, 73, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.; Gluckman, P. Endothelial dysfunction and cardiovascular disease: The role of predictive adaptive responses. Heart 2005, 91, 864–866. [Google Scholar] [CrossRef] [PubMed]
- Bolad, I.; Delafontaine, P. Endothelial dysfunction: Its role in hypertensive coronary disease. Curr. Opin. Cardiol. 2005, 20, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Baretella, O.; Meyer, M.R. Obesity and risk of vascular disease: Importance of endothelium-dependent vasoconstriction. Br. J. Pharmacol. 2012, 165, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Trojan, P.; Janik, M.; Przybyło, M. Śródbłonek-niedoceniany organ. 1. Budowa i rola w procesach fizjologicznych. Kosm. Probl. Nauk Biol. 2014, 4, 555–568. [Google Scholar]
- Tam, J.; Duda, D.G.; Perentes, J.Y.; Quadri, R.S.; Fukumura, D.; Jain, R.K. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: Role of local versus bone marrow-derived endothelial cells. PLoS ONE 2009, 4, e4974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.L.; Vita, J.A. Effects of systemic inflammation on endothelium—Dependent vasodilation. Trends Cardiovasc. Med. 2006, 16, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Ben-Aicha, S.; Badimon, L. New insights into the role of adipose tissue in thrombosis. Thromb. Haemost. 2013, 110, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Alessi, M.C. Thrombosis in central obesity and metabolic syndrome: Mechanisms and epidemiology. Cardiovasc. Res. 2017, 113, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Natural anticoagulant inhibitors activated protein C. Best Pract. Res. Clin. Hematol. 2004, 17, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 1998, 67, 395–424. [Google Scholar] [CrossRef] [PubMed]
- Cieślik-Guerra, U. Methods of arterial stiffness measurement. Arter. Hypertens. 2011, 15, 42–48. [Google Scholar]
- Lane, H.A.; Smith, J.C.; Davies, J.S. Noninvasive assessment of preclinical atherosclerosis. Vasc. Health Risk Manag. 2006, 2, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdański, P.; Suliburska, J.; Szulińska, M.; Sikora, M.; Walkowiak, J.; Jakubowski, H. L-Arginine and vitamin C attenuate pro-atherogenic effects of high-fat diet on biomarkers of endothelial dysfunction in rats. Biomed. Pharmacother. 2015, 76, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Szulinska, M.; Suliburska, J.; Pupek-Musialik, D.; Jablecka, A.; Witmanowski, H. Supplementation with L-arginine favorably influences plasminogen activator inhibitor type 1 concentration in obese patients. A randomized, double blind trial. J. Endocrinol. Investig. 2013, 36, 221–226. [Google Scholar]
- Miczke, A.; Szulińska, M.; Hansdorfer-Korzon, R.; Kręgielska-Narożna, M.; Sluliburska, J.; Walkowiak, J.; Bogdański, P. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: A doubleblind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 150–156. [Google Scholar] [PubMed]
- Szulińska, M.; Kręgielska-Narożna, M.; Świątek, J.; Styś, P.; Kuźnar-Kamińska, B.; Jakubowski, H.; Walkowiak, J.; Bogdański, P. Garlic extract favorably modifies markers of endothelial function in obese patients-randomized double blind placebo-controlled nutritional intervention. Biomed. Pharmacother. 2018, 102, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Stępień, M.; Kręgielska-Narożna, M.; Suliburska, J.; Skrypnik, D.; Bąk-Sosnowska, M.; Kujawska-Łuczak, M.; Grzymisławska, M.; Bogdański, P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl-induced hypertensive rat model. Food Nutr. Res. 2017, 61, 1295525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, S.; Ormel, G. Influence of the multispecies probiotic Ecologic® BARRIER on parameters of intestinal barrier function. Food Nutr. Sci. 2014, 5, 1739–1745. [Google Scholar]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.; Yakout, S.; Alnaami, M.; Alokail, M.; McTernan, F. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-dependent effects of multispecies probiotic supplementation on the Lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: A 12-Week randomized clinical trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, G.; Brown, R.; Salciccioli, L. Relationship between aortic atherosclerosis and non-invasive measures of arterial stiffness. Atherosclerosis 2007, 195, E190–E194. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T. Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [PubMed]
- O’Rourke, M.F. Wave travel and reflection in the arterial system. J. Hypertens. Suppl. 1999, 17, S45–S47. [Google Scholar] [PubMed]
- Edgell, C.J.; McDonald, C.C.; Graham, J.B. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 1983, 80, 3734–3737. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Witowski, J.; Korybalska, K.; Wisniewska, J.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jörres, A. Effect of glucose degradation products on human peritoneal mesothelial cell function. J. Am. Soc. Nephrol. 2000, 11, 729–739. [Google Scholar] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Aortic stiffness for cardiovascular risk prediction: Just measure it, just do it! J. Am. Coll. Cardiol. 2014, 63, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Kingwell, B.; Bank, A.; Weber, M.; Struijker-Boudier, H. Clinical applications of arterial stiffness: Therapeutics and pharmacology. Am. J. Hypertens. 2002, 15, 453–458. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Staessen, J.A.; Vlachopoulos, C.; Duprez, D.; Plante, G.E. Clinical applications of arterial stiffness; definitions and reference values. Am. J. Hypertens. 2002, 15, 426–444. [Google Scholar] [CrossRef] [Green Version]
- Pannier, B.M.; Avolio, A.P.; Hoeks, A.; Mancia, G.; Takazawa, K. Methods and devices for measuring arterial compliance in humans. Am. J. Hypertens. 2002, 15, 743–753. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. European network for non-invasive investigation of large arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Ebel, B.; Lemetis, G.; Beney, L.; Cachon, R.; Sokol, H.; Langella, P.; Gervais, P. Impact of probiotics on risk factors for cardiovascular diseases. A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Porteri, E.; Guelfi, D.; Muiesan, M.L.; Valentini, U.; Cimino, A.; Girelli, A.; Rodella, L.; Bianchi, R.; Sleiman, I.; et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulindependent diabetes mellitus. Circulation 2001, 103, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Rahman, A.R.; Rasool, A.H. Effect of angiotensin II on pulse wave velocity in humans is mediated through angiotensin II type 1 (AT(1)) receptors. J. Hum. Hypertens. 2002, 16, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Gøbel, R.J.; Larsen, N.; Jakobsen, M.; Mølgaard, C.; Michaelsen, K.F. Probiotics to adolescents with obesity: Effects on inflammation and metabolic syndrome. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. mBio 2014, 5, e01011-14. [Google Scholar] [CrossRef] [PubMed]
- Finney, A.C.; Orr, A.W. Guidance Molecules in Vascular Smooth Muscle. Front. Physiol. 2018, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Venugopal, J.; Wang, J.; Kleiman, K.; Guo, C.; Eitzman, D.T. Obesity-induced endothelial dysfunction is prevented by neutrophil extracellular trap inhibition. Sci. Rep. 2018, 8, 4881. [Google Scholar] [CrossRef] [PubMed]
- Korybalska, K.; Luczak, J.; Swora-Cwynar, E.; Kanikowska, A.; Czepulis, N.; Kanikowska, D.; Skalisz, H.; Breborowicz, A.; Grzymislawski, M.; Witowski, J. Weight loss-dependent and -independent effects of moderate calorie restriction on endothelial cell markers in obesity. J. Physiol. Pharmacol. 2017, 68, 597–608. [Google Scholar] [PubMed]
- De Andrés, J.; Manzano, S.; García, C.; Rodríguez, J.M.; Espinosa-Martos, I.; Jiménez, E. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef. Microbes 2018, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F. Effects if multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Am. Coll. Nutr. 2017, 36, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mahrabany, E.; Alipour, B.; Homayouni-Rad, A. Probiotic supplementation improves inflammatory status in patient with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Martio, J.; Korpela, M. Effects of probiotic therapy on the acticity and activation of mild rheumatoid arthritis—A pilot study. Scand. J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Villaret, A.; Galitzky, J.; Decaunes, P.; Esteve, D.; Marques, M.A.; Sengenès, C.; Chiotasso, P.; Tchkonia, T.; Lafontan, M.; Kirkland, J.L.; et al. Adipose tissue endothelial cells from obese human subjects: Differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes 2010, 59, 2755–2763. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell. Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Patterson, C.; Perrella, M.A.; Endege, W.O.; Yoshizumi, M.; Lee, M.E.; Haber, E. Downregulation of Vascular Endothelial Growth Factor Receptors by Tumor Necrosis Factor-a in Cultured Human Vascular Endothelial Cells. J. Clin. Investig. 1996, 98, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Maeda, K.; Kuriyama, H.; Okamoto, Y.; Hotta, K.; Nishida, M.; Takahashi, M.; Nakamura, T.; et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation 1999, 100, 2473–2476. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Wunsch, E.; Mydlowska, M.; Milkiewicz, M.; Serwin, K.; Mularczyk, M.; Milkiewicz, P.; Raszeja-Wyszomirska, J. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J. Physiol. Pharmacol. 2016, 67, 867–877. [Google Scholar] [PubMed]
- Li, W.; Yang, S.; Kim, S.O.; Reid, G.; Challis, J.R.; Bocking, A.D. Lipopolysaccharide-Induced Profiles of Cytokine, Chemokine, and Growth Factors Produced by Human Decidual Cells Are Altered by Lactobacillus rhamnosus GR-1 Supernatant. Reprod. Sci. 2014, 21, 939–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yang, G.; Song, J.H.; Xu, H.; Li, D.; Goldsmith, J.; Zeng, H.; Parsons-Wingerter, P.A.; Reinecker, H.C.; Kelly, C.P. Probiotic yeast inhibits VEGFR signaling and angiogenesis in intestinal inflammation. PLoS ONE 2013, 8, e64227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, D.T.; Farb, M.G.; Kikuchi, R.; Karki, S.; Tiwari, S.; Bigornia, S.J.; Bates, D.O.; LaValley, M.P.; Hamburg, N.M.; Vita, J.A.; et al. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 2014, 130, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Fukuda, S.; Ohno, H.; Yamamoto, N. Exposure to probiotic Lactobacillus acidophilus L-92 modulates gene expression profiles of epithelial Caco-2 cells. J. Med. Food 2012, 15, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Seljeflot, I.; Sommervoll, L.; Christensen, B.; Otterstad, J.E.; Arnesen, H. Increased levels of endothelial haemostatic markers in patients with coronary heart disease. Thromb. Res. 2002, 105, 25–31. [Google Scholar] [CrossRef]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Agüero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef] [PubMed]
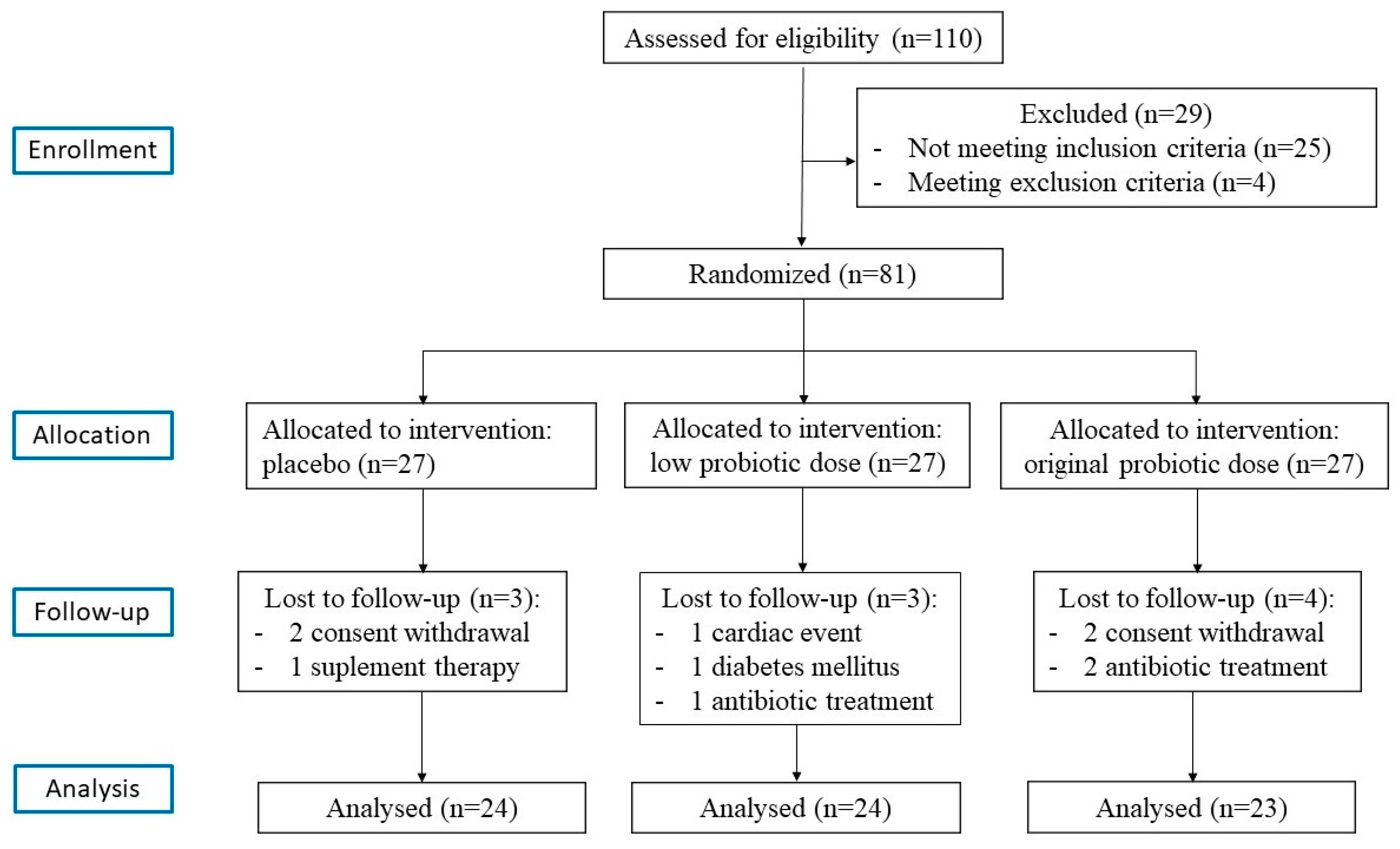

| Variable | Group | Mean ± SD | SMD | p-Value | p-Value Post-Hoc Test |
|---|---|---|---|---|---|
| BMI (kg/m2) | HD | 36.57 ± 5.95 | 0.09 * | ns | |
| LD | 36.00 ± 5.20 | −0.02 † | 0.9365 | ||
| Placebo | 36.10 ± 11.93 | 0.10 # | |||
| Age (years) | HD | 55.16 ± 6.87 | −0.50 * | ns | |
| LD | 56.38 ± 6.55 | −0.35 † | 0.2977 | ||
| Placebo | 58.72 ± 7.25 | −0.18 # | |||
| Functional parameters | |||||
| SBP (mm Hg) | HD | 134.80 ± 10.10 | 0.10 * | ns | |
| LD | 133.50 ± 10.86 | −0.01 † | 0.7391 | ||
| Placebo | 133.64 ± 12.20 | 0.12 # | |||
| DBP (mm Hg) | HD | 79.88 ± 8.05 | −0.51 * | ns | |
| LD | 82.46 ± 5.53 | −0.20 † | 0.1446 | ||
| Placebo | 83.76 ± 7.26 | −0.37 # | |||
| PWA SP (mm Hg) | HD | 131.32 ± 7.50 | 0.40 * | ns | |
| LD | 126.56 ± 12.81 | −0.05 † | 0.1964 | ||
| Placebo | 127.23 ± 12.43 | 0.45 # | |||
| PWA PP (mm Hg) | HD | 46.44 ± 11.18 | 0.01 * | ns | |
| LD | 41.88 ± 10.93 | −0.41 † | 0.1515 | ||
| Placebo | 46.35 ± 10.65 | 0.41 # | |||
| PWA Alx | HD | 33.28 ± 13.30 | 0.06 * | ns | |
| LD | 32.36 ± 11.19 | −0.02 † | 0.8618 | ||
| Placebo | 33.62 ± 10.30 | 0.08 # | |||
| PWV (m/s) | HD | 7.32 ± 0.90 | 0.46 * | ns | |
| LD | 6.92 ± 1.78 | 0.01 † | 0.2248 | ||
| Placebo | 6.90 ± 0.94 | 0.28 # | |||
| Biochemical parameters | |||||
| IL-6 (pg/mL) | HD | 4.50 ± 0.51 | 0.08 * | ns | |
| LD | 4.71 ± 0.49 | 0.48 † | 0.1887 | ||
| Placebo | 4.46 ± 0.56 | −0.42 # | |||
| VEGF (pg/mL) | HD | 155.04 ± 19.38 | 0.81 * | * 0.0192 | |
| LD | 142.46 ± 27.24 | 0.19 † | 0.0034 | ||
| Placebo | 137.64 ± 23.20 | 0.53 # | |||
| TNF (pg/L) | HD | 1.04 ± 0.34 | −0.03 * | ns | |
| LD | 1.24 ± 0.36 | 0.54 † | 0.0663 | ||
| Placebo | 1.05 ± 0.34 | −0.57 # | |||
| TM (ng/mL) | HD | 4.20 ± 0.73 | 0.02 * | ns | |
| LD | 4.01 ± 0.58 | −0.30 † | 0.5170 | ||
| Placebo | 4.19 ± 0.64 | 0.29 # | |||
| vWF (ng/mL) | HD | 84.86 ± 6.40 | 0.11 * | ns | |
| LD | 83.90 ± 5.71 | −0.05 † | 0.8522 | ||
| Placebo | 84.18 ± 6.47 | 0.16 # | |||
| Variable | Group | Baseline | After 3 Months | SMD | p-Value |
|---|---|---|---|---|---|
| BMI | HD | 36.57 ± 5.95 | 36.22 ± 5.29 | 0.31 | 0.3165 |
| LD | 36.00 ± 5.20 | 35.51 ± 5.16 | 0.39 | 0.1209 | |
| Placebo | 36.10 ± 4.37 | 36.04 ± 4.32 | 0.07 | 0.9612 | |
| Functional parameters | |||||
| SBP (mm Hg) | HD | 134.80 ± 10.10 | 131.40 ± 9.41 | 0.39 | 0.0359 |
| LD | 133.50 ± 10.86 | 130.88 ± 9.42 | 0.26 | 0.0498 | |
| Placebo | 133.64 ± 12.20 | 131.52 ± 12.31 | 0.17 | 0.1795 | |
| DBP (mm Hg) | HD | 79.88 ± 8.05 | 79.36 ± 7.42 | 0.07 | 0.7031 |
| LD | 82.46 ± 5.53 | 81.73 ± 6.40 | 0.12 | 0.5095 | |
| Placebo | 83.76 ± 7.26 | 81.88 ± 7.20 | 0.26 | 0.2927 | |
| PWA SP (mm Hg) | HD | 131.32 ± 7.50 | 121.75 ± 11.14 | 1.01 | 0.0004 |
| LD | 126.56 ± 12.81 | 127.28 ± 16.53 | −0.05 | 0.6997 | |
| Placebo | 127.23 ± 12.43 | 127.92 ± 16.79 | −0.04 | 0.8078 | |
| PWA PP (mm Hg) | HD | 46.44 ± 11.18 | 40.63 ± 8.65 | 0.67 | 0.0103 |
| LD | 41.88 ± 10.93 | 41.45 ± 14.07 | 0.03 | 0.7989 | |
| Placebo | 46.35 ± 10.65 | 44.16 ± 8.50 | 0.26 | 0.3219 | |
| PWA Alx | HD | 33.28 ± 13.30 | 27.56 ± 9.94 | 0.58 | 0.0021 |
| LD | 32.36 ± 11.19 | 33.48 ± 11.12 | −0.10 | 0.1711 | |
| Placebo | 33.62 ± 10.30 | 33.19 ± 11.41 | 0.04 | 0.8751 | |
| PWV (m/s) | HD | 7.32 ± 0.90 | 6.45 ± 0.77 | 1.13 | 0.0013 |
| LD | 6.92 ± 1.78 | 6.74 ± 1.29 | 0.14 | 0.3536 | |
| Placebo | 6.90 ± 0.94 | 6.79 ± 0.95 | 0.12 | 0.3221 | |
| Biochemical parameters | |||||
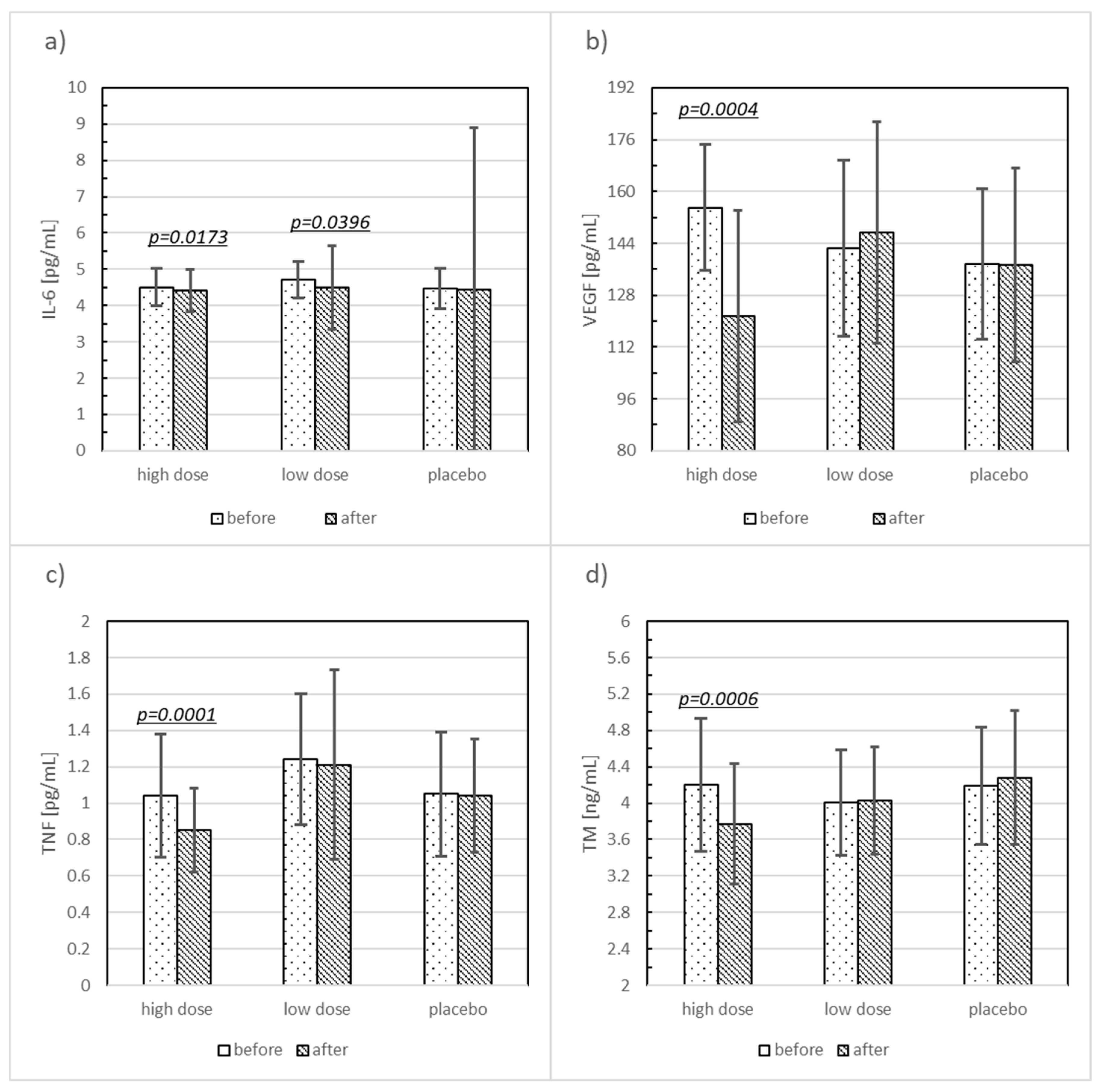
| IL-6 (pg/mL) | HD | 4.50 ± 0.51 | 4.41 ± 0.59 | 0.15 | 0.0173 |
| LD | 4.71 ± 0.49 | 4.50 ± 1.15 | 0.43 | 0.0396 | |
| Placebo | 4.46 ± 0.56 | 4.45 ± 4.45 | 0.02 | 0.3282 | |
| VEGF (pg/mL) | HD | 155.04 ± 19.38 | 121.52 ± 32.66 | 1.73 | 0.0004 |
| LD | 142.46 ± 27.24 | 147.35 ± 34.19 | −0.18 | 0.5991 | |
| Placebo | 137.64 ± 23.20 | 137.32 ± 29.94 | 0.01 | 0.7467 | |
| TNF (pg/mL) | HD | 1.04 ± 0.34 | 0.85 ± 0.23 | 0.56 | 0.0001 |
| LD | 1.24 ± 0.36 | 1.21 ± 052 | 0.08 | 0.2427 | |
| Placebo | 1.05 ± 0.34 | 1.04 ± 0.31 | 0.03 | 0.7610 | |
| TM (ng/mL) | HD | 4.20 ± 0.73 | 3.77 ± 0.66 | 0.59 | 0.0006 |
| LD | 4.01 ± 0.58 | 4.03 ± 0.59 | −0.03 | 0.8081 | |
| Placebo | 4.19 ± 0.64 | 4.28 ± 0.74 | −0.14 | 0.5938 | |
| vWF (ng/mL) | HD | 84.86 ± 6.40 | 84.80 ± 6.45 | 0.01 | 0.8929 |
| LD | 83.90 ± 5.71 | 84.16 ± 5.58 | −0.05 | 0.8081 | |
| Placebo | 84.18 ± 6.47 | 83.55 ± 5.96 | 0.10 | 0.9544 | |
| Variable | Group | Mean ± SD | SMD | p-Value | p-Value Post-Hoc Test |
|---|---|---|---|---|---|
| ΔBMI | HD | −0.35 ± 1.29 | −0.26 * | ns | |
| LD | −0.49 ± 1.29 | −0.38 † | 0.6960 | ||
| Placebo | −0.06 ± 0.87 | 0.11 # | |||
| Functional parameters | |||||
| ΔSBP (mm Hg) | HD | −3.76 ± 8.47 | −0.21 * | ns | |
| LD | −2.62 ± 9.63 | −0.06 † | 0.7789 | ||
| Placebo | −2.12 ± 6.83 | −0.13 # | |||
| ΔDBP (mm Hg) | HD | −0.52 ± 6.74 | 0.17 * | ns | |
| LD | −0.73 ± 5.57 | 0.16 † | 0.7677 | ||
| Placebo | −1.88 ± 8.74 | 0.03 # | |||
| Δ PWA SP (mm Hg) | HD | −9.57 ± 10.87 | −1.00 * | * 0.0054 # 0.0057 | |
| LD | 0.72 ± 12.49 | −0.01 † | 0.0016 | ||
| Placebo | 0.69 ± 10.61 | −0.91 # | |||
| ΔPWA PP (mm Hg) | HD | −5.92 ± 10.55 | −0.37 * | ||
| LD | −1.70 ± 9.97 | 0.02 † | 0.1439 | ns | |
| Placebo | −1.88 ± 11.10 | −0.41 # | |||
| ΔPWA Alx | HD | −5.72 ± 8.84 | −0.55 * | * 0.0079 | |
| LD | 1.12 ± 4.73 | 0.19 † | 0.0084 | ||
| Placebo | −0.43 ± 10.45 | −0.97 # | |||
| ΔPWV (m/s) | HD | −0.87 ± 1.69 | −0.82 * | * 0.0045 # 0.0189 | |
| LD | −0.19 ± 1.76 | −0.07 † | 0.0028 | ||
| Placebo | −0.10 ± 0.58 | −0.55 # | |||
| Biochemical parameters | |||||
| ΔIL-6 (pg/mL) | HD | −0.09 ± 0.02 | −0.38 * | ns | |
| LD | −0.21 ± 0.99 | −0.10 † | 0.2461 | ||
| Placebo | −0.009 ± 0.08 | −0.09 # | |||
| ΔVEGF (pg/mL) | HD | −33.52 ± 36.84 | −1.09 * | * 0.0001 # 0.0007 | |
| LD | 4.88 ± 33.08 | 0.20 † | 0.0001 | ||
| Placebo | −0.58 ± 21.75 | −1.10 # | |||
| ΔTNF (pg/mL) | HD | −0.20 ± 0.22 | −1.03 * | * 0.0009 # 0.0471 | |
| LD | −0.03 ± 0.28 | −0.09 † | 0.0012 | ||
| Placebo | −0.01 ± 0.14 | −0.68 # | |||
| ΔTM (ng/mL) | HD | −0.43 ± 0.52 | −0.78 * | * 0.0194 | |
| LD | −0.14 ± 1.03 | −0.25 † | 0.0172 | ||
| Placebo | 0.09 ± 0.79 | −0.36 # | |||
| ΔvwF (ng/mL) | HD | −0.07 ± 6.64 | 0.07 * | ns | |
| LD | 0.12 ± 5.13 | 0.11 † | 0.9915 | ||
| Placebo | −0.44 ± 4.64 | −0.03 # | |||
| Variable | Study Population | |||
|---|---|---|---|---|
| Group | Mean ± SD | SMD | p-Value | |
| % of control before | HD | 144.03 ± 43.18 | −0.67 * | 0.1042 |
| LD | 160.83 ± 32.07 | −0.30 † | ||
| Placebo | 171.23 ± 37.51 | −0.44 # | ||
| % of control after | HD | 159.68 ± 38.42 | 0.22 * | 0.4502 |
| LD | 163.50 ± 33.88 | 0.33 † | ||
| Placebo | 151.12 ± 39.96 | −0.11 # | ||
| Mean Δ | HD | 15.65 ± 54.28 | 0.72 * | 0.0529 |
| LD | 2.67 ± 30.78 | 0.60 † | ||
| Placebo | −20.11 ± 44.17 | 0.29 # | ||
| % of change | HD | 27.94 ± 77.65 | 0.65 * | 0.0513 |
| LD | 3.28 ± 20.48 | 0.57 † | ||
| Placebo | −9.77 ± 25.29 | 0.43 # | ||
| % of control before vs. after | HD | 144.03 vs. 159.68 | −0.27 | 0.1821 |
| LD | 160.83 vs. 163.50 | −0.09 | 0.7716 | |
| Placebo | 171.23 vs. 151.12 | 0.44 | 0.0520 | |
| Group | Correlated Parameters | p | r |
|---|---|---|---|
| LD | SBP II & PWV II | 0.0181 | 0.46 |
| DBP II & Δ PWV | 0.0252 | −0.43 | |
| IL-6 II & Δ TM | 0.0482 | −0.39 | |
| sTM II & PWA Alx II | 0.0401 | −0.41 | |
| HD | SBP II & PWA PP II | 0.0174 | 0.54 |
| DBP II & TNF II | 0.0121 | 0.49 | |
| IL-6 II & PWA SP II | 0.0229 | 0.46 | |
| IL-6 II & Δ TNF | 0.0249 | −0.48 | |
| TM II & SBP II | 0.0278 | 0.41 | |
| TM II & DBP II | 0.0058 | 0.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, 1672. https://doi.org/10.3390/nu10111672
Szulińska M, Łoniewski I, Skrypnik K, Sobieska M, Korybalska K, Suliburska J, Bogdański P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients. 2018; 10(11):1672. https://doi.org/10.3390/nu10111672
Chicago/Turabian StyleSzulińska, Monika, Igor Łoniewski, Katarzyna Skrypnik, Magdalena Sobieska, Katarzyna Korybalska, Joanna Suliburska, and Paweł Bogdański. 2018. "Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study" Nutrients 10, no. 11: 1672. https://doi.org/10.3390/nu10111672
APA StyleSzulińska, M., Łoniewski, I., Skrypnik, K., Sobieska, M., Korybalska, K., Suliburska, J., & Bogdański, P. (2018). Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients, 10(11), 1672. https://doi.org/10.3390/nu10111672








