Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Diet
2.4. Characterization of Animals
2.5. Hepatic Glycogen Measurement
2.6. Determination of Hepatic Total Lipid and Protein Contents
2.7. Liver Histochemical Analysis
2.8. Liver Perfusion Experiments
2.9. Analytical Assays of Liver Perfusion Experiments
2.10. Multiple-Indicator Dilution Experiments
2.11. Calculation of Multiple-Indicator Dilution Experiments
2.12. Mitochondria Isolation
2.13. Determination of Oxygen Consumption by Isolated Mitochondria Oxidizing Fatty Acids
2.14. Statistical Analysis
3. Results
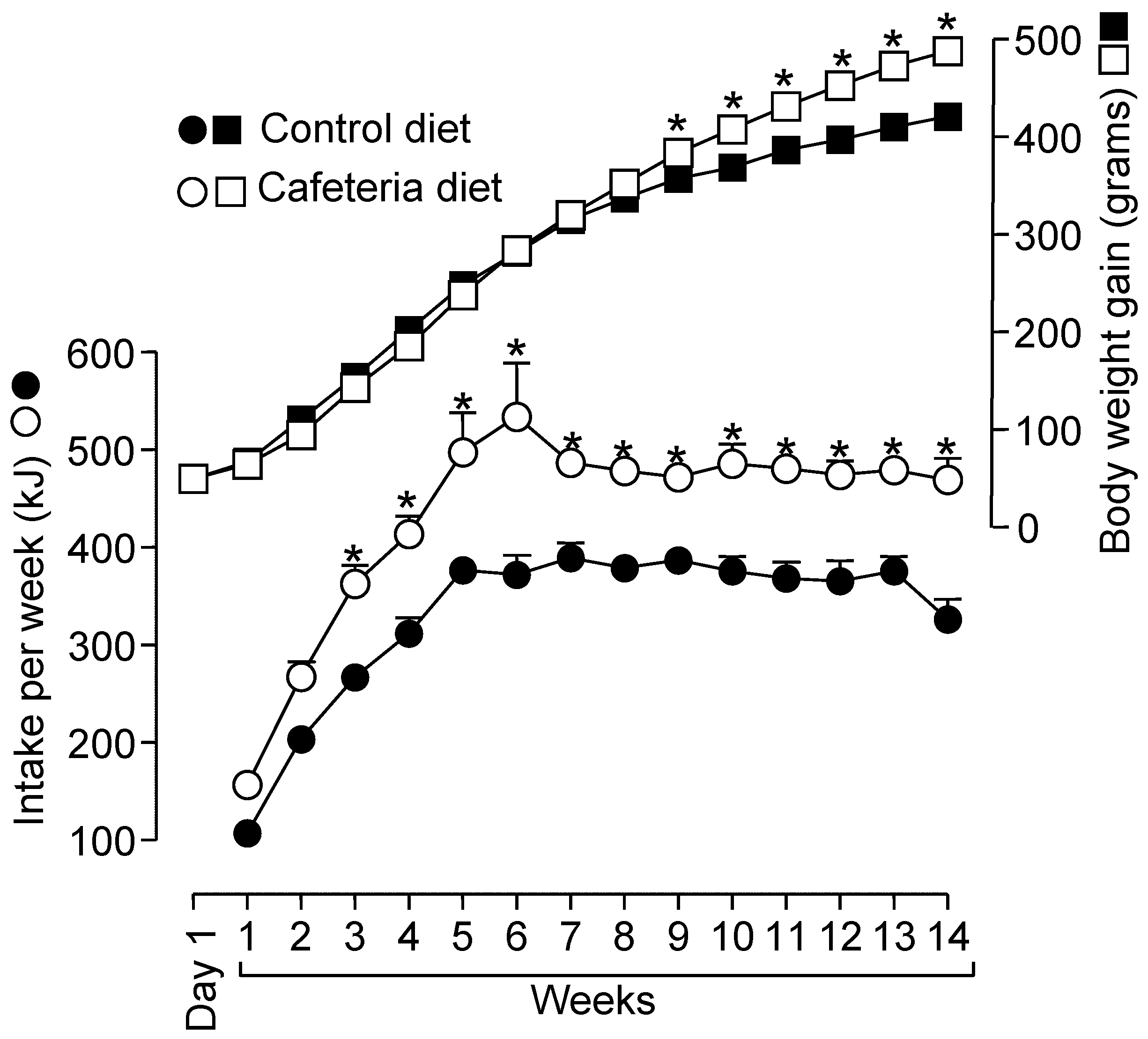
3.1. Biometrical Parameters, Hepatic Glycogen Measurements, and Determination of Hepatic Total Lipid and Protein Contents in Rats Fed a Standard or a Cafeteria Diet
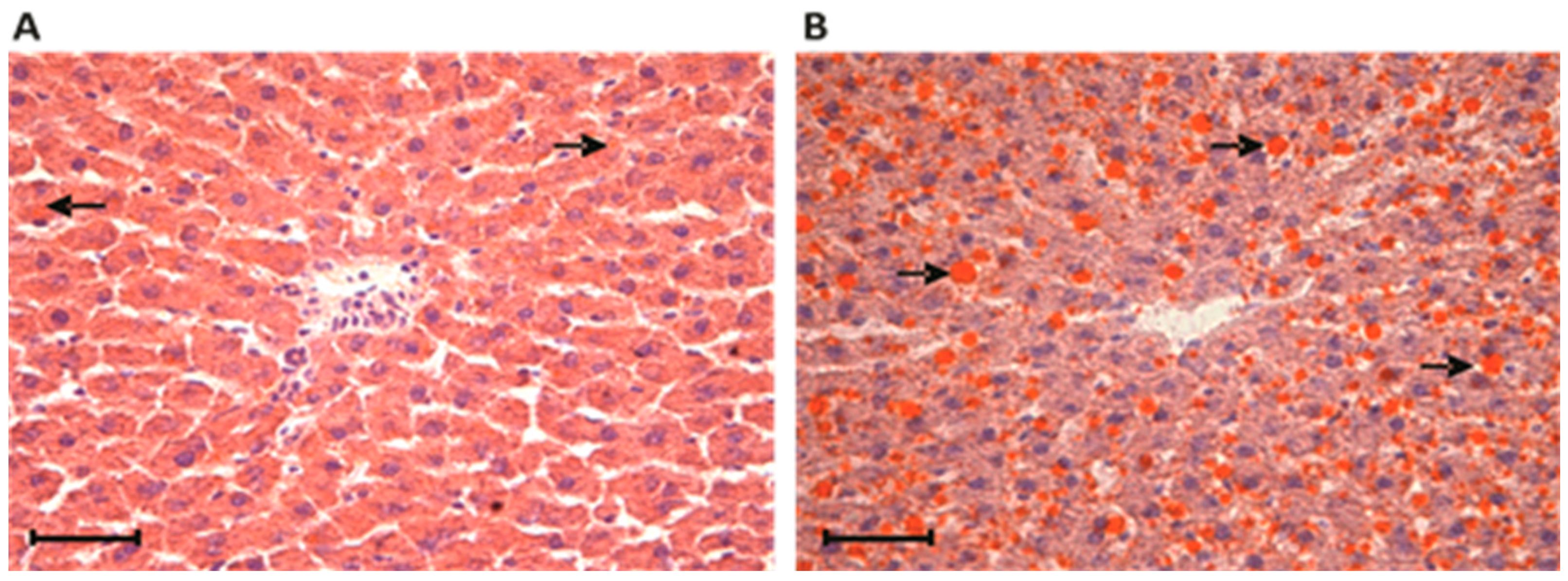
3.2. Liver Histochemical Analysis in Rats Fed a Standard or a Cafeteria Diet
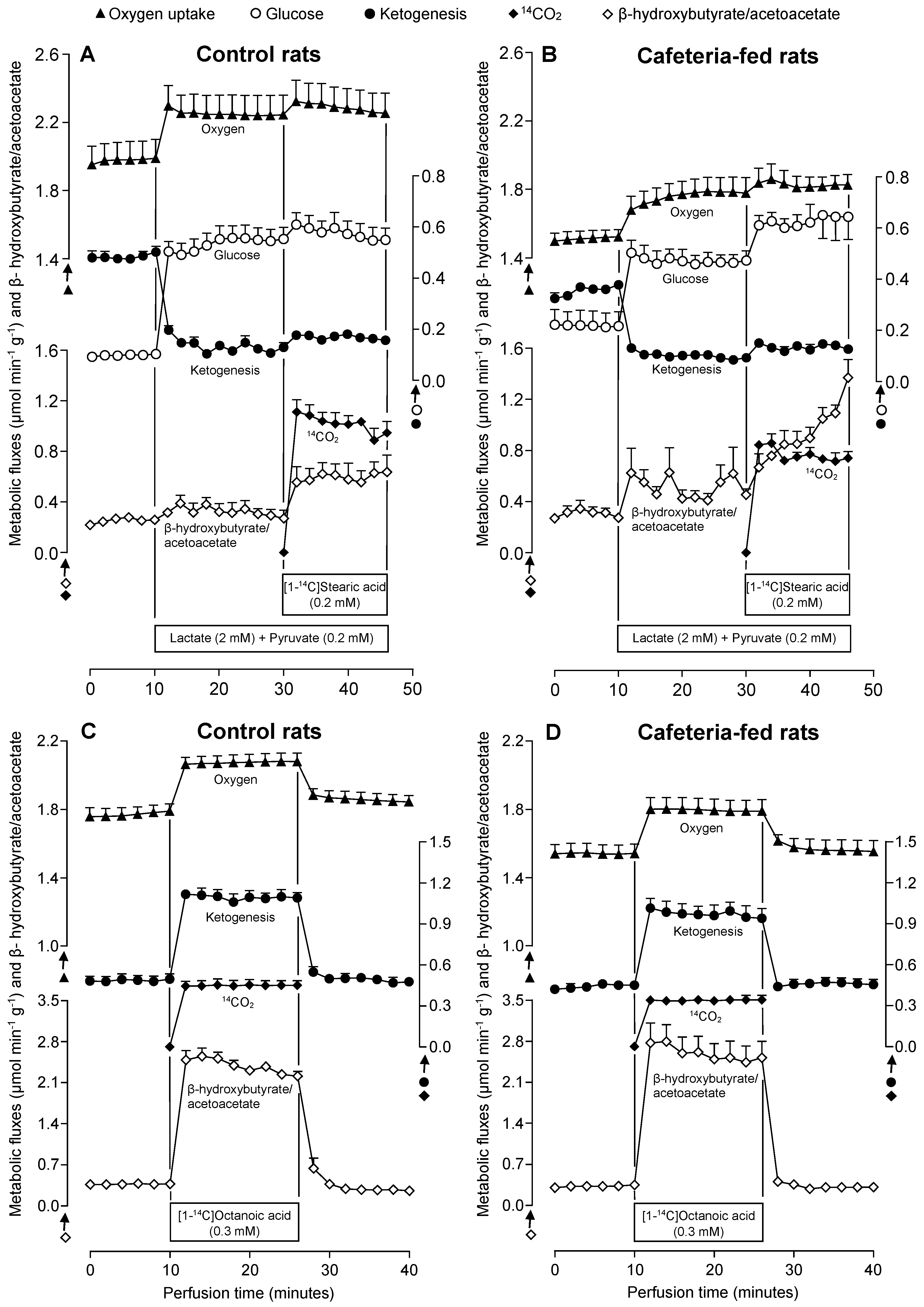
3.3. Metabolic Fluxes Due to Lactate Plus Pyruvate Infusion in the Absence and Presence of Stearate in Livers of Rats Fed a Standard or a Cafeteria Diet
3.4. Metabolic Fluxes Due to Octanoate Infusion in Livers of Rats Fed a Standard or a Cafeteria Diet
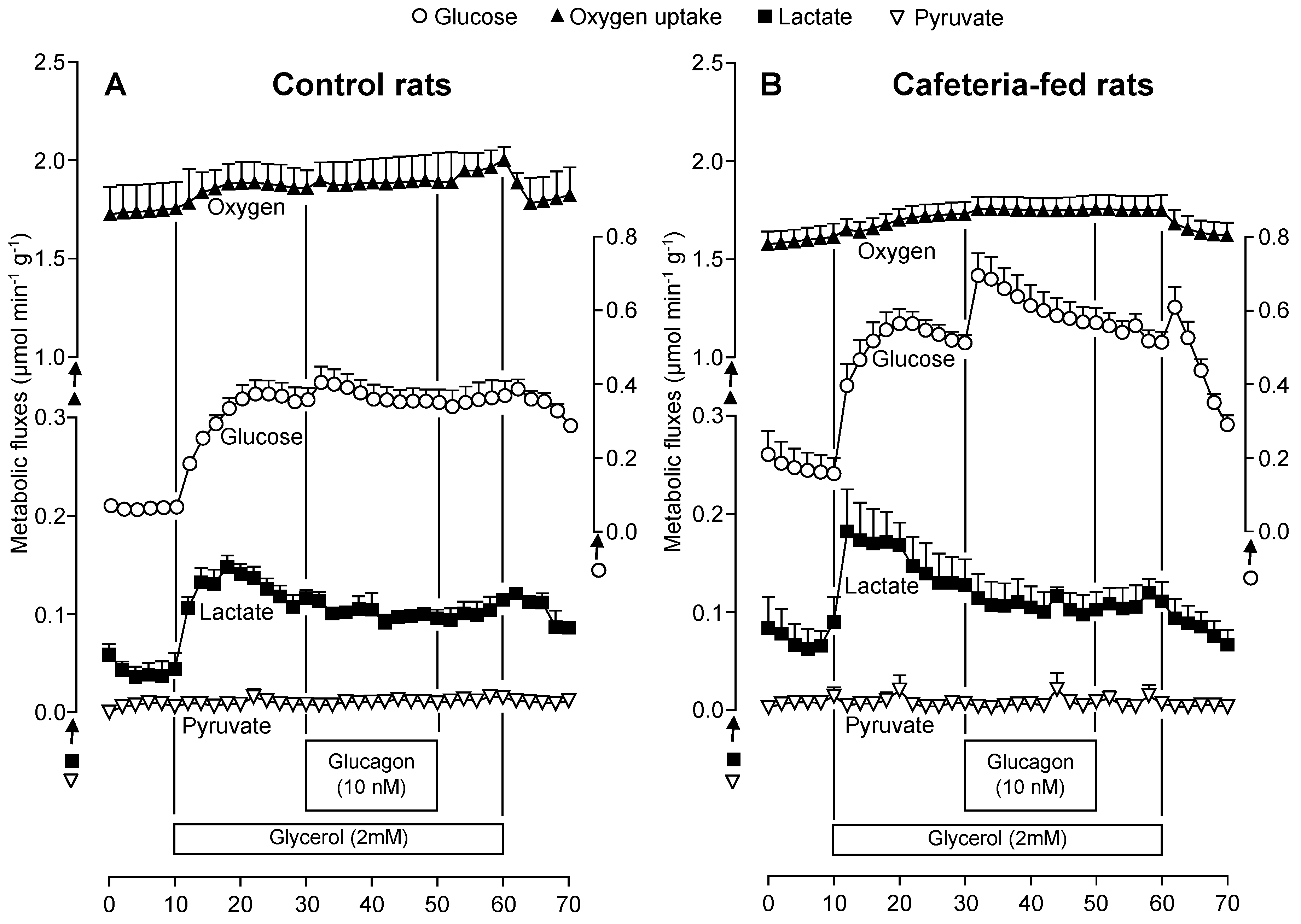
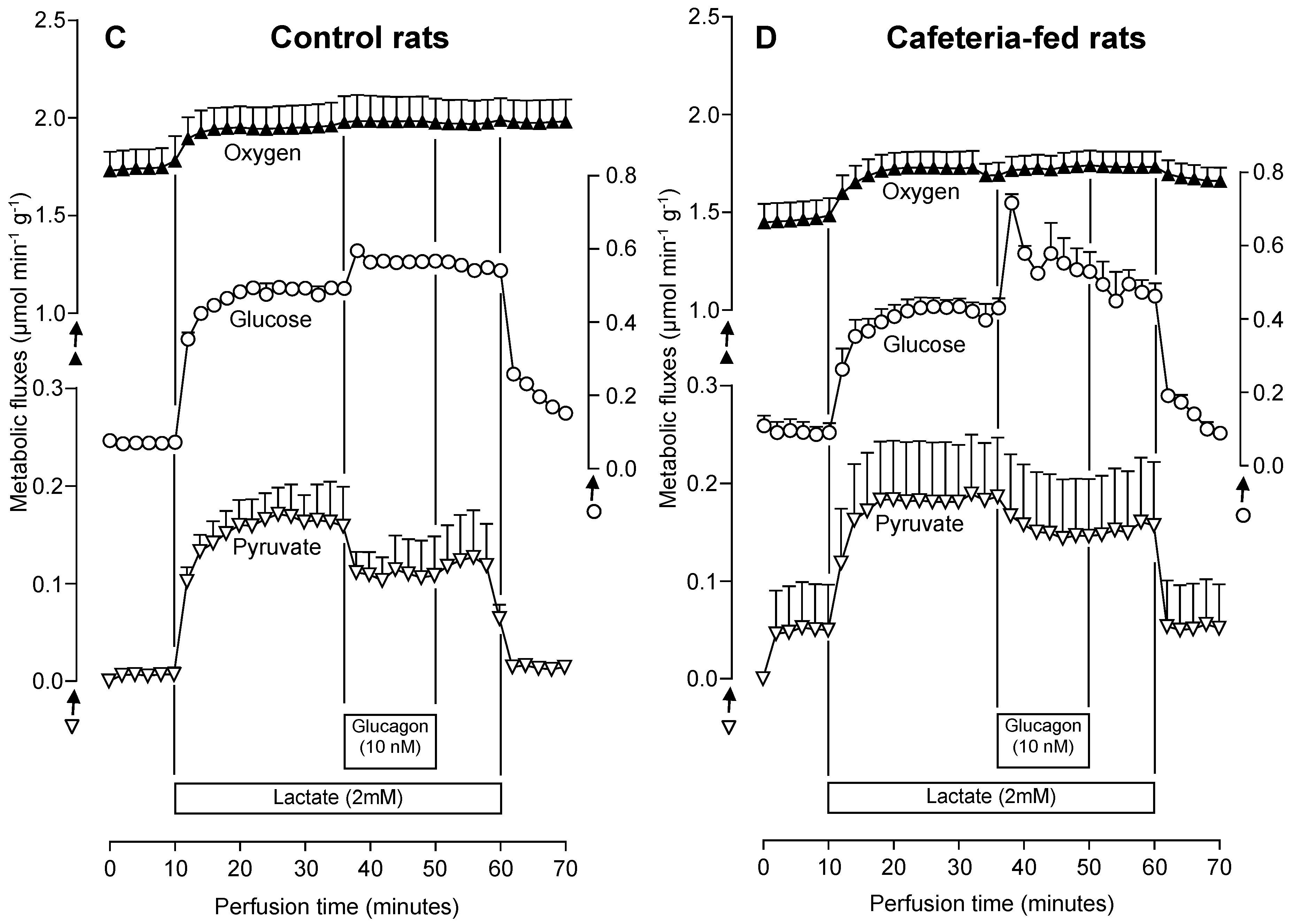
3.5. The Glucagon Effects on Gluconeogenesis Due to Lactate or Glycerol Infusion in Livers of Rats Fed a Standard or a Cafeteria Diet
3.6. Fatty Acid Oxidation in Isolated Mitochondria from Livers of Rats Fed a Standard or a Cafeteria Diet
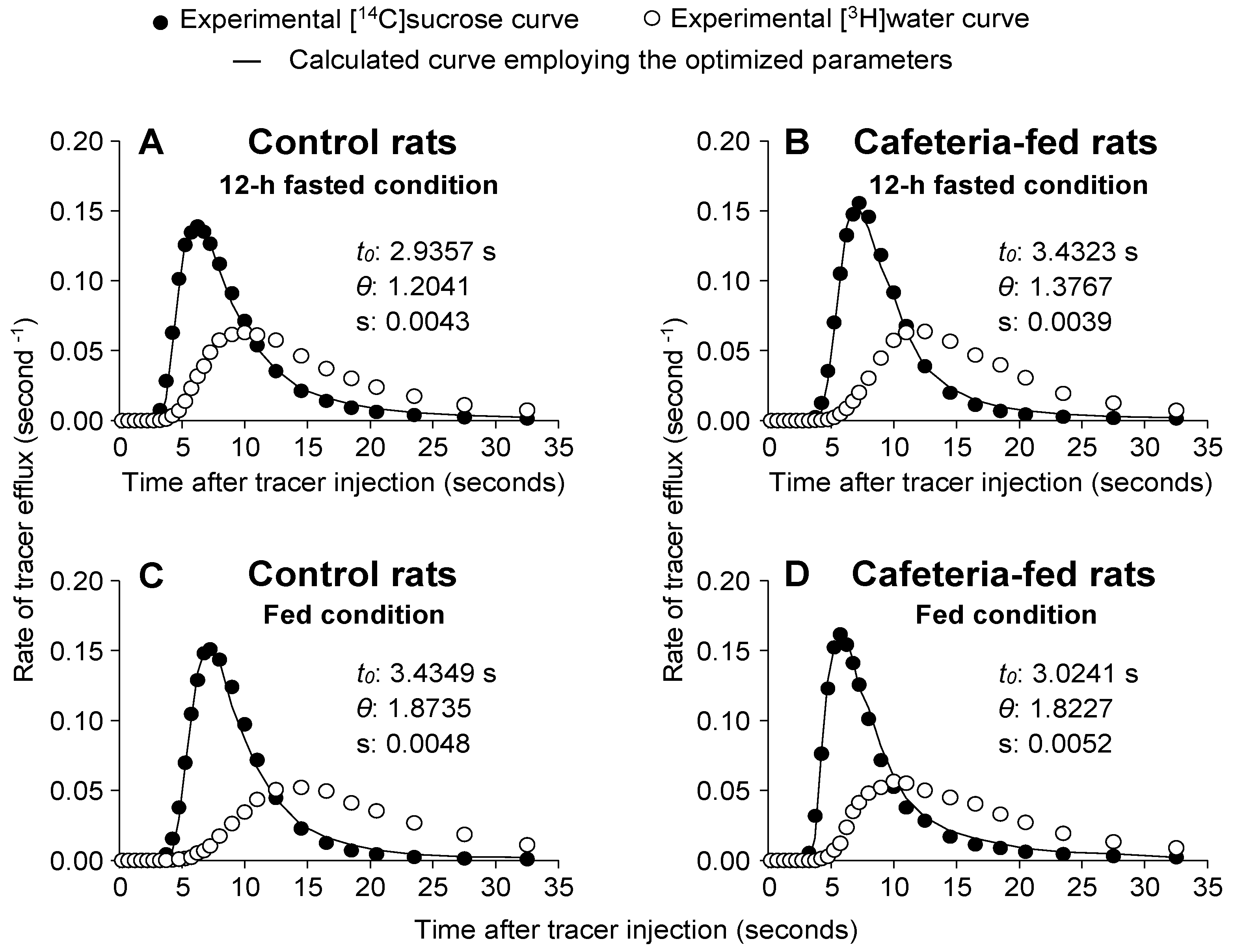
3.7. [14C]sucrose and [3H]water Outflow Profiles and Related Parameters in Livers of Rats Fed a Standard or a Cafeteria Diet
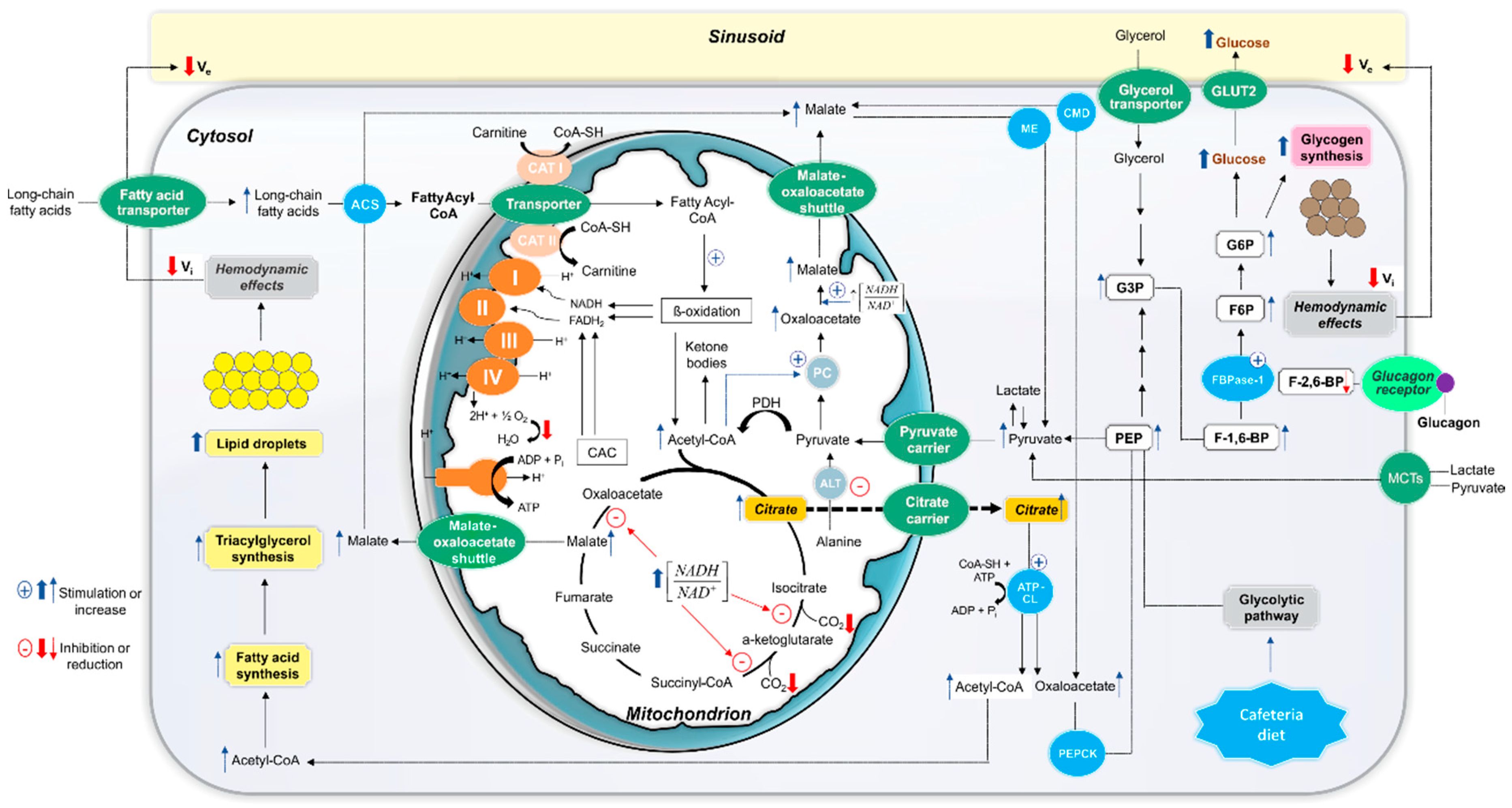
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hebestreit, A.; Bornhorst, C.; Barba, G.; Siani, A.; Huybrechts, I.; Tognon, G.; Eiben, G.; Moreno, L.A.; Fernandez Alvira, J.M.; Loit, H.M.; et al. Associations between energy intake, daily food intake and energy density of foods and BMI z-score in 2-9-year-old European children. Eur. J. Nutr. 2014, 53, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rivera, C.; Gupta, A.; Feberova, J.; de Nanassy, J.A.; Boland, M.P. Successful treatment of neonatal hemochromatosis as gestational alloimmune liver disease with intravenous immunoglobulin. J. Neonatal. Perinat. Med. 2014, 7, 301–304. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Ward, L.C.; Waanders, J.; Brown, L. Modulation of tissue fatty acids by L-carnitine attenuates metabolic syndrome in diet-induced obese rats. Food Funct. 2015, 6, 2496–2506. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gong, S.; Ye, S.Q.; Lyman, B.; Geng, L.; Chen, P.; Li, D.Y. Non-alcoholic fatty liver disease in children: Focus on nutritional interventions. Nutrients 2014, 6, 4691–4705. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Ballestri, S.; Marchesini, G.; Angulo, P.; Loria, P. Nonalcoholic fatty liver disease: A precursor of the metabolic syndrome. Dig. Liver Dis. 2015, 47, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuman, M.G.; French, S.W.; Zakhari, S.; Malnick, S.; Seitz, H.K.; Cohen, L.B.; Salaspuro, M.; Voinea-Griffin, A.; Barasch, A.; Kirpich, I.A.; et al. Alcohol, microbiome, life style influence alcohol and non-alcoholic organ damage. Exp. Mol. Pathol. 2017, 102, 162–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Yki-Jarvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Gruben, N.; Shiri-Sverdlov, R.; Koonen, D.P.; Hofker, M.H. Nonalcoholic fatty liver disease: A main driver of insulin resistance or a dangerous liaison? Biochim. Biophys. Acta 2014, 1842, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Diacylglycerol activation of protein kinase Cepsilon and hepatic insulin resistance. Cell Metab. 2012, 15, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Owen, O.E.; Cahill, G.F., Jr. Role of substrate in the regulation of hepatic gluconeogenesis in fasting man. Adv. Enzyme Regul. 1969, 7, 41–46. [Google Scholar] [CrossRef]
- Chevalier, S.; Burgess, S.C.; Malloy, C.R.; Gougeon, R.; Marliss, E.B.; Morais, J.A. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes 2006, 55, 675–681. [Google Scholar] [CrossRef] [PubMed]
- De Castro Ghizoni, C.V.; Gasparin, F.R.; Junior, A.S.; Carreno, F.O.; Constantin, R.P.; Bracht, A.; Ishii Iwamoto, E.L.; Constantin, J. Catabolism of amino acids in livers from cafeteria-fed rats. Mol. Cell Biochem. 2013, 373, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Junker, A.E.; Gluud, L.; Holst, J.J.; Knop, F.K.; Vilsboll, T. Diabetic and nondiabetic patients with nonalcoholic fatty liver disease have an impaired incretin effect and fasting hyperglucagonaemia. J. Intern. Med. 2016, 279, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2017. [Google Scholar] [CrossRef] [PubMed]
- Suppli, M.P.; Lund, A.; Bagger, J.I.; Vilsboll, T.; Knop, F.K. Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Med. Hypotheses 2016, 86, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.; Xu, C.; Hong, Y.; Lu, H.; Wu, J.; Chen, Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Sci. Rep. 2014, 4, 5832. [Google Scholar] [CrossRef] [PubMed]
- Gasparin, F.R.S.; Carreno, F.O.; Mewes, J.M.; Gilglioni, E.H.; Pagadigorria, C.L.S.; Natali, M.R.M.; Utsunomiya, K.S.; Constantin, R.P.; Ouchida, A.T.; Curti, C.; et al. Sex differences in the development of hepatic steatosis in cafeteria diet-induced obesity in young mice. Biochim. Biophys. Acta 2018, 1864, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Scoaris, C.R.; Rizo, G.V.; Roldi, L.P.; de Moraes, S.M.; de Proenca, A.R.; Peralta, R.M.; Natali, M.R. Effects of cafeteria diet on the jejunum in sedentary and physically trained rats. Nutrition 2010, 26, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1974. [Google Scholar]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Arlington, VA, USA, 1990. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 2008. [Google Scholar]
- Scholz, R.; Bücher, T. Hemoglobin-free perfusion of rat liver. In Control of Energy Metabolism; Academic Press: New York, NY, USA, 1965; pp. 393–414. [Google Scholar]
- Soboll, S.; Heldt, H.W.; Scholz, R. Changes in the subcellular distribution of metabolites due to ethanol oxidation in the perfused rat liver. Hoppe Seylers Z. Physiol. Chem. 1981, 362, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.J. Monitor and control of blood and tissue oxygenation. Trans. Am. Soc. Artif. Intern. Organ. 1956, 2, 41–45. [Google Scholar]
- Scholz, R.; Olson, M.S.; Schwab, A.J.; Schwabe, U.; Noell, C.; Braun, W. The effect of fatty acids on the regulation of pyruvate dehydrogenase in perfused rat liver. Eur. J. Biochem. 1978, 86, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Bracht, A.; Schwab, A.J.; Scholz, R. Untersuchung von Flußgeschwindigkeiten in der isolierten perfundierten Rattenleber durch Pulsmarkierung mit radioaktiven Substraten und mathematischer Analyse der Auswaschkinetiken. Hoppe Seylers Z. Physiol. Chem. 1980, 361, 357–378. [Google Scholar] [CrossRef] [PubMed]
- Babcock, M.B.; Cardell, R.R., Jr. Hepatic glycogen patterns in fasted and fed rats. Am. J. Anat. 1974, 140, 299–337. [Google Scholar] [CrossRef] [PubMed]
- Goresky, C.A. A linear method for determining liver sinusoidal and extravascular volumes. Am. J. Physiol. 1963, 204, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Björck, Å.; Dahlquist, G. Numerische Methoden; Oldenburg Verlag: Munich, Germany, 1972. [Google Scholar]
- Meier, P.; Zierler, K.L. On the theory of the indicator-dilution method for measurement of blood flow and volume. J. Appl. Physiol. 1954, 6, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Voss, D.O.; Campello, A.P.; Bacila, M. The respiratory chain and the oxidative phosphorylation of rat brain mitochondria. Biochem. Biophys. Res. Commun. 1961, 4, 48–51. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Hoyumpa, A.M., Jr.; Greene, H.L.; Dunn, G.D.; Schenker, S. Fatty liver: Biochemical and clinical considerations. Am. J. Dig. Dis. 1975, 20, 1142–1170. [Google Scholar] [CrossRef] [PubMed]
- Aas, M. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim. Biophys. Acta 1971, 231, 32–47. [Google Scholar] [CrossRef]
- Varin, F.; Huet, P.M. Hepatic microcirculation in the perfused cirrhotic rat liver. J. Clin. Investig. 1985, 76, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.T.; Malinowska, E.; Jura, M.; Kozak, L.P. C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiol. Rep. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, J.; Zhou, X.; Louis, O.; Velkeniers, B.; Hooghe-Peters, E.; Vanhaelst, L. The weight gain and ultimate adiposity in cafeteria diet-induced obesity is unrelated to the central serotoninergic tonus. Eat. Weight Disord. 1997, 2, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sistare, F.D.; Haynes, R.C., Jr. The interaction between the cytosolic pyridine nucleotide redox potential and gluconeogenesis from lactate/pyruvate in isolated rat hepatocytes. Implications for investigations of hormone action. J. Biol. Chem. 1985, 260, 12748–12753. [Google Scholar] [PubMed]
- Dynnik, V.V.; Temnov, A.V. A mathematical model of the pyruvate oxidation in liver mitochondria. 1. Regulation of the Krebs cycle by adenine and pyridine nucleotides. Biokhimiia 1977, 42, 1030–1044. [Google Scholar] [PubMed]
- Kimura, R.E.; Warshaw, J.B. Control of fatty acid oxidation by intramitochondrial [NADH]/[NAD+] in developing rat small intestine. Pediatr. Res. 1988, 23, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Van de Wier, B.; Balk, J.M.; Haenen, G.R.; Giamouridis, D.; Bakker, J.A.; Bast, B.C.; den Hartog, G.J.; Koek, G.H.; Bast, A. Elevated citrate levels in non-alcoholic fatty liver disease: The potential of citrate to promote radical production. FEBS Lett. 2013, 587, 2461–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Byrne, C.D. Clinical Review: Nonalcoholic fatty liver disease: A novel cardiometabolic risk factor for type 2 diabetes and its complications. J. Clin. Endocrinol. Metab. 2013, 98, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.A. Rate control of the tricarboxylic acid cycle. Adv. Enzyme Regul. 1970, 8, 335–353. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.S.; Mietus-Snyder, M.; Valente, A.; Schwarz, J.M.; Lustig, R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Manchon, C.; Martin-Requero, A.; Ayuso, M.S.; Parrilla, R. Role of endogenous fatty acids in the control of hepatic gluconeogenesis. Arch. Biochem. Biophys. 1992, 292, 95–101. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; St Maurice, M.; Rayment, I.; Cleland, W.W.; Wallace, J.C.; Attwood, P.V. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 2008, 413, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.R.; Browning, E.T.; Scholz, R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J. Biol. Chem. 1969, 244, 4607–4616. [Google Scholar] [PubMed]
- LaNoue, K.F.; Williamson, J.R. Interrelationships between malate-aspartate shuttle and citric acid cycle in rat heart mitochondria. Metabolism 1971, 20, 119–140. [Google Scholar] [CrossRef]
- Lopes-Cardozo, M.; van den Bergh, S.G. Ketogenesis in isolated rat liver mitochondria. I. Relationships with the citric acid cycle and with the mitochondrial energy state. Biochim. Biophys. Acta 1972, 283, 1–15. [Google Scholar] [CrossRef]
- Berk, P.D.; Zhou, S.; Bradbury, M.W. Increased hepatocellular uptake of long chain fatty acids occurs by different mechanisms in fatty livers due to obesity or excess ethanol use, contributing to development of steatohepatitis in both settings. Trans. Am. Clin. Climatol. Assoc. 2005, 116, 335–344; discussion 345. [Google Scholar] [PubMed]
- Bechmann, L.P.; Gieseler, R.K.; Sowa, J.P.; Kahraman, A.; Erhard, J.; Wedemeyer, I.; Emons, B.; Jochum, C.; Feldkamp, T.; Gerken, G.; et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010, 30, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Hannivoort, R.A.; Gerken, G.; Hotamisligil, G.S.; Trauner, M.; Canbay, A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 2012, 56, 952–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieghs, V.; Wouters, K.; van Gorp, P.J.; Gijbels, M.J.; de Winther, M.P.; Binder, C.J.; Lutjohann, D.; Febbraio, M.; Moore, K.J.; van Bilsen, M.; et al. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology 2010, 138, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Korbonits, M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Proietto, J. The biochemical basis of increased hepatic glucose production in a mouse model of type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1995, 38, 1389–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Andrikopoulos, S.; Filippis, C.; Thorburn, A.W.; Khan, D.; Proietto, J. Mechanism of fat-induced hepatic gluconeogenesis: Effect of metformin. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E275–E282. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, A.C.; D’Ávila, R.B.; Ferrari, A.G.; Kelmer-Bracht, A.M.; Constantin, J.; Yamamoto, N.S.; Bracht, A. Ca2+ dependence of gluconeogenesis stimulation by glucagon at different cytosolic NAD+-NADH redox potentials. Braz. J. Med. Biol. Res. 1997, 30, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.D.; Schaeffer, L.; Shragg, P.; Kolterman, O.G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 1987, 36, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Bernsmeier, C.; Meyer-Gerspach, A.C.; Blaser, L.S.; Jeker, L.; Steinert, R.E.; Heim, M.H.; Beglinger, C. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS ONE 2014, 9, e87488. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Martini, M.; Suzuki-Kemmelmeier, F.; de Oliveira, D.S.; Comar, J.F.; Bracht, A. Responses of the perfused liver of neonatal type 2 diabetic rats to gluconeogenic and ammoniogenic substrates. Health 2010, 2, 477–483. [Google Scholar] [CrossRef]
- Magnusson, I.; Chandramouli, V.; Schumann, W.C.; Kumaran, K.; Wahren, J.; Landau, B.R. Pathways of hepatic glycogen formation in humans following ingestion of a glucose load in the fed state. Metabolism 1989, 38, 583–585. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, M.B.; Garvey, D. Studies on mechanisms of hepatic insulin resistance in cafeteria-fed rats. Am. J. Physiol. 1993, 264, E18–E23. [Google Scholar] [CrossRef] [PubMed]
- Pinto Júnior, D.A.C.; Seraphim, P.M. Cafeteria diet intake for fourteen weeks can cause obesity and insulin resistance in Wistar rats. Rev. Nutr. 2012, 25, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Sabater, D.; Agnelli, S.; Arriaran, S.; Romero Mdel, M.; Fernandez-Lopez, J.A.; Alemany, M.; Remesar, X. Cafeteria diet induce changes in blood flow that are more related with heat dissipation than energy accretion. PeerJ 2016, 4, e2302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaccari, A.; Rossetti, L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J. Clin. Investig. 1992, 89, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Shimomura, I. Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, S.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Impairment of hepatic microcirculation in fatty liver. Microcirculation 2003, 10, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Sato, N.; Meren, H.; Inoue, A.; Kasahara, A.; Yoshihara, H.; Sasaki, Y.; Hayashi, N.; Fusamoto, H.; Abe, H.; et al. Hepatic tissue oxygen tension and regional hepatic blood volume in alcoholic fatty liver. Nihon Shokakibyo Gakkai Zasshi 1984, 81, 1019–1023. [Google Scholar] [PubMed]
- Seifalian, A.M.; Piasecki, C.; Agarwal, A.; Davidson, B.R. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation 1999, 68, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, J.P.; Dagenais, M.; Huet, P.M.; Roy, A.; Lapointe, R.; Marleau, D. The hepatic microcirculation in the isolated perfused human liver. Hepatology 1996, 23, 24–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumida, K.D.; Urdiales, J.H.; Donovan, C.M. Lactate delivery (not oxygen) limits hepatic gluconeogenesis when blood flow is reduced. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E192–E198. [Google Scholar] [CrossRef] [PubMed]
- Botini, F.F.; Suzuki-Kemmelmeier, F.; Nascimento, E.A.; Ide, L.T.; Bracht, A. Zonation of alanine metabolism in the bivascularly perfused rat liver. Liver Int. 2005, 25, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Constantin, J.; Ishii-Iwamoto, E.; Suzuki-Kemmelmeier, F.; Bracht, A. Zonation of the action of glucagon on gluconeogenesis studied in the bivascularly perfused rat liver. FEBS Lett. 1994, 352, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, T.; Kashiwagi, T.; Meren, H.; Thurman, R.G. Gluconeogenesis predominates in periportal regions of the liver lobule. Eur. J. Biochem. 1984, 144, 409–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilglioni, E.H.; Chang, J.C.; Duijst, S.; Go, S.; Adam, A.A.A.; Hoekstra, R.; Verhoeven, A.J.; Ishii-Iwamoto, E.L.; Oude Elferink, R.P.J. Improved oxygenation dramatically alters metabolism and gene expression in cultured primary mouse hepatocytes. Hepatol. Commun. 2018, 2, 299–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | Control Rats | Cafeteria-Fed Rats |
|---|---|---|
| Initial body weight (g) | 50.70 ± 2.00 (n = 21) | 51.10 ± 2.60 (n = 21) |
| Final body weight (g) | 416.00 ± 4.50 (n = 21) | 485.00 ± 6.60 * (n = 21) |
| Body weight gain (g) | 365.00 ± 4.40 (n = 21) | 434.00 ± 8.20 * (n = 21) |
| Adiposity index (g × 100 g of body weight−1) | 4.45 ± 0.28 (n = 10) | 8.96 ± 0.49 * (n = 10) |
| Liver wet weight (g) | 11.90 ± 0.37 (n = 10) | 14.05 ± 0.50 * (n = 10) |
| Hepatic glycogen in fed rats (µmol glucosyl units × g−1) | 139.10 ± 18.90 (n = 7) | 256.00 ± 14.90 * (n = 4) |
| Hepatic glycogen in 12-h fasted rats (µmol glucosyl units × g−1) | 6.69 ± 1.72 (n = 10) | 14.30 ± 1.45 * (n = 9) |
| Hepatic total lipid content (mg × g liver wet weight−1) | 19.16 ± 0.77 (n = 4) | 58.72 ± 7.82 * (n = 4) |
| Hepatic total protein content (mg × g liver wet weight−1) | 177.50 ± 6.33 (n = 4) | 173.50 ± 4.39 (n = 4) |
| Parameter | [Lactate] + [Pyruvate] (mM) | [Fatty Acid] (mM) | Δ Metabolite Production (μmol × g−1) | |
|---|---|---|---|---|
| Control Rats | Cafeteria-Fed Rats | |||
| Glucose | 0 | 0 | 0.98 ± 0.05 (n = 4) | 2.18 ± 0.56 (n = 4) |
| 2.0 + 0.2 | 0 | 7.84 ± 0.82 (n = 4) | 4.58 ± 0.40 * (n = 4) | |
| 2.0 + 0.2 | 0.2 Stearate | 0.51 ± 0.10 (n = 4) | 2.25 ± 0.70 * (n = 4) | |
| Ketone bodies | 0 | 0 | 4.84 ± 0.19 (n = 8) | 4.04 ± 0.21 * (n = 9) |
| 2.0 + 0.2 | 0 | −6.25 ± 0.23 (n = 4) | −4.72 ± 0.05 * (n = 4) | |
| 2.0 + 0.2 | 0.2 Stearate | 0.64 ± 0.19 (n = 4) | 0.56 ± 0.10 (n = 4) | |
| 0 | 0.3 Octanoate | 8.49 ± 0.90 (n = 4) | 7.26 ± 1.20 (n = 5) | |
| O2 consumption | 0 | 0 | 18.74 ± 0.66 (n = 8) | 15.30 ± 0.34 * (n = 9) |
| 2.0 + 0.2 | 0 | 4.78 ± 0.37 (n = 4) | 4.38 ± 0.81 (n = 4) | |
| 2.0 + 0.2 | 0.2 Stearate | 0.70 ± 0.15 (n = 4) | 0.84 ± 0.25 (n = 4) | |
| 0 | 0.3 Octanoate | 4.23 ± 0.81 (n = 4) | 3.57 ± 0.35 (n = 5) | |
| CO2 | 2.0 + 0.2 | 0.2 Stearate | 14.22 ± 1.02 (n = 4) | 10.70 ± 0.60 * (n = 4) |
| 0 | 0.3 Octanoate | 6.30 ± 0.54 (n = 4) | 4.75 ± 0.40 * (n = 5) | |
| Conditions | Δ Metabolite Production (μmol × g−1) | |||||||
| Control Rats | Cafeteria-Fed Rats | |||||||
| Glycerol Gluconeogenesis | Glicose | O2 Consumption | Lactate | Pyruvate | Glicose | O2 Consumption | Lactate | Pyruvate |
| Basal production | 0.64 ± 0.09 | 17.41 ± 1.37 | 0.41 ± 0.10 | — | 1.74 ± 0.51 | 15.96 ± 0.62 | 0.71 ± 0.20 | — |
| Glycerol (2 mM) | 4.75 ± 0.65 | 1.97 ± 0.56 | 1.54 ± 0.38 | — | 6.62 ± 0.45 * | 1.48 ± 0.46 | 1.25 ± 0.35 | — |
| Glycerol (2 mM) + Glucagon (10 nM) | 0.41 ± 0.13 | 0.86 ± 0.50 | −0.29 ± 0.04 | — | 2.29 ± 0.50 * | 0.39 ± 0.10 | −0.42 ± 0.12 | — |
| Conditions | Δ Metabolite Production (μmol × g−1) | |||||||
| Control Rats | Cafeteria-Fed Rats | |||||||
| Lactate Gluconeogenesis | Glicose | O2 Consumption | Lactate | Pyruvate | Glicose | O2 Consumption | Lactate | Pyruvate |
| Basal production | 0.70 ± 0.03 | 17.45 ± 1.00 | — | 0.05 ± 0.04 | 0.92 ± 0.27 | 14.62 ± 0.92 | — | 0.44 ± 0.41 |
| Lactate (2 mM) | 9.59 ± 0.17 | 3.97 ± 0.46 | — | 3.55 ± 0.68 | 7.51 ± 0.15 * | 5.34 ± 0.61 | — | 3.06 ± 0.42 |
| Lactate (2 mM) + Glucagon (10 nM) | 0.90 ± 0.07 | 0.09 ± 0.03 | — | −0.60 ± 0.11 | 1.62 ± 0.25 * | 0.42 ± 0.11 * | — | −0.42 ± 0.07 |
| Group (n) | Octanoyl-CoA + l-Carnitine | Palmitoyl-CoA + l-Carnitine | Octanoyl l-Carnitine | Palmitoyl l-Carnitine |
|---|---|---|---|---|
| Control rats (6) | 40.5 ± 4.3 | 59.0 ± 3.3 | 61.3 ± 2.6 | 77.0 ± 5.9 |
| Cafeteria-fed rats (6) | 37.0 ± 3.2 | 64.5 ± 3.1 | 63.5 ± 3.9 | 88.5 ± 5.8 |
| Parameter | 12-h Fasted Rats | Fed Rats | ||
|---|---|---|---|---|
| Control Rats (n = 6) | Cafeteria-Fed Rats (n = 6) | Control Rats (n = 5) | Cafeteria-Fed Rats (n = 5) | |
| t0 (seconds) | 3.06 ± 0.29 | 2.99 ± 0.21 | 3.08 ± 0.29 | 2.91 ± 0.12 |
| θ | 1.18 ± 0.07 * | 1.20 ± 0.06 # | 1.80 ± 0.12 | 1.88 ± 0.10 |
| (seconds) | 10.48 ± 0.58 | 9.73 ± 0.35 | 10.14 ± 0.49 | 9.78 ± 0.16 |
| (seconds) | 19.24 ± 0.70 * | 17.64 ± 0.38 # | 22.57 ± 0.55 | 22.47 ± 0.81 |
| Ve as flow × (mL/g) | 0.33 ± 0.03 | 0.28 ± 0.02 # | 0.25 ± 0.02 | 0.19 ± 0.01 ° |
| Vi as flow × (mL/g) | 0.38 ± 0.01 * | 0.33 ± 0.01 ∆ | 0.44 ± 0.02 | 0.36 ± 0.02 |
| Vi/Ve | 1.20 ± 0.08 * | 1.20 ± 0.08 # | 1.81 ± 0.11 | 1.85 ± 0.08 |
| Vv as flow × t0 (mL/g) | 0.14 ± 0.02 | 0.12 ± 0.01 # | 0.11 ± 0.01 | 0.08 ± 0.00 ° |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda Júnior, A.S.; Constantin, J.; Utsunomiya, K.S.; Gilglioni, E.H.; Gasparin, F.R.S.; Carreño, F.O.; De Moraes, S.M.F.; Rocha, M.; Natali, M.R.M.; Ghizoni, C.V.d.C.; et al. Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence. Nutrients 2018, 10, 1571. https://doi.org/10.3390/nu10111571
Maeda Júnior AS, Constantin J, Utsunomiya KS, Gilglioni EH, Gasparin FRS, Carreño FO, De Moraes SMF, Rocha M, Natali MRM, Ghizoni CVdC, et al. Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence. Nutrients. 2018; 10(11):1571. https://doi.org/10.3390/nu10111571
Chicago/Turabian StyleMaeda Júnior, Antonio Sueiti, Jorgete Constantin, Karina Sayuri Utsunomiya, Eduardo Hideo Gilglioni, Fabiana Rodrigues Silva Gasparin, Fernando Olinto Carreño, Solange Marta Franzói De Moraes, Márcio Rocha, Maria Raquel Marçal Natali, Cristiane Vizioli de Castro Ghizoni, and et al. 2018. "Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence" Nutrients 10, no. 11: 1571. https://doi.org/10.3390/nu10111571
APA StyleMaeda Júnior, A. S., Constantin, J., Utsunomiya, K. S., Gilglioni, E. H., Gasparin, F. R. S., Carreño, F. O., De Moraes, S. M. F., Rocha, M., Natali, M. R. M., Ghizoni, C. V. d. C., Bracht, A., Ishii-Iwamoto, E. L., & Constantin, R. P. (2018). Cafeteria Diet Feeding in Young Rats Leads to Hepatic Steatosis and Increased Gluconeogenesis under Fatty Acids and Glucagon Influence. Nutrients, 10(11), 1571. https://doi.org/10.3390/nu10111571






