Microbial and Nutritional Programming—The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies
Abstract
1. Introduction
2. Gut Microbiota and Allergic Conditions
3. The Impact of Gut Microbiota on Immune System Development
4. Factors Affecting Gut Microbiota Formation
5. The Impact of the Mode of Delivery on the Gut Microbiome and Allergy Development
6. The Effect of Gut Microbiome and Breastfeeding on Allergy Development
7. Infant Formulas Supplemented with Prebiotics and Probiotics in Allergy Prevention
8. The Effect of Nutritional Programming on Allergy Development
9. Conclusions and Recommendations
Funding
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CM | Cow milk |
| CS | Cesarean section |
| GF | Germ-free |
| HMOs | Human milk oligosaccharides |
| IgA (E) | immunoglobulins A (E) |
| IL | interleukin |
| lcFOS | long chain fructo-oligosaccharides |
| OR | Odds ratio |
| pHF | partially hydrolyzed formula |
| RR | Relative risk |
| scGOS | short chain galacto-oligosaccharides |
| TGF | transforming growth factor |
| Th | T helper |
| Treg | regulatory T cells |
| WAO | World Allergy Organization |
References
- Von Mutius, E. The Rising Trends in Asthma and Allergic Diseases. Clin. Exp. Allergy 1998, 28 (Suppl. 5), 45–49. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Inouye, M.; Logan, A.C.; Prescott, S.L.; Sly, P.D. An Exposome Perspective: Early-Life Events and Immune Development in a Changing World. J. Allergy Clin. Immunol. 2017, 140, 24–40. [Google Scholar] [CrossRef]
- Shreiner, A.; Huffnagle, G.B.; Noverr, M.C. The “Microflora Hypothesis” of Allergic Diseases. Adv. Exp. Med. Biol. 2008, 635, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The First Thousand Days—Intestinal Microbiology of Early Life: Establishing a Symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. Effects of Early Nutrition Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Timing of Introduction of Complementary Foods, and Hydrolyzed Formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium, Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 11635–11638. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Cernada, M.; Baüerl, C.; Vento, M.; Pérez-Martínez, G. Microbial Ecology and Host-Microbiota Interactions During Early Life Stages. Gut Microbes 2012, 3, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220. [Google Scholar] [CrossRef] [PubMed]
- Björksten, B.; Naaber, P.; Sepp, E.; Mikelsaar, M. The Intestinal Microflora in Allergic Estonian and Swedish 2-year-old Children. Clin. Exp. Allergy 1999, 29, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low Diversity of the Gut Microbiota in Infants with Atopic Eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Kalliomäki, M.; Kirjavainen, P.; Kero, P.; Salminen, S.; Isolauri, E. Distinct Patterns of Neonatal Gut Microflora in Infants in Whom Atopy Was Not Developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.; Skov, T.; Paudan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced Diversity of the Intestinal Microbiota During Infancy Is Associated With Increased Risk of Allergic Disease at School Age. J. Allergy Clin. Immunol. 2011, 128, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low Gut Microbiota Diversity in Early Infancy Precedes Asthma at School Age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, Y.M.; Jenmalm, M.C.; Böttcher, M.F.; Björkstén, B.; Sverremark-Ekström, E. Altered Early Infant Gut Microbiota in Children Developing Allergy up to 5 Years of Age. Clin. Exp. Allergy 2009, 39, 518–526. [Google Scholar] [CrossRef]
- Brugman, S.; Perdijk, O.; van Neerven, R.J.; Savelkoul, H.F. Mucosal Immune Development in Early Life: Setting the Stage. Arch. Immunol. Ther. Exp. 2015, 63, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Tlaskalová-Hogenová, H.; Stepánková, R.; Hudcovic, T.; Tucková, L.; Cukrowska, B.; Lodinová-Zádníková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D.; et al. Commensal Bacteria (Normal Microflora), Mucosal Immunity and Chronic Inflammatory and Autoimmune Diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Kozakova, H.; Rehakova, Z.; Sinkora, J.; Tlaskalova-Hogenova, H. Specific Antibody and Immunoglobulin Responses after Intestinal Colonization of Germ-Free Piglets with Non-Pathogenic Escherichia coli O86. Immunobiology 2001, 204, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kozakova, H.; Schwarzer, M.; Tuckova, L.; Srutkova, D.; Czarnowska, E.; Rosiak, I.; Hudcovic, T.; Schabussova, I.; Hermanova, P.; Zakostelska, Z.; et al. Colonization of Germ-Free Mice with a Mixture of Three Lactobacillus Strains Enhances the Integrity of Gut Mucosa and Ameliorates Allergic Sensitization. Cell. Mol. Immunol. 2016, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of Immune Tolerance to Allergens: Role of IL-10 and Tregs. J. Clin. Investig. 2014, 124, 4678–4680. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The Placenta Harbors a Unique Microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human Gut Colonisation May be Initiated in Utero by Distinct Microbial Communities in the Placenta and Amniotic Fluid. Sci. Rep. 2016, 22, 23129. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A Critical Assessment of the “Sterile Womb” and “in Utero Colonization” Hypotheses: Implications for Research on the Pioneer Infant Microbiome. Microbiome 2017, 28, 48. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Larcombe, D.L.; Logan, A.C.; West, C.; Burks, W.; Caraballo, L.; Levin, M.; Etten, E.V.; Horwitz, P.; Kozyrskyj, A.; et al. The Skin Microbiome: Impact of Modern Environments on Skin Ecology, barrier Integrity, and Systemic Immune Programming. World Allergy Organ. J. 2017, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Makino, H.; Cetinyurek Yavuz, A.; Ben-Amor, K.; Roelofs, M.; Ishikawa, E.; Kubota, H.; Swinkels, S.; Sakai, T.; Oishi, K.; et al. Early-Life Events, Including Mode of Delivery and Type of feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE 2016, 11, e0158498. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A. Neuropeptides and the Microbiota-Gut-Brain Axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota Across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Biasucci, G.; Benenati, B.; Morelli, L.; Bessi, E.; Boehm, G. Cesarean Delivery May Affect the Early Biodiversity of Intestinal Bacteria. J. Nutr. 2008, 138, 1796S–1800S. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased Gut Microbiota Diversity, Delayed Bacteroidetes Colonisation and Reduced Th1 Responses in Infants Delivered by Caesarean Section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Lechowicz, M.; Konopka, E.; Majewska, U.; Borszewska-Kornacka, M.; Mikula, M.; Cukrowska, B.; et al. Effect of Saccharomyces boulardii and Mode of Delivery on the Early Development of the Gut Microbial Community in Preterm Infants. PLoS ONE 2016, 11, e0150306. [Google Scholar] [CrossRef] [PubMed]
- Papathoma, E.; Triga, M.; Fouzas, S.; Dimitriou, G. Cesarean Section Delivery and Development of Food Allergy and Atopic Dermatitis in Early Childhood. Pediatr. Allergy Immunol. 2016, 27, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Håberg, S.E.; Stigum, H.; Nafstad, P.; London, S.J.; Vangen, S.; Nystad, W. Delivery by Cesarean Section and Early Childhood Respiratory Symptoms and Disorders the Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2011, 174, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Guibas, G.V.; Moschonis, G.; Xepapadaki, P.; Roumpedaki, E.; Androutsos, O.; Manios, Y.; Papadopoulos, N.G. Conception via in vitro Fertilization and Delivery by Caesarean Section Are Associated with Paediatric Asthma Incidence. Clin. Exp. Allergy 2013, 43, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Tollånes, M.C.; Moster, D.; Daltveit, A.K.; Irgens, L.M. Cesarean Section and Risk of Severe Childhood Asthma: A Population-Based Cohort Study. J. Pediatrics 2008, 153, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Feldman, A.S.; Rosas-Salazar, C.; James, K.; Escobar, G.; Gebretsadik, T.; Li, S.X.; Carroll, K.N.; Walsh, E.; Mitchel, E.; et al. Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS ONE 2016, 11, e0151705. [Google Scholar] [CrossRef] [PubMed]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial Effects of Human Milk Oligosaccharides on Gut Microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Human Milk Oligosaccharides (HMOS): Structure, Function, and Enzyme-Catalyzed Synthesis. Adv. Carbohydr. Chem. Biochem. 2015, 72, 113–190. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Langa, S.; Martín, V.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The Microbiota of Human Milk in Healthy Women. Cell. Mol. Biol. 2013, 59, 31–42. [Google Scholar] [PubMed]
- Martín, R.; Jiménez, E.; Heilig, H.; Fernández, L.; Marín, M.L.; Zoetendal, E.G.; Rodríguez, J.M. Isolation of Bifidobacteria from Breast Milk and Assessment of the Bifidobacterial Population by PCR-Denaturing Gradient Gel Electrophoresis and Quantitative Real-Time PCR. Appl. Environ. Microbiol. 2009, 75, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of Lactation Stage, Gestational age and Mode of Delivery on Breast Milk Microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E. Maternal Weight and Excessive Weight Gain During Pregnancy Modify the Immunomodulatory Potential of Breast Milk. Pediatr. Res. 2012, 72, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Schanler, R.J.; Lee, M.L.; Rechtman, D.J. Greater Mortality and Morbidity in Extremely Preterm Infants Fed a Diet Containing Cow Milk Protein Products. Breastfeed. Med. 2014, 9, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; McGuire, W. Formula versus Donor Breast Milk for Feeding Preterm or Low Birth Weight Infants. Cochrane Database Syst. Rev. 2018, 20, CD002971. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.S. Breastfeeding and Allergy: The Evidence. Ann. Nutr. Metab. 2011, 59 (Suppl. 1), 20–26. [Google Scholar] [CrossRef] [PubMed]
- Klopp, A.; Vehling, L.; Becker, A.B.; Subbarao, P.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; CHILD Study Investigators; Azad, M.B. Modes of Infant Feeding and the Risk of Childhood Asthma: A Prospective Birth Cohort Study. J. Pediatr. 2017, 190, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Chen, Q.; Chen, Y.; Bao, Y.; Wu, M.; Zhang, J. Cesarean Section Without Medical Indication and Risk of Childhood Asthma, and Attenuation by Breastfeeding. PLoS ONE 2017, 18, e0184920. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.E.; Mosca, F.; Miniello, V.; Fanaro, S.; Jelinek, J.; Stahl, B.; Boehm, G. Effects of a New Mixture of Prebiotics on Faecal Flora and Stools in Term Infants. Acta Paediatr. 2003, 91, 77–79. [Google Scholar] [CrossRef]
- Knol, J.; Scholtens, P.; Kafka, C.; Steenbakkers, J.; Gro, S.; Helm, K.; Klarczyk, M.; Schöpfer, H.; Böckler, H.M.; Wells, J. Colon Microflora in Infants Fed Formula with Galacto- and Fructo-Oligosaccharides: More Like Breast-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.C.; Ben-Amor, K.; Lay, C.; Neo, A.G.E.; Chiang, W.C.; Rao, R.; Chew, C.; Chaithongwongwatthana, S.; Khemapech, N.; Knol, J.; et al. Effect of Synbiotic on the Gut Microbiota of Cesarean Delivered Infants: A Randomized, Double-Blind, Multicenter Study. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Kosuwon, P.; Lao-Araya, M.; Uthaisangsook, S.; Lay, C.; Bindels, J.; Knol, J.; Chatchatee, P. A Synbiotic Mixture of scGOS/lcFOS and Bifidobacterium breve M-16V Increases Faecal Bifidobacterium in Healthy Young Children. Benef. Microbes 2018, 9, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Arslanoglu, S.; Stahl, B.; Jelinek, J.; Wahn, U.; Boehm, G. A Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Atopic Dermatitis During the First Six Months of Age. Arch. Dis. Child. 2006, 91, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections During the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Boehm, G.; Wienz, F.; Stahl, B.; Bertino, E. Early Neutral Prebiotic Oligosaccharide Supplementation Reduces the Incidence of Some Allergic Manifestations in the First 5 Years of Life. J. Biol. Regul. Homeost. Agents 2012, 26 (Suppl. 3), 49–59. [Google Scholar] [PubMed]
- Cuello-Garcia, C.A.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Gandhi, S.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P.): Prebiotics. World Allergy Organ J. 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P.): Probiotics. World Allergy Organ J. 2015, 27, 4. [Google Scholar] [CrossRef] [PubMed]
- Elazab, N.; Mendy, A.; Gasana, J.; Vieira, E.R.; Quizon, A.; Forno, E. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-analysis of Clinical Trials. Pediatrics 2013, 132, e666–e676. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Horvath, A. Lactobacillus rhamnosus GG in the Primary Prevention of Eczema in Children: A Systematic Review and Meta-Analysis. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased Food Diversity in the First Year of Life is Inversely Associated with Allergic Diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Pitt, T.J.; Becker, A.B.; Chan-Yeung, M.; Chan, E.S.; Watson, W.T.A.; Chooniedass, R.; Azad, M.B. Reduced Risk of Peanut Sensitization Following Exposure through Breast-Feeding and Early Peanut Introduction. J. Allergy Clin. Immunol. 2018, 141, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; Molgaard, C. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Horvath, A. A Partially Hydrolyzed 100% Whey Formula and the Risk of Eczema and Any Allergy: An Updated Meta-analysis. World Allergy Organ J. 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y. Prevention and Management of Cow’s Milk Allergy in Non-Exclusively Breastfed Infants. Nutrients 2017, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Gouw, J.W.; Jo, J.; Meulenbroek, L.A.P.M.; Heijjer, S.; Kremer, E.; Sandalova, E.; Knulst, A.C.; Jeurink, P.; Garssen, J.; Rijnierse, A.; Knippels, L.M.J. Identification of Peptides with Tolerogenic Potential in a Hydrolyzed Whey-Based Infant Formula. Clin. Exp. Allergy 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Esch, B.C.; Schouten, B.; de Kivit, S.; Hofman, G.A.; Knippels, L.M.; Willemsen, L.E.; Garssen, J. Oral Tolerance Induction by Partially Hydrolyzed Whey Protein in Mice is Associated with Enhanced Numbers of Foxp3+ Regulatory T-Cells in the Mesenteric Lymph Nodes. Pediatr. Allergy Immunol. 2011, 22, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.J.; Tang, M.L.; Chiang, W.C.; Chua, M.C.; Ismail, I.; Nauta, A.; Hourihane, J.O.B.; Smith, P.; Gold, M.; Ziegler, J.; et al. Prebiotic-Supplemented Partially Hydrolysed Cow’s Milk Formula for the Prevention of Eczema in High-Risk Infants: A Randomized Controlled Trial. Allergy 2016, 71, 701–710. [Google Scholar] [CrossRef] [PubMed]
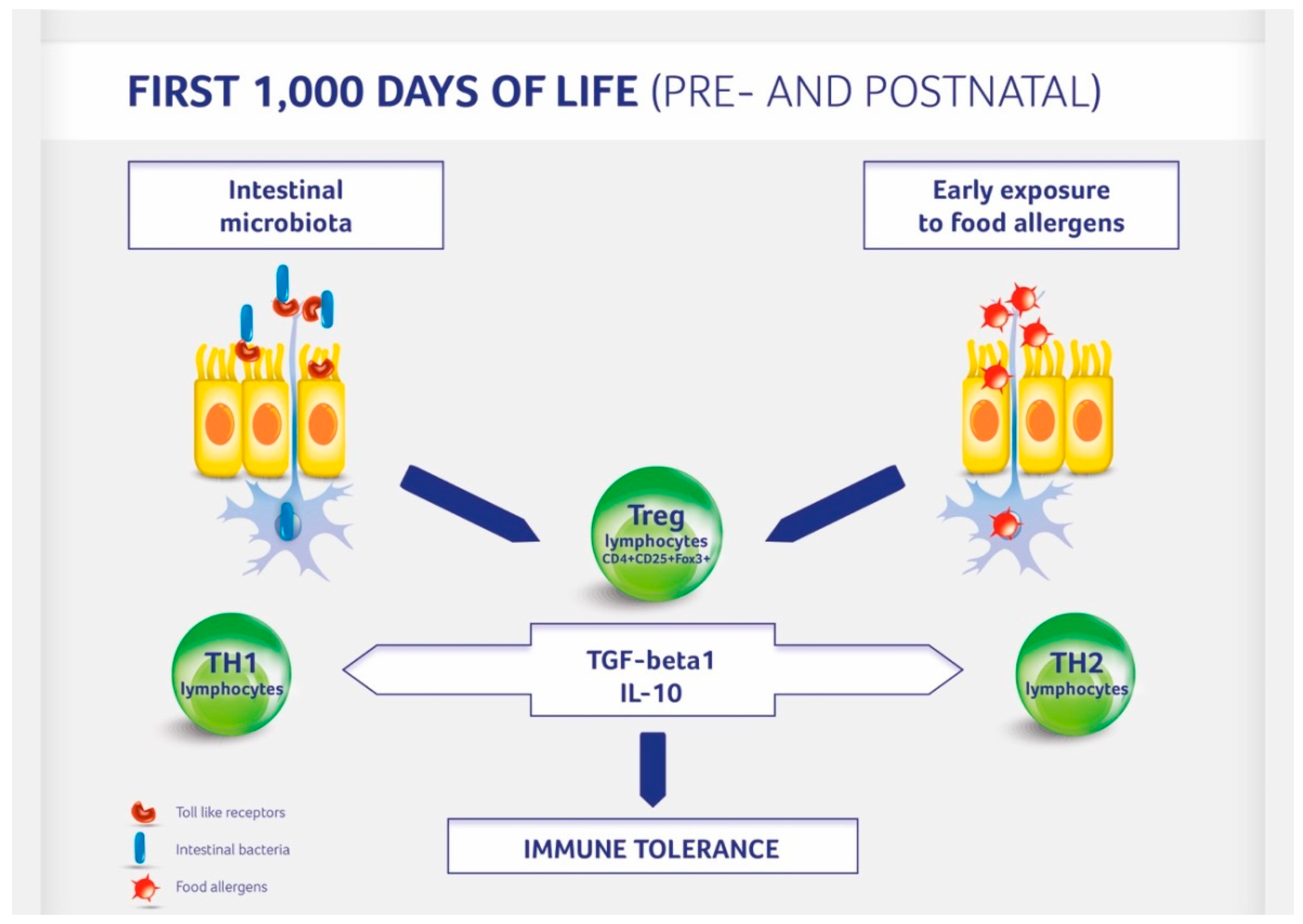
| Prenatal Period | Postnatal Period |
|---|---|
|
|
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cukrowska, B. Microbial and Nutritional Programming—The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies. Nutrients 2018, 10, 1541. https://doi.org/10.3390/nu10101541
Cukrowska B. Microbial and Nutritional Programming—The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies. Nutrients. 2018; 10(10):1541. https://doi.org/10.3390/nu10101541
Chicago/Turabian StyleCukrowska, Bożena. 2018. "Microbial and Nutritional Programming—The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies" Nutrients 10, no. 10: 1541. https://doi.org/10.3390/nu10101541
APA StyleCukrowska, B. (2018). Microbial and Nutritional Programming—The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies. Nutrients, 10(10), 1541. https://doi.org/10.3390/nu10101541




