The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
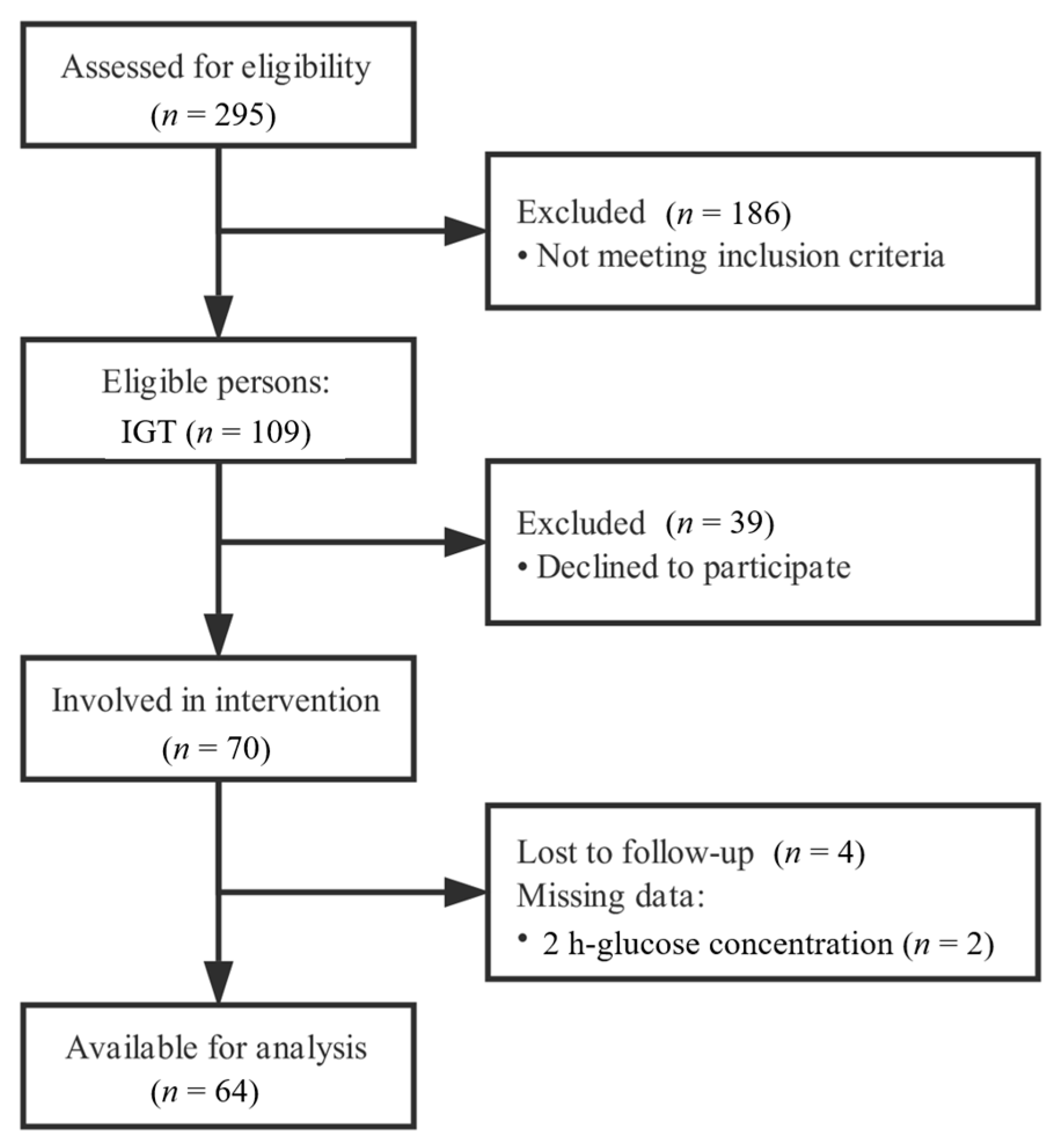
2.1. Subjects
2.2. Intervention
2.3. Samples Collection and Outcomes Measurement
2.4. Statistical Analysis
3. Results
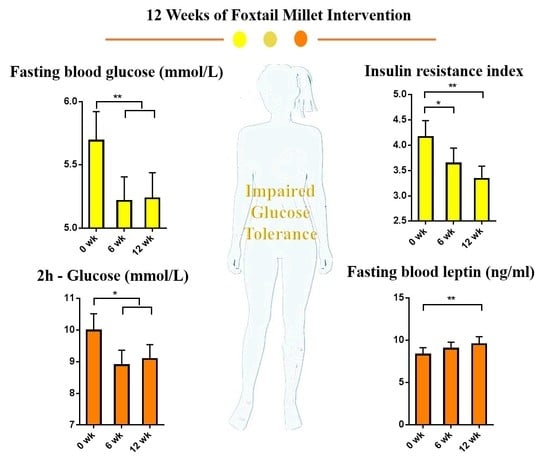
3.1. Effect of Foxtail Millet Intervention on Blood Glucose
3.2. Effect of Foxtail Millet Intake on Blood Lipid and Blood Pressure
3.3. Effect of Foxtail Millet Intake on Anthropometric Indicators
3.4. Effect of Foxtail Millet Intake on Cytokines
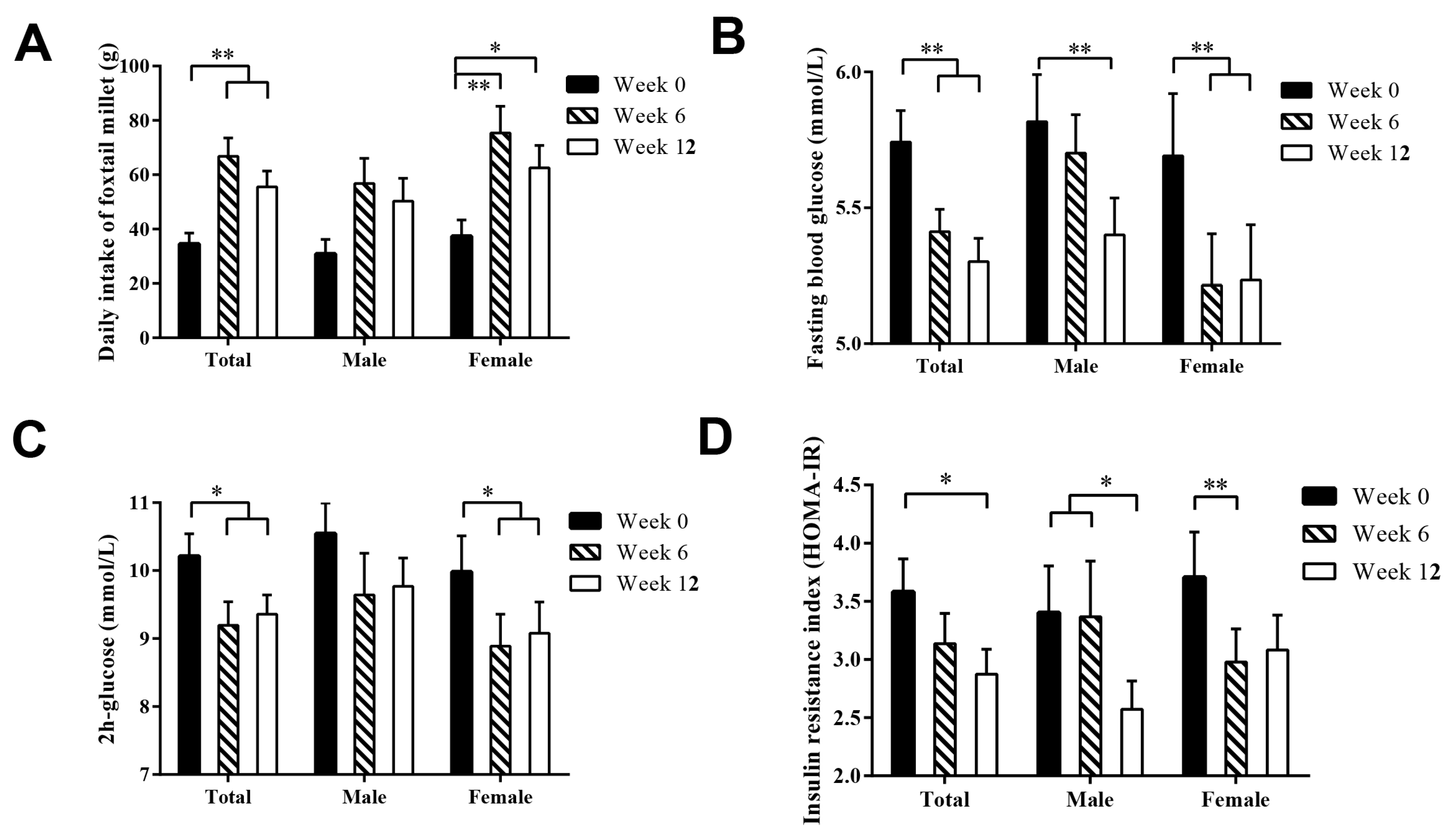
3.5. Sex-Dependent Difference in the Glucose-Lowering Effect of Foxtail Millet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-R.; Li, G.-W.; Hu, Y.-H.; Wang, J.-X.; Yang, W.-Y.; An, Z.-X.; Hu, Z.-X.; Xiao, J.-Z.; Cao, H.-B.; Liu, P.-A. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Ajala, O.; English, P.; Pinkney, J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am. J. Clin. Nutr. 2013, 97, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Xi, P.; Liu, R.H. Whole food approach for type 2 diabetes prevention. Mol. Nutr. Food Res. 2016, 60, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Qi, L.; Fahey, G.C., Jr.; Klurfeld, D.M. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; Pereira, M.A.; Liu, S.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Whole-grain intake and the risk of type 2 diabetes: A prospective study in men. Am. J. Clin. Nutr. 2002, 76, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet grains: Nutritional quality, processing, and potential health benefits. Compr. Rev. Food Sci. F 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Muninarayana, C.; Balachandra, G.; Hiremath, S.; Iyengar, K.; Anil, N. Prevalence and awareness regarding diabetes mellitus in rural Tamaka, Kolar. Int. J. Diabetes Dev. Ctries 2010, 30, 18. [Google Scholar] [CrossRef] [PubMed]
- Cisse, F.; Erickson, D.P.; Hayes, A.; Opekun, A.R.; Nichols, B.L.; Hamaker, B.R. Traditional Malian Solid Foods Made from Sorghum and Millet Have Markedly Slower Gastric Emptying than Rice, Potato, or Pasta. Nutrients 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.L.; Sumathi, S. Effect of consumption of finger millet on hyperglycemia in non-insulin dependent diabetes mellitus (NIDDM) subjects. Plant Food Hum. Nutr. 2002, 57, 205–213. [Google Scholar] [CrossRef]
- Ugare, R.; Chimmad, B.; Naik, R.; Bharati, P.; Itagi, S. Glycemic index and significance of barnyard millet (Echinochloa frumentacae) in type II diabetics. J. Food Sci. Technol. 2014, 51, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.; Shen, Q. In vitro starch digestibility and in vivo glycemic response of foxtail millet and its products. Food Funct. 2016, 7, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-Y.; Osada, K.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of dietary protein of Korean foxtail millet on plasma adiponectin, HDL-cholesterol, and insulin levels in genetically type 2 diabetic mice. Biosci. Biotechnol. Biochem. 2005, 69, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M.; Schmitter, M.; Tu, Y.-K. Assessment of replication of research evidence from animals to humans in studies on peri-implantitis therapy. J. Dent. 2009, 37, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Haddad, P.; Amouzgar-Hashemi, F.; Samsami, S.; Chinichian, S.; Oghabian, M.A. Aloe vera for prevention of radiation-induced dermatitis: A self-controlled clinical trial. Curr. Oncol. 2013, 20, 345–348. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Available online: http://apps.who.int/iris/bitstream/handle/10665/66040/WHO_NCD_NCS_99.2.pdf;jsessionid=9C91D94A2CCE91A06F9E772A20ABC984?sequence=1 (accessed on 7 May 2013).
- Ren, X.; Chen, J.; Wang, C.; Molla, M.M.; Diao, X.; Shen, Q. In vitro starch digestibility, degree of gelatinization and estimated glycemic index of foxtail millet-derived products: Effect of freezing and frozen storage. J. Cereal Sci. 2016, 69, 166–173. [Google Scholar] [CrossRef]
- Xue, Y.; Lee, E.; Ning, K.; Zheng, Y.; Ma, D.; Gao, H.; Yang, B.; Bai, Y.; Wang, P.; Zhang, Y. Prevalence of picky eating behaviour in Chinese school-age children and associations with anthropometric parameters and intelligence quotient. A cross-sectional study. Appetite 2015, 91, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lyu, J.; He, P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 961. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, Y.; Kasetti, R.B.; Nabi, S.A.; Swapna, S.; Apparao, C. Antihyperglycemic and hypolipidemic activities of Setaria italica seeds in STZ diabetic rats. Pathophysiology 2011, 18, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, J.; Sanjeevi, V.; Rohini, U.; Trueman, P.; Viswanathan, V. Postprandial glycaemic response of foxtail millet dosa in comparison to a rice dosa in patients with type 2 diabetes. Indian J. Med. Res. 2016, 144, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Louheranta, A.; Mannelin, M.; Rastas, M.; Salminen, V.; Eriksson, J.; Uusitupa, M.; Tuomilehto, J. The Finnish Diabetes Prevention Study (DPS) Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003, 26, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Nutrition, D.; Trial, C. adherence to the ADA nutritional recommendations, targets of metabolic control, and onset of diabetes complications. A 7-year, prospective, population-based, observational multicenter study. J. Diabetes Complicat. 2006, 20, 361–366. [Google Scholar]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits and uses. Food Rev. Int. 2017, 34. [Google Scholar] [CrossRef]
- Harreiter, J.; Kautzkywiller, A. Sex and Gender Differences in Prevention of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Long, N.; Diwan, V.; Winkvist, A. Attitudes to compliance with tuberculosis treatment among women and men in Vietnam. Int. J. Tuberc. Lung Dis. 1999, 3, 862–868. [Google Scholar] [PubMed]
- Pan, A.; Lin, X.; Hemler, E.; Hu, F.B. Diet and Cardiovascular Disease: Advances and Challenges in Population-Based Studies. Cell. Metab. 2018, 27, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.T.; Fletcher, E.A.; Galbraith, C.E.; Clifton, P.M. Patient freedom to choose a weight loss diet in the treatment of overweight and obesity: A randomized dietary intervention in type 2 diabetes and pre-diabetes. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.J.; Smit, E.; Snelling, A.; Sempos, C.T.; Andersen, R.E. Hormone replacement therapy and its relationship to lipid and glucose metabolism in diabetic and nondiabetic postmenopausal women results from the third national health and nutrition examination survey (NHANES III). Diabetes Care 2002, 25, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Goodwin, E.; Channer, K.; Jones, T. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2006, 154, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Campesi, I.; Occhioni, S.; Tonolo, G. Sex-gender differences in diabetes vascular complications and treatment. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Crepaldi, G.; Manzato, E.; Mancini, M.; Rivellese, A.; Paoletti, R.; Pazzucconi, F.; Pamparana, F.; Stragliotto, E. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis 1998, 137, 419–427. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kraemer, H.C. Monounsaturated versus polyunsaturated dietary fat and serum lipids. A meta-analysis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Wei, D.; Xin, R.; Chao, W.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2016, 56, 1–10. [Google Scholar]
- Jéquier, E. Leptin signaling, adiposity, and energy balance. Ann. N. Y. Acad. Sci. 2002, 967, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Call, M.C. Inflammation and type 2 diabetes. Diabetes Metab. 2012, 38, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Okhovat, A.; Berjis, N.; Okhovat, H.; Malekpour, A.; Abtahi, H. Low-level laser for treatment of tinnitus: A self-controlled clinical trial. J. Res. Med. Sci. 2011, 16, 33–38. [Google Scholar] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405. [Google Scholar] [CrossRef] [PubMed]
| Indices | Week 0 | Week 6 | Week 12 | p-Value 2 |
|---|---|---|---|---|
| Blood glucose | ||||
| FBG (mmol/L) | 5.7 ± 0.9 a | 5.4 ± 0.7 b | 5.3 ± 0.7 b | <0.001 |
| 2 h-Glucose (mmol/L) | 10.2 ± 2.6 a | 9.2 ± 2.8 b | 9.4 ± 2.3 b | 0.003 |
| FINS (pmol/L) | 94.7 ± 49.2 | 89.3 ± 52.4 | 84.0 ± 47.5 | 0.141 |
| 2 h-Insulin (pmol/L) | 805.3 ± 540.0 | 736.7 ± 581.1 | 759.1 ± 460.3 | 0.529 |
| Fasting C-peptide (pmol/L) | 657.4 ± 276.4 | 626.9 ± 289.0 | 598.9 ± 256.9 | 0.149 |
| Fructosamine (μmol/L) | 264.6 ± 25.4 | 268.7 ± 23.4 | 266.4 ± 26.1 | 0.293 |
| HOMA-IR | 3.6 ± 2.3 a | 3.1 ± 2.1 ab | 2.9 ± 1.7 b | 0.007 |
| HOMA-IS | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.6 | 0.085 |
| Blood lipid | ||||
| TC (mmol/L) | 5.2 ± 1.0 | 5.2 ± 1.0 | 5.2 ± 1.0 | 0.953 |
| TAG (mmol/L) | 2.0 ± 1.2 | 2.0 ± 1.2 | 2.1 ± 1.1 | 0.421 |
| HDL-C (mmol/L) | 1.2 ± 0.3 a | 1.3 ± 0.3 b | 1.3 ± 0.3 a | 0.044 |
| LDL-C (mmol/L) | 3.3 ± 0.9 | 3.4 ± 0.9 | 3.2 ± 0.9 | 0.056 |
| ApoA1 (mmol/L) | 1.6 ± 0.3 a | 1.6 ± 0.3 b | 1.6 ± 0.3 ab | 0.013 |
| ApoB (mmol/L) | 1.0 ± 0.3 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.140 |
| ApoA1/ApoB | 1.6 ± 0.6 | 1.6 ± 0.4 | 1.6 ± 0.5 | 0.684 |
| Blood pressure | ||||
| SBP (mmHg) | 125.0 ± 13.2 | 126.6 ± 14.5 | 128.3 ± 13.2 | 0.155 |
| DBP (mmHg) | 84.9 ± 8.5 a | 83.9 ± 8.4 a | 81.6 ± 7.8 b | 0.003 |
| Cytokine | ||||
| TNF-α (pg/mL) | 2.6 ± 5.5 | 1.3 ± 0.6 | 1.4 ± 0.5 | 0.088 |
| IL-6 (pg/mL) | 6.2 ± 9.4 a | 4.2 ± 5.3 b | 4.8 ± 5.5 ab | 0.084 |
| Hormones | ||||
| Leptin (ng/mL) | 8.3 ± 6.4 a | 9.0 ± 6.1 ab | 9.6 ± 7.0 b | 0.012 |
| Adiponectin (ug/mL) | 2.1 ± 1.6 | 1.8 ± 1.6 | 1.8 ± 2.1 | 0.301 |
| GLP-1 (pg/mL) | 23.6 ± 6.1 | 23.9 ± 7.0 | 24.8 ± 9.0 | 0.437 |
| Anthropometric indices | ||||
| Weight (kg) | 69.1 ± 11.6 a | 69.4 ± 11.7 ab | 68.2 ± 11.2 b | 0.004 |
| BMI | 26.0 ± 3.5 a | 25.8 ± 3.4 ab | 25.5 ± 3.2 b | <0.001 |
| Waist circumference (cm) | 90.3 ± 8.7 | 90.4 ± 9.5 | 89.6 ± 8.5 | 0.296 |
| Hip circumference (cm) | 100.3 ± 7.7 | 100.0 ± 7.1 | 100.0 ± 6.9 | 0.400 |
| Waist-hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.0 | 0.644 |
| Body composition | ||||
| Body fat percentage (%) | 31.9 ± 8.1 a | 31.4 ± 8.0 ab | 30.9 ± 7.7 b | 0.132 |
| Body fat mass (kg) | 22.1 ± 7.1 a | 21.9 ± 7.2 ab | 21.1 ± 6.2 b | 0.086 |
| Fat-free mass (kg) | 47.0 ± 9.4 | 47.4 ± 9.2 | 47.2 ± 9.7 | 0.670 |
| Obesity degree | 110.0 ± 15.2 a | 110.0 ± 15.4 a | 108.3 ± 13.8 b | 0.005 |
| Muscle mass (kg) | 44.4 ± 9.1 | 44.8 ± 8.8 | 44.6 ± 9.3 | 0.677 |
| Protein (kg) | 10.5 ± 3.3 | 10.5 ± 3.1 | 10.6 ± 3.2 | 0.585 |
| Mineral (kg) | 2.6 ± 0.4 | 2.6 ± 0.4 | 2.6 ± 0.4 | 0.587 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients 2018, 10, 1509. https://doi.org/10.3390/nu10101509
Ren X, Yin R, Hou D, Xue Y, Zhang M, Diao X, Zhang Y, Wu J, Hu J, Hu X, et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients. 2018; 10(10):1509. https://doi.org/10.3390/nu10101509
Chicago/Turabian StyleRen, Xin, Ruiyang Yin, Dianzhi Hou, Yong Xue, Min Zhang, Xianmin Diao, Yumei Zhang, Jihong Wu, Jinrong Hu, Xiaosong Hu, and et al. 2018. "The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial" Nutrients 10, no. 10: 1509. https://doi.org/10.3390/nu10101509
APA StyleRen, X., Yin, R., Hou, D., Xue, Y., Zhang, M., Diao, X., Zhang, Y., Wu, J., Hu, J., Hu, X., & Shen, Q. (2018). The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients, 10(10), 1509. https://doi.org/10.3390/nu10101509