Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Dietary Intervention
2.4. Anthropometric, Clinical and Biochemical Measurements
2.5. Physical Activity
2.6. Dietary Intake Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, M.P.; Hennigan, K.; Gorman, C.S.O.; Mccarron, L. Obesity, diet and lifestyle in 9-year-old children with parentally reported chronic diseases: Findings from the growing up in Ireland longitudinal child cohort study. Ir. J. Med. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Boirie, Y.; Cederholm, T.; Chourdakis, M.; Cuerda, C.; Delzenne, N.M.; Deutz, N.E.; Fouque, D.; Genton, L.; Gil, C.; et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin. Nutr. 2017, 36, 917–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, S.V.; Rodríguez-Martín, A.; Ruiz, J.P.N.; Nieto, J.M.M.; Campoy, J.L.L. Hábitos y estilos de vida modificables en niños con sobrepeso y obesidad. Nutr. Hosp. 2010, 25, 823–831. [Google Scholar] [CrossRef]
- Al-Khudairy, L.; Loveman, E.; Colquitt, J.; Mead, E.; Johnson, R.; Fraser, H.; Olajide, J.; Murphy, M.; Velho, R.M.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev. 2017, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Mead, E.; Brown, T.; Rees, K.; Azevedo, L.B.; Whittaker, V.; Jones, D.; Olajide, J.; Mainardi, G.M.; Corpeleijn, E.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T. Lifestyle intervention in childhood obesity: Changes and challenges. Nat. Rev. Endocrinol. 2013, 9, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Morell-Azanza, L.; Azcona-Sanjulián, M.C.; Martínez, J.A.; Ramírez, M.J.; Marti, A. Reduced serotonin levels after a lifestyle intervention in obese children: Association with glucose and anthropometric measurements. Nutr. Hosp. 2018, 35, 279–285. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Etayo, P.; Moreno, L.A.; Santabarbara, J.; Martín-matillas, M.; Piqueras, M.J.; Rocha-silva, D.; Marti, A.; Campoy, C.; Marcos, A.; Garagorri, J.M.; et al. Anthropometric indices to assess body-fat changes during a multidisciplinary obesity treatment in adolescents: EVASYON Study. Clin. Nutr. 2015, 34, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rendo-Urteaga, T.; Puchau, B.; Chueca, M.; Oyarzabal, M.; Azcona-Sanjulián, M.C.; Martínez, J.A.; Marti, A. Total antioxidant capacity and oxidative stress after a 10-week dietary intervention program in obese children. Eur. J. Pediatr. 2014, 173, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Morell-Azanza, L.; García-Calzón, S.; Rendo-Urteaga, T.; Martin-Calvo, N.; Chueca, M.; Martínez, J.A.; Azcona-Sanjulián, M.C.; Marti, A. Serum oxidized low-density lipoprotein levels are related to cardiometabolic risk and decreased after a weight loss treatment in obese children and adolescents. Pediatr. Diabetes 2016, 18, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Waling, M.; Lind, T.; Hernell, O.; Larsson, C. A one-year intervention has modest effects on energy and macronutrient intakes of overweight and obese Swedish children. J. Nutr. 2010, 140, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Waling, M.; Larsson, C. Improved dietary intake among overweight and obese children followed from 8 to 12 years of age in a randomised controlled trial. J. Nutr. Sci. 2012, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vyncke, K.; Cruz Fernandez, E.; Fajó-Pascual, M.; Cuenca-García, M.; De Keyzer, W.; Gonzalez-Gross, M.; Moreno, L.A.; Beghin, L.; Breidenassel, C.; Kersting, M.; et al. Validation of the diet quality index for adolescents by comparison with biomarkers, nutrient and food intakes: The HELENA study. Br. J. Nutr. 2013, 109, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Kourlaba, G.; Grammatikaki, E.; Koubitski, A.; Siatitsa, P.E.; Vandorou, A.; Kyriakou, K.; Dede, V.; Moschonis, G. Development of a lifestyle-diet quality index for primary schoolchildren and its relation to insulin resistance: The healthy lifestyle-diet index. Eur. J. Clin. Nutr. 2010, 64, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J. Hum. Nutr. Diet. 2014, 27, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Asghari, G.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. A systematic review of diet quality indices in relation to obesity. Br. J. Nutr. 2017, 117, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Lorenzo, H.; Vrotsou, K.; Aresti, U.; Rica, I.; Sánchez, E. Estudio de Crecimiento de Bilbao. Curvas y Tablas de Crecimiento (Estudio Transversal). Available online: http://www.fundacionorbegozo.com/wp-content/uploads/pdf/estudios_2011.pdf (accessed on 3 February 2014).
- Zazpe, I.; Sánchez-Taínta, A.; Santiago, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Martínez, J.A.; Martínez-González, M.Á. Association between dietary carbohydrate intake quality and micronutrient intake adequacy in a Mediterranean cohort: The SUN (Seguimiento Universidad de Navarra) project. Br. J. Nutr. 2014, 111, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Aranceta Batrina, J.; Arija Val, V.; Maíz Aldalur, E.; Martínez de Victoria Muñoz, E.; Ortega Anta, R.M.; Pérez Rodrigo, C.; Quiles Izquierdo, J.; Rodríguez Martín, A.; Román Viñas, B.; Salvador i Castell, G.; et al. Guías alimentarias para la población española (SENC, diciembre 2016); la nueva pirámide de la alimentación saludable. Nutr. Hosp. 2015, 31, 1–145. [Google Scholar]
- Marqués, M.; Moleres, A.; Rendo-Urteaga, T.; Gómez-Martínez, S.; Zapatera, B.; Romero, P.; de Miguel-Etayo, P.; Campoy, C.; Alfredo Martínez, J.; Azcona-San Julián, C.; et al. Design of the nutritional therapy for overweight and obese Spanish adolescents conducted by registered dieticians: The EVASYON study TT—Diseño de terapia nutricional para adolescentes españoles con sobrepeso y obesidad realizado por dietistas titulados. Nutr. Hosp. 2012, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Serra Majem, L. Objetivos nutricionales para la población española: Consenso de la Sociedad Española de Nutrición Comunitaria 2011. Rev. Esp. Nutr. Comunitaria 2011, 17, 178–199. [Google Scholar]
- Willett, W.C.; Sacks, F.M.; Trichopoulou, A.; Drescher, D.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Daldalian, M.C.; Mayfield, C.A.; Dean, K.; Black, W.R.; Sampilo, M.L.; Gonzalez-Mijares, M.; Suminski, R. Outcomes from an urban pediatric obesity program targeting minority youth: the healthy hawks program. Child. Obes. 2013, 9, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Evenson, K.R.; Catellier, D.J.; Gill, K.; Ondrak, K.S.; McMurray, R.G. Calibration of two objective measures of physical activity for children. J. Sports Sci. 2008, 26, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Fuente-Arrillaga, C.; Vzquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- VIG. De actieve voedingsdriehoek: een praktische voedings-en beweeggids. Available online: https://www.ikhebeenvraag.be/mediastorage/FSDocument/233/actieve_voedingsdriehoek_maart_2012.pdf (accessed on 3 October 2017).
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean diet quality index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Rendo-Urteaga, T.; Garcia-Calzon, S.; Gonzalez-Muniesa, P.; Milagro, F.I.; Chueca, M.; Oyarzabal, M.; Azcona-Sanjulian, M.C.; Martinez, J.A.; Marti, A. Peripheral blood mononuclear cell gene expression profile in obese boys who followed a moderate energy-restricted diet: Differences between high and low responders at baseline and after the intervention. Br. J. Nutr. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Moleres, A.; Marcos, A.; Campoy, C.; Moreno, L.A.; Azcona-Sanjulián, M.C.; Martínez-González, M.A.; Martínez, A.; Zalba, G.; Marti, A. Telomere length as a biomarker for adiposity changes after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS ONE 2014, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Abellana Sangra, R.; Farran Codina, A. The identification, impact and management of missing values and outlier data in nutritional epidemiology. Nutr. Hosp. 2015, 31 (Suppl. 3), 189–195. [Google Scholar] [CrossRef]
- Heaney, R.P.; Weaver, C.M. Calcium and Vitamin D. Endocrinol. Metab. Clin. North Am. 2003, 32, 181–194. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Chung, S.T.; Onuzuruike, A.U.; Magge, S.N. Cardiometabolic risk in obese children. Ann. N. Y. Acad. Sci. 2018, 1411, 166–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinehr, T.; Kleber, M.; Toschke, A.M. Lifestyle intervention in obese children is associated with a decrease of the metabolic syndrome prevalence. Atherosclerosis 2009, 207, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M. Iodine deficiency and excess in children: Worldwide status in 2013. Endocr. Pract. 2013, 19, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Mouratidou, T.; Vicente-Rodriguez, G.; Gracia-Marco, L.; Huybrechts, I.; Sioen, I.; Widhalm, K.; Valtueña, J.; González-Gross, M.; Moreno, L.A. Associations of dietary calcium, vitamin D., milk intakes, and 25-Hydroxyvitamin D with bone mass in Spanish adolescents: The HELENA study. J. Clin. Densitom. 2013, 16, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, L.; Pezzella, V.; Nocerino, R.; Di Costanzo, M.; Coruzzo, A.; Passariello, A.; Leone, L.; Savoia, M.; Del Puente, A.; Esposito, A.; et al. Calcium and vitamin D intakes in children: A randomized controlled trial. BMC Pediatr. 2013, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported dietary intake, disparity between the reported consumption and the level needed for adequacy and food sources of calcium, phosphorus, magnesium and vitamin D in the Spanish population: Findings from the ANIBES study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas-Barba, L.; Pérez-Rodrigo, C.; Bartrina, J.A. Nutrient adequacy in Spanish children and adolescents. Br. J. Nutr. 2006, 96, S49–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Sobaler, A.M.; Aparicio, A.; González-Rodríguez, L.G.; Cuadrado-Soto, E.; Rubio, J.; Marcos, V.; Sanchidrián, R.; Santos, S.; Pérez-Farinós, N.; Dal Re, M.Á.; et al. Adequacy of usual vitamin and mineral intake in Spanish children and adolescents: ENALIA study. Nutrients 2017, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Royo-Bordonada, M.A.; Gorgojo, L.; de Oya, M.; Garcés, C.; Rodríguez-Artalejo, F.; Rubio, R.; del Barrio, J.L.; Martín-Moreno, J.M. Food sources of nutrients in the diet of Spanish children: The four provinces study. Br. J. Nutr. 2003, 89, 105. [Google Scholar] [CrossRef] [PubMed]
- Buendia, J.R.; Bradlee, M.L.; Daniels, S.R.; Singer, M.R.; Moore, L.L. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015, 169, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Z.; Kuklina, E.V.; Fang, J.; Ayala, C.; Hong, Y.; Loustalot, F.; Dai, S.; Gunn, J.P.; Tian, N.; et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics 2012, 130, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Couch, S.C.; Saelens, B.E.; Levin, L.; Dart, K.; Falciglia, G.; Daniels, S.R. The efficacy of a clinic-based behavioral nutrition intervention emphasizing a DASH-type diet for adolescents with elevated blood pressure. J. Pediatr. 2008, 152, 494–501. [Google Scholar] [CrossRef] [PubMed]
- López-Sobaler, A.M.; Aparicio, A.; Rubio, J.; Marcos, V.; Sanchidrián, R.; Santos, S.; Pérez-Farinós, N.; Dal-Re, M.Á.; Villar-Villalba, C.; Yusta-Boyo, M.J.; et al. Adequacy of usual macronutrient intake and macronutrient distribution in children and adolescents in Spain: A national dietary survey on the child and adolescent population, Enalia 2013–2014. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Grammatikaki, E.; Papoutsou, S.; Liarigkovinos, T.; Kondaki, K.; Moschonis, G. Nutrient intakes of toddlers and preschoolers in Greece: The GENESIS Study. J. Am. Diet. Assoc. 2008, 108, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Nemet, D.; Barkan, S.; Epstein, Y.; Friedland, O.; Kowen, G.; Eliakim, A. Short- and long-term beneficial effects of a combined dietary-behavioral-physical activity intervention for the treatment of childhood obesity. Pediatrics 2005, 115, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bel, S.; Michels, N.; De Vriendt, T.; Patterson, E.; Cuenca-García, M.; Diethelm, K.; Gutin, B.; Grammatikaki, E.; Manios, Y.; Leclercq, C.; et al. Association between self-reported sleep duration and dietary quality in European adolescents. Br. J. Nutr. 2013, 110, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Cuenca-García, M.; Labayen, I.; Esteban-Cornejo, I.; Henriksson, H.; Kersting, M.; Vanhelst, J.; Widhalm, K.; Gottrand, F.; Moreno, L.A.; et al. Diet quality and attention capacity in European adolescents: The healthy lifestyle in Europe by nutrition in adolescence (HELENA) study. Br. J. Nutr. 2017, 117, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Etayo, P.; Moreno, L.A.; Santabárbara, J.; Martín Matillas, M.; Azcona-SanJulian, C.; Marti del Moral, A.; Campoy, C.; Marcos, A.; Garagorri, J.M.; Marcos, A.; et al. Diet quality index is a good predictor of treatment efficacy in overweight and obese adolescents: The EVASYON study. Clin. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Papandreou, C.; Politidou, E.; Naoumi, A.; Peppas, D.; Mavrogianni, C.; Lionis, C.; Chrousos, G.P. Revised healthy lifestyle-diet index and associations with obesity and iron deficiency in schoolchildren: The healthy growth study. J. Hum. Nutr. Diet. 2015, 28, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Ertaş Öztürk, Y.; Bozbulut, R.; Döğer, E.; Bideci, A.; Köksal, E. The relationship between diet quality and insulin resistance in obese children: Adaptation of the healthy lifestyle-diet index in Turkey. J. Pediatr. Endocrinol. Metab. 2018, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Da-Silva, L.; Rêgo, C.; Pietrobelli, A. The diet of preschool children in the Mediterranean countries of the European Union: A systematic review. Int. J. Environ. Res. Public Health 2016, 13, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in children and adolescents: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, C.; Pippi, R.; Buratta, L.; Aiello, C.; Gianfredi, V.; Piana, N.; Reginato, E. Effects of an intensive lifestyle intervention to treat overweight/obese children and adolescents. Biomed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Ribas-Barba, L.; Pérez-Rodrigo, C.; Aranceta, J. Nutrient adequacy and Mediterranean diet in Spanish school children and adolescents. Eur. J. Clin. Nutr. 2003, 57, S35–S39. [Google Scholar] [CrossRef] [PubMed]
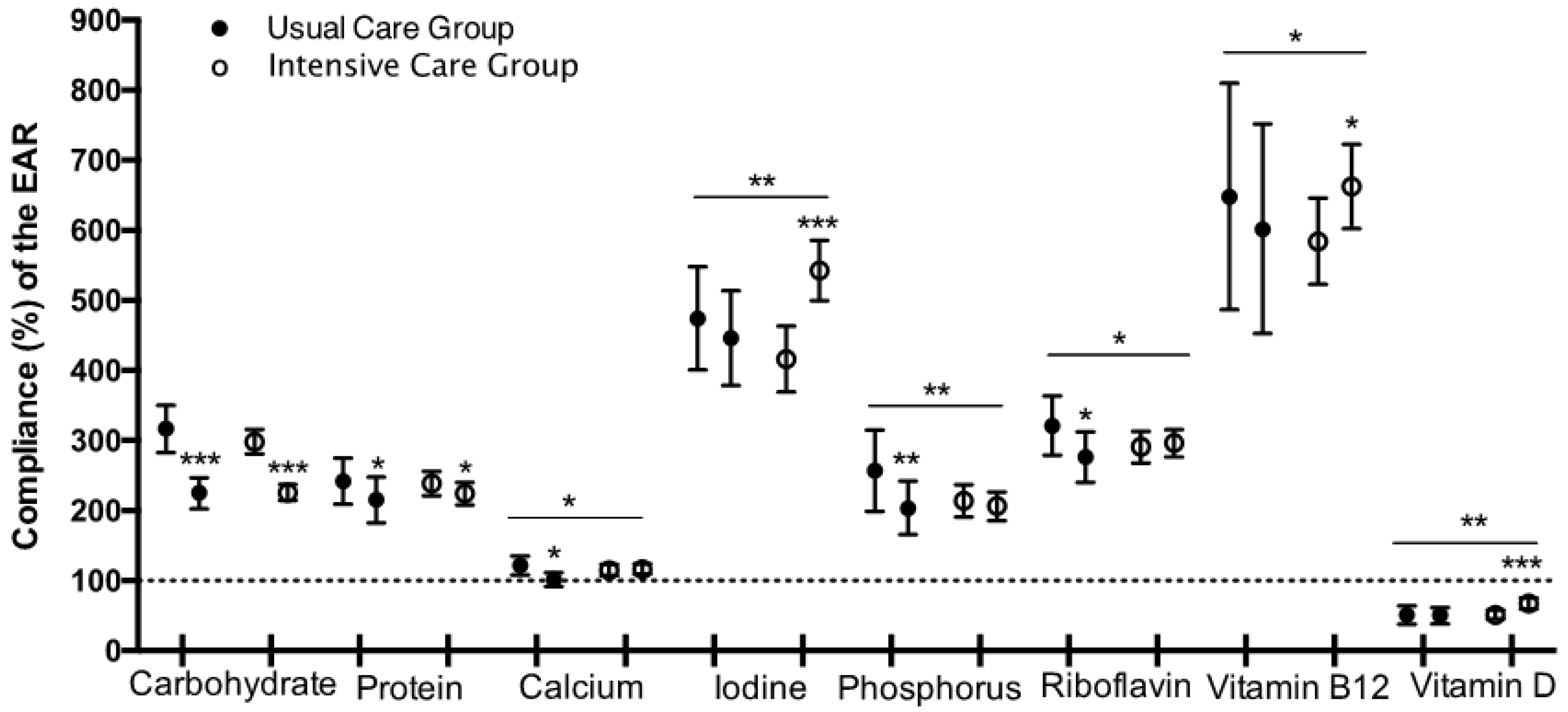
| Baseline | |||||
|---|---|---|---|---|---|
| Changes within group after eight weeks | |||||
| Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Changes between Groups (Intensive vs. Usual Care) | |
| Mean (SD) or Percentage | Mean (95% CI) | Mean (95% CI) | |||
| Age (years) | 10.7 (2.4) | 11.49 (2.5) | |||
| Sex (F) | 18 (69) | 49 (61) | |||
| Tanner (1/2/3/4/5) | 1(38) 2(8) 3(15) 4(4) 5(23) | 1(33) 2(19) 3(14) 4(6) 5(25) | |||
| Weight (kg) | 62.7 (17.9) | 67.27 (19.7) | −2.2 (−3.1, −1.3) *** | −2.6 (−3.1, −2.2) *** | −0.4 (−1.4, 0.5) |
| Height (cm) | 147.9 (12.9) | 151.75 (13.3) | 1.2 (0.9, 1.5) *** | 0.9 (0.8, 1.0) *** | −0.3 (−0.6, −0.00) * |
| BMI | 28.1 (4.5) | 28.52 (4.6) | −1.4 (−1.8, −0.9) *** | −1.5 (−1.7, −1.3) *** | −0.1 (−0.6, 0.3) |
| BMI-SDS | 2.9 (1.2) | 2.86 (1.0) | −0.5 (−0.8, −0.2 ) ** | −0.5 (−0.6, −0.4) *** | −0.0 (−0.4,0.2) |
| Glucose (mg/dL) | 91.2 (6.05) | 88.0 (6.3) * | −5.8 (−8.9, −2.7) *** | −2.0 (−3.8, −0.3) * | 3.8 (0.3, 7.2) * |
| Insulin (µU/mL) | 19.9 (20.5) | 16.3 (8.5) | −3.5 (−8.3, 1.2) | −2.1 (−3.7, −0.6) ** | 1.4 (−2.4, 5.1) |
| Total Cholesterol (mg/dL) | 156.8 (22.6) | 164.8 (27.3) | −11.4 (−18.4, −4.4) ** | −11.9 (−17.2, −6.6) *** | −0.6 (−10.3, 9.2) |
| Systolic BP (mmHg) | 113.3 (11.5) | 119.0 (12.0) * | 0.76 (−4.6, 6.2) | −6.6 (−9.0, −4.3) *** | −7.4 (−12.5, −2.3) ** |
| Diastolic BP (mmHg) | 71.5 (8.1) | 72.8 (8.2) | 1.68 (−4.2, 7.5) | −3.5 (−5.4, −1.6) *** | −5.2 (−9.8, −0.6) * |
| Fruits (g/day) | 254.0 (135.5) | 253.4 (177.3) | 9.4 (−78.9, 97.8) | 70.4 (20.6, 120.2) ** | 61.0 (−39.1, 161.0) |
| Vegetables (g/day) | 262.0 (156.1) | 313.1 (162.9) | 60.0 (−31.7, 151.7) | 68.1 (28.3, 107.8) ** | 8.1 (−77.7, 93.9,) |
| Legumes (g/day) | 17.6 (10.2) | 20.2 (11.1) | −1.3 (−5.6, 2.9) | 0.7 (−2.2, 3.6) | 2.0 (−3.6, 7.6,) |
| Dairy Products (g/day) | 497.4 (198.3) | 457.1 (192.7) | −83.3 (−181.9, 15.3) | 93.6 (43.2, 144.0) *** | 176.9 (73.3, 280.5) ** |
| Meat (g/day) | 180.0 (44.9) | 205.8 (60.1) * | −23.2 (−47.3, 1.0) | −53.6 (−68.4, −38.9) *** | −30.5 (−59.6, −1.3,) * |
| Sausages (g/day) | 9.7 (11.0) | 21.1 (21.6) * | −5.5 (−10.5, −0.6) * | −19.0 (−23.6, −14.4) *** | −13.5 (−22.1, −4.9,) ** |
| Fish (g/day) | 70.2 (42.4) | 71.2 (36.3) | 12.7 (−2.4, 27.9) | 31.7 (22.5, 40.9) *** | 19.0 (0.8, 37.1) * |
| Whole Grains (g/day) | 3.7 (10.5) | 21.3 (54.3) | 48.6 (17.1, 80.0) ** | 28.0 (11.4, 44.6) ** | −20.6 (−54.5, 13.3,) |
| Refined Grains (g/day) | 181.31 (76.3) | 163.5 (92.8) | −97.2 (−126.9, −67.5) *** | −74.4 (−95.9, −52.9) *** | 22.8 (8.4, −64.0) |
| Eggs (g/day) | 23.4 (10.9) | 24.3 (9.8) | −1.1 (−5.3, 3.1) | −0.7 (−2.8, 1.3) | 0.4 (−3.9, 4.7) |
| Nuts (g/day) | 0.1 (0.1) | 0.1 (0.1) | 0.4 (−1.2, 2.0) | −0.9 (−1.7, 0.0) | −1.3 (−3.0, 0.5) |
| Olive Oil (g/day) | 30.2 (15.5) | 29.4 (16.9) | −6.1 (−13.2, 1.1) | −4.7 (−8.5, −0.9) * | 1.4 (−6.4, 9.1) |
| Sweets (g/day) | 120.9 (75.6) | 100.4 (70.5) | −57.6 (−91.1, −24.2) ** | −48.5 (−63.6, −33.4) *** | 9.1 (−23.1, 41.3) |
| MVPA (min/day) | 45.7 (23.8) | 43.7 (23.9) | 0.6 (−10.5, 11.6) | 5.5 (0.6, 10.3) * | 4.9 (−5.6, 15.3) |
| Baseline | |||||
|---|---|---|---|---|---|
| Changes within group after eight weeks | |||||
| Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Changes between Groups (Intensive vs. Usual Care) | |
| mean (SD) | mean (95% CI) | mean (95% CI) | |||
| Carbohydrate (g/day) | 317.1 (83.2) | 298.2 (80.1) | −91.3 (−124.4, −58.3) *** | −71.7 (−88.96, −54.50) *** | 19.6 (−15.7, 54.9) |
| Fibre (g/day) | 21.8 (6.0) | 23.6 (7.5) | 2.1 (−1.9, 6.0) | 3.7 (1.8, 5.6) *** | 1.6 (−2.4, 5.6) |
| Protein (g/day) | 105.7 (18.0) | 110.8 (20.2) | −14.6 (−23.7, −5.6) ** | −11.1 (−16.1, −6.1) *** | 3.6 (−6.5, 13.7) |
| Total Fat (g/day) | 115.4 (29.9) | 116.1 (33.0) | −38.0 (−49.7, −26.4) *** | −44.4 (−51.9, −36.9) *** | −6.4 (−21.1, 8.4) |
| SFA (g/day) | 34.9 (10.7) | 35.3 (13.5) | −12.0 (−16.3, −7.8) *** | −15.3 (−18.7, −12.0) *** | −3.3 (−9.5, 2.9) |
| MUFA (g/day) | 50.5 (13.9) | 51.6 (16.6) | −14.0 (−20.8, −7.2) *** | −19.2 (−23.6, −14.8) *** | −5.2 (−13.7, 3.2) |
| PUFA (g/day) | 15.5 (6.1) | 14.9 (5.4) | −4.6 (−7.3, −1.8) ** | −6.1 (−7.4, −4.7) *** | −1.5 (−4.3, 1.3) |
| Trans Fat (g/day) | 0.8 (0.5) | 1.0 (0.5) | −0.4 (−0.6, −0.2) *** | −0.6 (−0.7, −0.4) *** | −0.2 (−0.4, 0.1) |
| Cholesterol (mg/day) | 435.5 (106.1) | 444.5 (115.9) | −72.7 (−110.5, −34.9) *** | −78.0 (−103.5, −52.6) *** | −5.4 (−54.7, 43.9) |
| Calcium (mg/day) | 1208.2 (315.3) | 1203.4 (361.7) | −165.7 (−324.1, −7.4) * | 35.5 (−53.8, 124.9) | 201.3 (22.0, 380.6) * |
| Iron (mg/day) | 17.8 (3.9) | 18.4 (4.0) | −2.3 (−4.3, −0.4) * | −2.6 (−3.5, −1.7) *** | −0.3 (−2.2, 1.7) |
| Iodine (μg/day) | 341.8 (126.2) | 315.5 (150.4) | −4.3 (−66.2, 57.6) | 104.1 (67.3, 141.0) *** | 108.4 (35.3, 181.5) ** |
| Magnesium (mg/day) | 383.6 (83.7) | 400.5 (92.3) | −23.6 (−67.4, 20.2) | −2.6 (−26.1, 20.9) | 21.0 (−26.7, 68.7) |
| Zinc (mg/day) | 13.5 (2.5) | 14.5 (3.3) | −1.8 (−3.3, −0.4) * | −2.1 (−2.9, −1.2) *** | −0.2 (−1.9, 1.5) |
| Sodium (mg/day) | 2914.1 (655.7) | 3098.7 (899.8) | −750.8 (−1023.8, −477.7) *** | −936.4 (−1155.9, −717.0) *** | −185.6 (−599.9, 228.7) |
| Potassium (g/day) | 4.2 (1.0) | 4.4 (1.0) | −0.3 (−0.8, 0.2) | 0.1 (−0.1, 0.4) | 0.4 (−1.0, 0.9) |
| Phosphorus (mg/day) | 1799.9 (339.5) | 1868.2 (405.3) | −191.4 (−337.9, −44.9) * | −9.3 (−106.7, 88.1) | 182.1 (−7.0, 371.3) |
| Selenium (μg/day) | 101.1 (30.2) | 106.7 (34.4) | −10.3 (−23.9, 3.3) | −5.7 (−14.3, 2.9) | 4.6 (−12.2, 21.4) |
| Thiamine (mg/day) | 2.4 (0.7) | 2.4 (0.6) | 0.1 (−0.4, 0.5) | 0.1 (−0.1, 0.2) | −0.0 (−0.4, 0.4) |
| Riboflavin (mg/day) | 2.2 (0.5) | 2.3 (0.6) | −0.2 (−0.4, 0.1) | 0.1 (−0.1, 0.2) | 0.3 (−0.0, 0.06) |
| Niacin (mg/day) | 41.7 (8.3) | 45.0 (8.9) | −2.1 (−6.5, 2.3) | −2.4 (−4.5, −0.2) * | −0.3 (−4.8, 4.3) |
| Vitamin B6 (mg/day) | 2.3 (0.5) | 2.4 (0.6) | −0.0 (−0.3, 0.3) | 0.1 (−0.0, 0.2) | 0.1 (−0.2, 0.4) |
| Folate (μg/day) | 344.1 (101.6) | 364.5 (99.1) | −2.3 (−52.9, 48.3) | 21.2 (−2.8, 45.1) | 23.5 (−26.8, 73.8) |
| Vitamin B12 (μg/day) | 8.4 (4.4) | 8.9 (3.7) | 0.1 (−1.4, 1.5) | 1.4 (0.5, 2.2) ** | 1.3 (−0.4, 3.0) |
| Vitamin C (mg/day) | 187.0 (73.2) | 189.2 (71.3) | −0.5 (−39.2, 38.2) | 23.6 (4.2, 43.0) * | 24.1 (−15.9, 64.1) |
| Vitamin A (μg/day) | 1411.8 (1199.8) | 1287.0 (768.5) | −276.9 (−735.3, 181.5) | 6.8 (−15.0, 163.3) | 283.7 (−87.1, 654.5) |
| Vitamin D (μg/day) | 5.1 (3.2) | 5.1 (2.8) | −0.0 (−1.3, 1.3) | 1.7 (0.9, 2.5) *** | 1.7 (0.1, 3.4) * |
| Vitamin E (mg/day) | 10.5 (4.1) | 10.8 (4.5) | −3.1 (−4.4, −1.7) *** | −2.8 (−3.7, −2.0) *** | 0.2 (−1.4, 1.9) |
| Baseline | ||||||
|---|---|---|---|---|---|---|
| AMDR | Changes within group after eight weeks | |||||
| Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Changes between Groups (Intensive vs. Usual Care) | ||
| mean (SD) or percentage | mean (95% CI) | mean (95% CI) | ||||
| Energy (kcal/day) | 2729.4 (572.6) | 2681.7 (585.6) | −766.1 (−997.9, −534.3) *** | −731.1 (862.4, −599.7) *** | 35.0 (−228.3, 298.4) | |
| Carbohydrates (% Energy) | 46.3 (5.3) | 44.3 (6.3) | −0.1 (−2.4, 2.2) | 2.0 (0.6, 3.4) ** | 2.1 (−0.7, 4.9) | |
| % Within AMDR | 45–65 | 54 | 47 | 0 | 11 | 11 |
| Protein (% Energy) | 15.7 (1.9) | 16.8 (2.4) * | 3.1 (2.1, 4.0) *** | 3.8 (3.2, 4.4) *** | 0.8 (−0.4, 1.9) | |
| % Within AMDR | 10–30 | 100 | 100 | 0 | 0 | 0 |
| Fat (% Energy) | 38.0 (5.3) | 38.9 (6.1) | −3.0 (−5.4, −0.6) * | −5.8 (−7.3, −4.3) *** | −2.9 (−5.8, 0.1) | |
| % Within AMDR | 25–35 | 31 | 30 | 27 * | 38 *** | 11 |
| Usual Care Group (n = 26) | Intensive Care Group (n = 81) | |||||||
|---|---|---|---|---|---|---|---|---|
| <% EAR | >% UL | <% EAR | >% UL | |||||
| Baseline | 8 Weeks | Baseline | 8 Weeks | Baseline | 8 Weeks | Baseline | 8 Weeks | |
| Calcium (mg/day) | 23.1 | 42.3 | - | - | 37.0 | 29.6 | - | - |
| Iron (mg/day) | - | - | - | - | - | - | - | - |
| Iodine (μg/day) | - | - | 7.7 | - | 2.5 | - | 4.9 | 7.4 |
| Magnesium (mg/day) | 7.7 | 11.5 | 73.1 | 65.6 | 6.2 | 2.5 | 70.4 | 76.5 |
| Zinc (mg/day) | - | - | 19.2 | 7.7 | - | - | 8.6 | 3.7 |
| Sodium (mg/day) # | - | 7.7 | 92.3 | 53.9 ** | 2.5 | 9.9 | 86.4 | 39.5 *** |
| Potassium (g/day) # | 53.9 | 80.8 | 59.3 | 58.0 | ||||
| Phosphorus (mg/day) | - | - | - | - | - | - | - | - |
| Selenium (μg/day) | - | - | - | - | 1.2 | 1.2 | - | - |
| Thiamine (mg/day) | - | - | - | - | ||||
| Riboflavin (mg/day) | - | - | - | - | ||||
| Niacin (mg/day) | - | - | 96.2 | 96.2 | - | - | 100.0 | 98.8 |
| Vitamin B6 (mg/day) | - | - | - | - | - | - | - | - |
| Folate (μg/day) | 23.1 | 19.2 | 11.5 | 11.5 | 18.5 | 11.1 | 9.9 | 6.2 |
| Vitamin B12 (μg/day) | - | - | - | - | ||||
| Vitamin C (mg/day) | - | - | - | - | - | - | - | - |
| Vitamin A (μg/day) | 3.9 | 15.4 | 38.5 | 15.4 | 7.4 | 1.2 §§ | 22.2 | 18.5 |
| Vitamin D (μg/day) | 88.5 | 92.3 | - | - | 92.5 | 71.6 **§ | - | - |
| Vitamin E (mg/day) | 30.8 | 65.4 ** | - | - | 45.7 | 77.8 *** | - | - |
| Baseline | |||||
|---|---|---|---|---|---|
| Changes within group after eight weeks | |||||
| Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Usual Care Group (n = 26) | Intensive Care Group (n = 81) | Changes between Groups (Intensive vs. Usual Care) | |
| mean (SD) | mean (95% CI) | mean (95% CI) | |||
| Total Diet Quality Index (DQI, −33% to 100%) | 26.3 (9.0) | 33.1 (7.4) | 6.8 (2.2, 11.3) ** | 12.1 (10.0, 14.2) *** | 5.3 (0.9, 9.8) * |
| Dietary Quality DQI (DQ, −100% to 100%) | 49.5 (18.5) | 71.9 (14.1) | 22.3 (15.5, 29.2) *** | 29.5 (25.8, 33.2) *** | 7.2 (−0.3, 14.7) |
| Dietary Diversity DQI (DD, 0 to 100%) | 25.5 (13.9) | 19.2 (14.7) | −6.3 (−15.0, 2.5) | 1.4 (−2.8, 5.5) | 7.6 (−1.1, 16.3) |
| Dietary Equilibrium DQI (DA, 0 to 100%) | 3.9 (5.0) | 8.1 (4.0) | 4.2 (2.2, 6.3) *** | 5.4 (4.2, 6.6) *** | 1.2 (−1.2, 3.6) |
| Healthy Lifestyle Diet Index # (HLDI, 0 to 38 points) | 18.3 (2.7) | 19.7 (2.8) | 1.4 (−0.1, 2.9) | 4.1 (3.3, 4.9) *** | 2.7 (1.0, 4.3) ** |
| Mediterranean Diet Quality Index (KIDMED, 0–12 points) | 5.2 (1.8) | 7.2 (1.6) | 2.0 (0.9, 3.0) *** | 3.0 (2.5, 3.5) *** | 1.0 (−0.1, 2.1) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Rodríguez, A.; Zazpe, I.; Morell-Azanza, L.; Chueca, M.J.; Azcona-sanjulian, M.C.; Marti, A. Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention. Nutrients 2018, 10, 1500. https://doi.org/10.3390/nu10101500
Ojeda-Rodríguez A, Zazpe I, Morell-Azanza L, Chueca MJ, Azcona-sanjulian MC, Marti A. Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention. Nutrients. 2018; 10(10):1500. https://doi.org/10.3390/nu10101500
Chicago/Turabian StyleOjeda-Rodríguez, Ana, Itziar Zazpe, Lydia Morell-Azanza, María J. Chueca, Maria Cristina Azcona-sanjulian, and Amelia Marti. 2018. "Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention" Nutrients 10, no. 10: 1500. https://doi.org/10.3390/nu10101500
APA StyleOjeda-Rodríguez, A., Zazpe, I., Morell-Azanza, L., Chueca, M. J., Azcona-sanjulian, M. C., & Marti, A. (2018). Improved Diet Quality and Nutrient Adequacy in Children and Adolescents with Abdominal Obesity after a Lifestyle Intervention. Nutrients, 10(10), 1500. https://doi.org/10.3390/nu10101500






