10,12 Conjugated Linoleic Acid-Driven Weight Loss Is Protective against Atherosclerosis in Mice and Is Associated with Alternative Macrophage Enrichment in Perivascular Adipose Tissue
Abstract
:1. Introduction
2. Materials and Methods
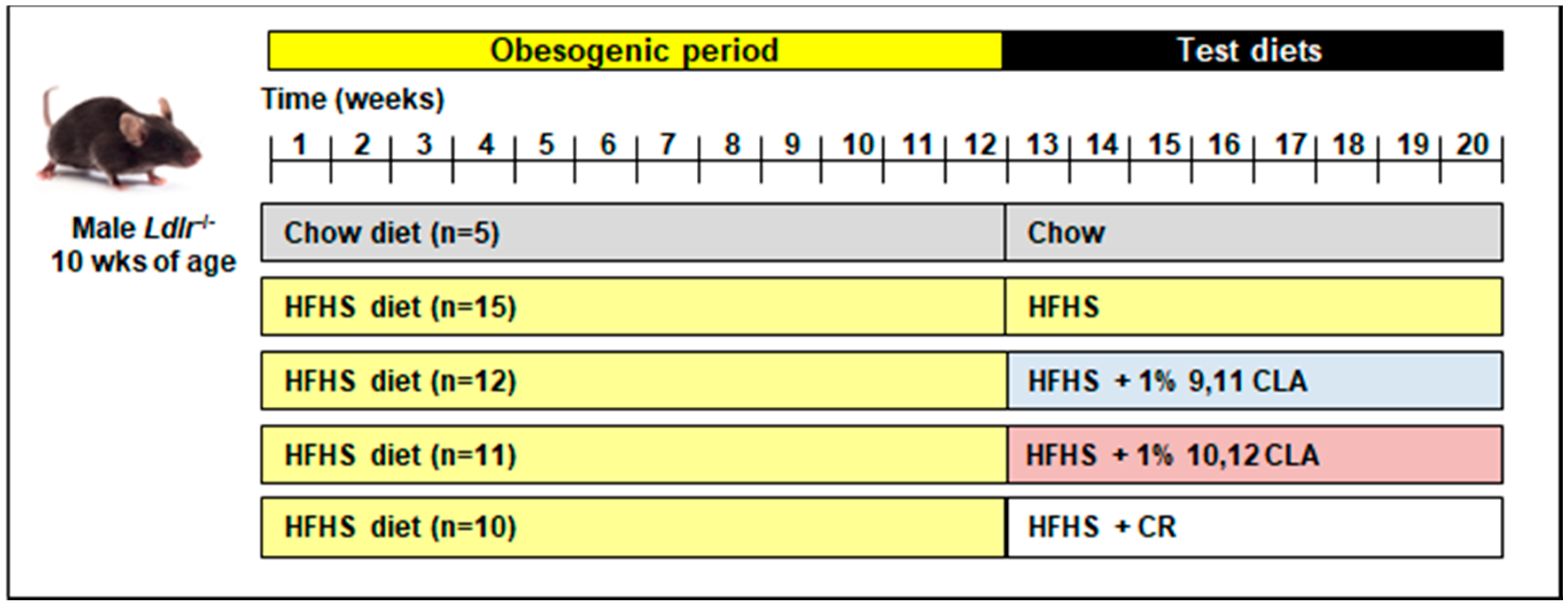
2.1. Mouse Study Design
2.2. Plasma Analyses
2.3. Atherosclerosis
2.4. Immunohistochemistry
2.5. Quantitative Real-Time PCR
2.6. Bone Marrow-Derived Macrophage Culture
2.7. Statistics
3. Results
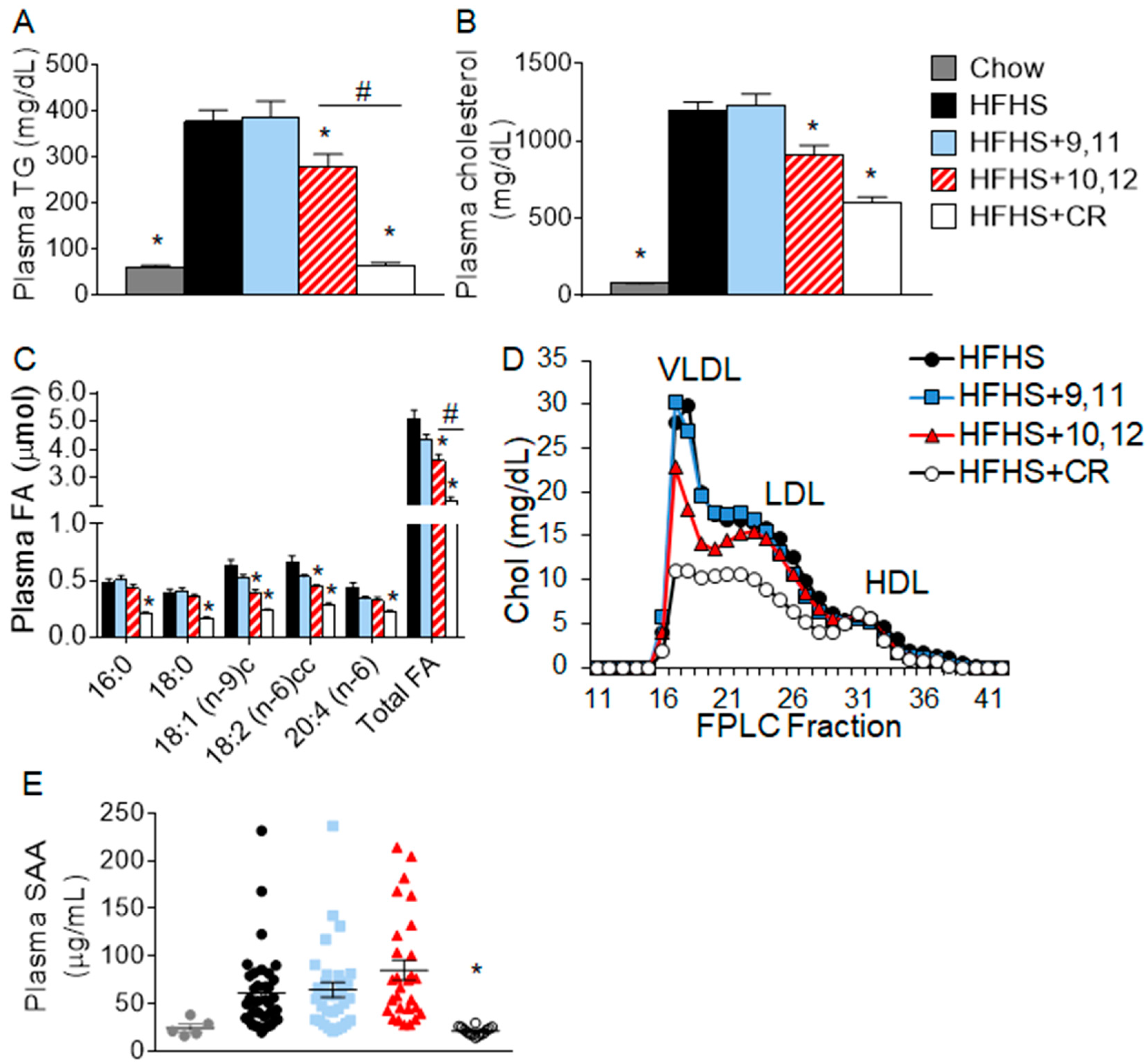
3.1. Weight Loss by 10,12 CLA and CR Improves Plasma Triglycerides, Cholesterol, Fatty Acids, and Lipoprotein Profiles
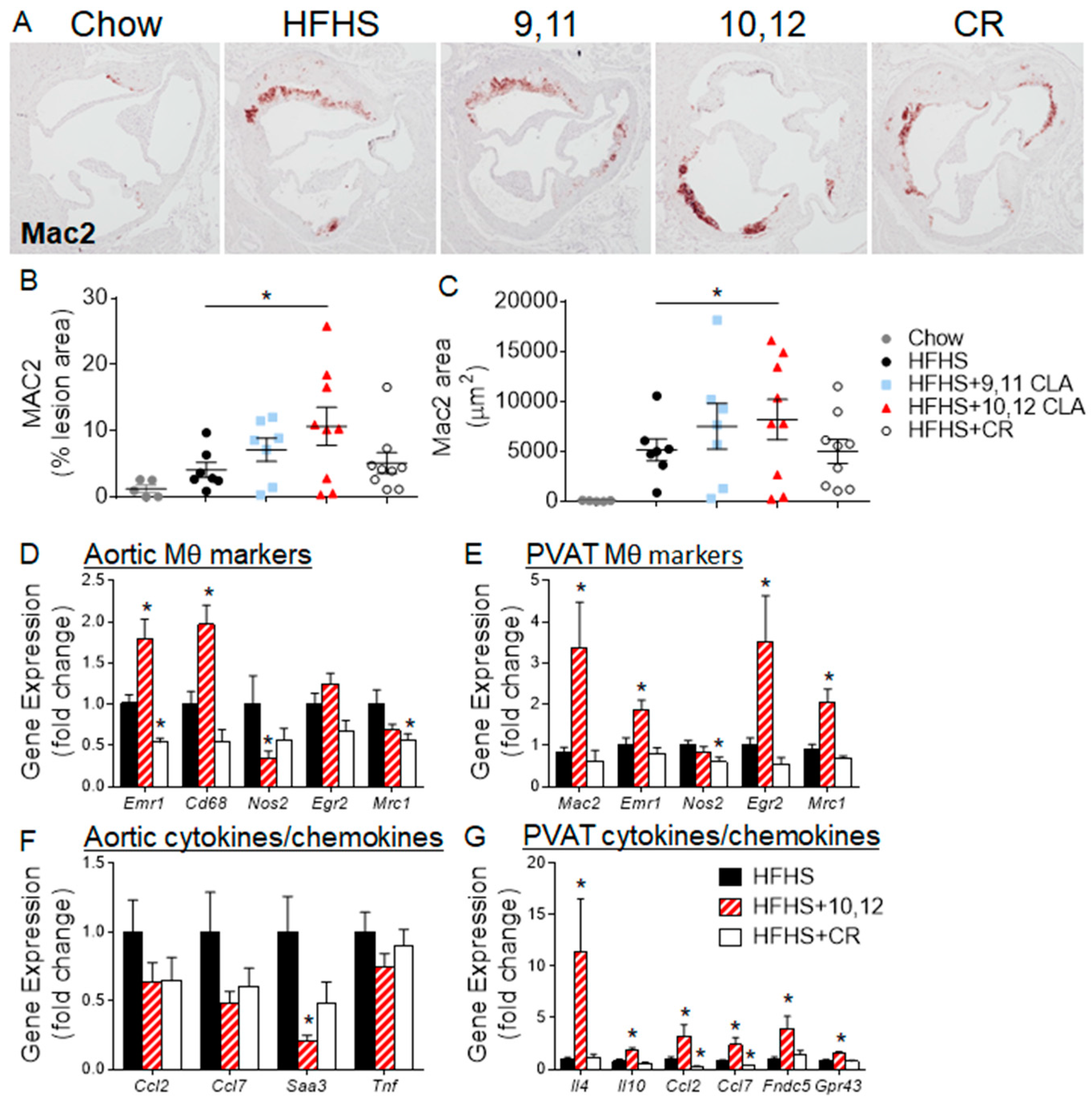
3.2. Weight Loss Following 10,12 CLA Supplementation Improves Atherosclerosis
3.3. Aortic Sinus Lesions Contain More Macrophages Following 10,12 CLA Supplementation
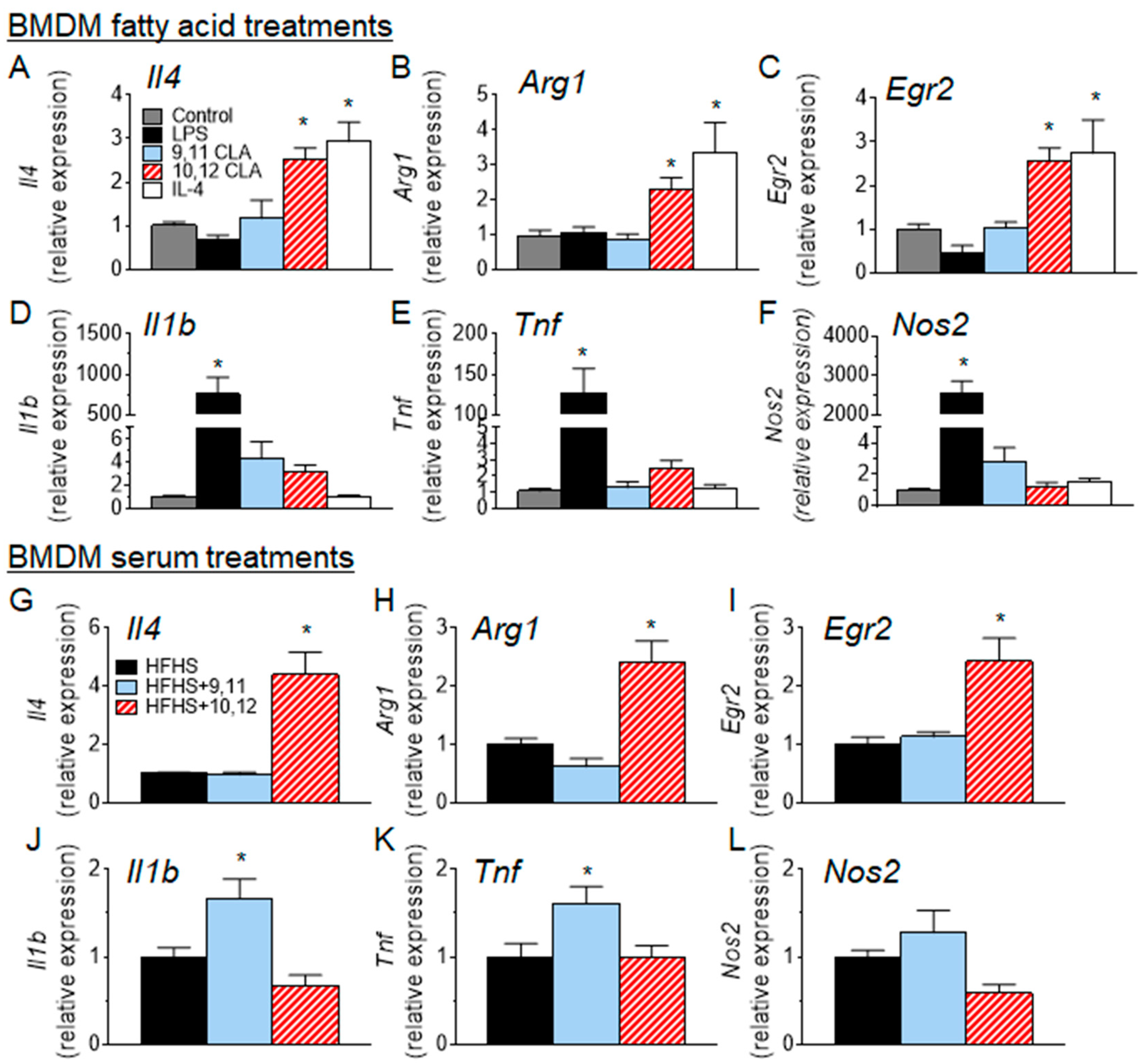
3.4. 10,12 CLA Induces a Resident Alternatively-Activated Macrophage Phenotype
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLA | conjugated linoleic acid |
| HFHS | high fat high sucrose |
| PVAT | perivascular adipose tissue |
| LDLR | low-density lipoprotein receptor |
| CR | caloric restriction |
| SAA | serum amyloid A |
| BMDM | bone marrow-derived macrophages |
| FPLC | fast phase liquid chromatography |
| TG | triglycerides |
| FA | fatty acids |
| SCFA | short-chain fatty acids |
References
- Ashwell, M.S.; Ceddia, R.P.; House, R.L.; Cassady, J.P.; Eisen, E.J.; Eling, T.E.; Collins, J.B.; Grissom, S.F.; Odle, J. Trans-10, cis-12-conjugated linoleic acid alters hepatic gene expression in a polygenic obese line of mice displaying hepatic lipidosis. J. Nutr. Biochem. 2010, 21, 848–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churruca, I.; Fernández-Quintela, A.; Zabala, A.; Macarulla, M.T.; Navarro, V.; Rodríguez, V.M.; Simón, E.; Milagro, F.; Portillo, M.P. The effect of trans-10, cis-12 conjugated linoleic acid on lipogenesis is tissue dependent in hamsters. Genes Nutr. 2007, 2, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, M.T.; Fernández-Quintela, A.; Zabala, A.; Navarro, V.; Echevarría, E.; Churruca, I.; Rodríguez, V.M.; Portillo, M.P. Effects of conjugated linoleic acid on liver composition and fatty acid oxidation are isomer-dependent in hamster. Nutrition 2005, 21, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; Wang, S.; Goodspeed, L.; Wietecha, T.; Houston, B.; Omer, M.; Ogimoto, K.; Subramanian, S.; Gowda, G.A.; O’Brien, K.D.; et al. Metabolically distinct weight loss by 10,12 CLA and caloric restriction highlight the importance of subcutaneous white adipose tissue for glucose homeostasis in mice. PLoS ONE 2017, 12, e0172912. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; Han, C.Y.; Wang, S.; Omer, M.; Chait, A. 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species. J. Lipid Res. 2013, 54, 2964–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.N.; Kritchevsky, D.; Pariza, M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Kritchevsky, D.; Tepper, S.A.; Wright, S.; Tso, P.; Czarnecki, S.K. Influence of conjugated linoleic acid (CLA) on establishment and progression of atherosclerosis in rabbits. J. Am. Coll. Nutr. 2000, 19, 472S–477S. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.L.; Karakach, T.K.; Currie, D.L.; McLeod, R.S. t-10, c-12 CLA Dietary Supplementation Inhibits Atherosclerotic Lesion Development Despite Adverse Cardiovascular and Hepatic Metabolic Marker Profiles. PLoS ONE 2012, 7, e52634. [Google Scholar] [CrossRef] [PubMed]
- Franczyk-Zarów, M.; Kostogrys, R.B.; Szymczyk, B.; Jawień, J.; Gajda, M.; Cichocki, T.; Wojnar, L.; Chlopicki, S.; Pisulewski, P.M. Functional effects of eggs, naturally enriched with conjugated linoleic acid, on the blood lipid profile, development of atherosclerosis and composition of atherosclerotic plaque in apolipoprotein E and low-density lipoprotein receptor double-knockout mice (apoE/LDLR−/−). Br. J. Nutr. 2008, 99, 49–58. [Google Scholar] [PubMed]
- Toomey, S.; Harhen, B.; Roche, H.M.; Fitzgerald, D.; Belton, O. Profound resolution of early atherosclerosis with conjugated linoleic acid. Atherosclerosis 2006, 187, 40–49. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Duffy, M.M.; Mooney, D.; James, W.G.; Griffin, M.D.; Fitzgerald, D.J.; Belton, O. IL-10 mediates the immunoregulatory response in conjugated linoleic acid-induced regression of atherosclerosis. FASEB J. 2013, 27, 499–510. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, M.; Alghamdi, K.; Marcone, S.; Belton, O. Conjugated linoleic acid induces an atheroprotective macrophage MΦ2 phenotype and limits foam cell formation. J. Inflamm. (Lond.) 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Kostogrys, R.B.; Franczyk-Żarów, M.; Maślak, E.; Gajda, M.; Mateuszuk, Ł.; Chłopicki, S. Effects of margarine supplemented with t10c12 and C9T11 CLA on atherosclerosis and steatosis in apoE/LDLR−/− mice. J. Nutr. Health Aging 2012, 16, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.H.; Miller, J.R.; Mitchell, P.L.; Currie, D.L.; McLeod, R.S. Conjugated linoleic acid isomers have no effect on atherosclerosis and adverse effects on lipoprotein and liver lipid metabolism in apoE−/− mice fed a high-cholesterol diet. Atherosclerosis 2008, 200, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.L.; Langille, M.A.; Currie, D.L.; McLeod, R.S. Effect of conjugated linoleic acid isomers on lipoproteins and atherosclerosis in the Syrian Golden hamster. Biochim. Biophys. Acta 2005, 1734, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Arbonés-Mainar, J.M.; Navarro, M.A.; Guzmán, M.A.; Arnal, C.; Surra, J.C.; Acín, S.; Carnicer, R.; Osada, J.; Roche, H.M. Selective effect of conjugated linoleic acid isomers on atherosclerotic lesion development in apolipoprotein E knockout mice. Atherosclerosis 2006, 189, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Do the Apoe−/− and Ldlr−/− Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Diet and murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Han, C.; Chiba, T.; McMillen, T.; Wang, S.; Haw, A.R.; Kirk, E.; O’Brien, K.; Chait, A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.E.; Kirk, E.A.; McDonald, T.O.; Wang, S.; Wight, T.N.; O’Brien, K.D.; Chait, A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation 2004, 110, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; Wang, S.; Goodspeed, L.; Ding, Y.; Averill, M.; Subramanian, S.; Wietecha, T.; O’Brien, K.D.; Chait, A. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS ONE 2014, 9, e108564. [Google Scholar] [CrossRef] [PubMed]
- Willecke, F.; Yuan, C.; Oka, K.; Chan, L.; Hu, Y.; Barnhart, S.; Bornfeldt, K.E.; Goldberg, I.J.; Fisher, E.A. Effects of High Fat Feeding and Diabetes on Regression of Atherosclerosis Induced by Low-Density Lipoprotein Receptor Gene Therapy in LDL Receptor-Deficient Mice. PLoS ONE 2015, 10, e0128996. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Subramanian, S.; Montes, V.N.; Goodspeed, L.; Wang, S.; Han, C.; Teresa, A.S.; Kim, J.; O’Brien, K.D.; Chait, A. Toll-like receptor 4 deficiency decreases atherosclerosis but does not protect against inflammation in obese low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Subramanian, S.; Ding, Y.; Goodspeed, L.; Wang, S.; Han, C.Y.; Teresa, A.S.; Kim, J.; O’Brien, K.D.; Chait, A. Inhibition of intestinal cholesterol absorption decreases atherosclerosis but not adipose tissue inflammation. J. Lipid Res. 2012, 53, 2380–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; He, X.; Shi, X.; Huang, C.; Liu, J.; Zhou, S.; Heng, C.K. Association between serum amyloid A and obesity: A meta-analysis and systematic review. Inflamm. Res. 2010, 59, 323–534. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L.J.; Gao, Z.; Goodspeed, L.; Wang, S.; Das, A.K.; Burant, C.F.; Chait, A.; Blaser, M.J. Obese Mice Losing Weight Due to trans-10,cis-12 Conjugated Linoleic Acid Supplementation or Food Restriction Harbor Distinct Gut Microbiota. J. Nutr. 2018, 148, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Toomey, S.; Roche, H.; Fitzgerald, D.; Belton, O. Regression of pre-established atherosclerosis in the apoE−/− mouse by conjugated linoleic acid. Biochem. Soc. Trans. 2003, 31, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E. Halting the progression of atherosclerosis with intensive lipid lowering: Results from the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) trial. Am. J. Med. 2005, 118, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Stock, J.K.; Ginsberg, H.N.; Forum, P. PCSK9 inhibitors and cardiovascular disease: Heralding a new therapeutic era. Curr. Opin. Lipidol. 2015, 26, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. Am. Heart J. 1999, 138, S419–S420. [Google Scholar] [CrossRef]
- Yamamoto, S.; Zhong, J.; Yancey, P.G.; Zuo, Y.; Linton, M.F.; Fazio, S.; Yang, H.; Narita, I.; Kon, V. Atherosclerosis following renal injury is ameliorated by pioglitazone and losartan via macrophage phenotype. Atherosclerosis 2015, 242, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.; Vengrenyuk, Y.; Ramsey, S.A.; Vila, N.R.; Girgis, N.M.; Liu, J.; Gusarova, V.; Gromada, J.; Weinstock, A.; Moore, K.J.; et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J. Clin. Investig. 2017, 127, 2904–2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, J.E.; Vengrenyuk, Y.; Reiser, V.; Wu, C.; Statnikov, A.; Aliferis, C.F.; Garabedian, M.J.; Fisher, E.A.; Puig, O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS ONE 2012, 7, e39790. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.N.; Shaked, I.; Hubbeling, H.G.; Punt, J.A.; Wu, R.; Herrley, E.; Zaugg, C.; Pei, H.; Geissmann, F.; Ley, K.; et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ. Res. 2012, 110, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Cardilo-Reis, L.; Gruber, S.; Schreier, S.M.; Drechsler, M.; Papac-Milicevic, N.; Weber, C.; Wagner, O.; Stangl, H.; Soehnlein, O.; Binder, C.J. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol. Med. 2012, 4, 1072–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, S.J.; Ruckerl, D.; Cook, P.C.; Jones, L.H.; Finkelman, F.D.; van Rooijen, N.; MacDonald, A.S.; Allen, J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011, 332, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J.; Pober, J.S. Interleukin-4 induces the synthesis and secretion of MCP-1/JE by human endothelial cells. Am. J. Pathol. 1991, 138, 1315–1319. [Google Scholar] [PubMed]
- Lee, Y.W.; Kühn, H.; Hennig, B.; Toborek, M. IL-4 induces apoptosis of endothelial cells through the caspase-3-dependent pathway. FEBS Lett. 2000, 485, 122–126. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Kühn, H.; Hennig, B.; Neish, A.S.; Toborek, M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell. Cardiol. 2001, 33, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Walch, L.; Massade, L.; Dufilho, M.; Brunet, A.; Rendu, F. Pro-atherogenic effect of interleukin-4 in endothelial cells: Modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis 2006, 187, 285–291. [Google Scholar] [CrossRef] [PubMed]
- King, V.L.; Szilvassy, S.J.; Daugherty, A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.; Tipping, P.G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2003, 163, 1117–1125. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lee, W.H.; Kim, P.H. Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium. Cytokine 2010, 49, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Villacorta, L.; Li, R.; Hamblin, M.; Xu, W.; Dou, C.; Zhang, J.; Wu, J.; Zeng, R.; Chen, Y.E. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 2012, 126, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ortega, M.; Somoza, B.; Huang, Y.; Gollasch, M.; Fernández-Alfonso, M.S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metab. 2015, 26, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.; Capettini, L.S.; Lemos, V.S.; Santos, R.A.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.C.; Santos, L.C.; Leonel, A.J.; de Oliveira, J.S.; Santos, E.A.; Navia-Pelaez, J.M.; da Silva, J.F.; Mendes, B.P.; Capettini, L.S.; Teixeira, L.G.; et al. Oral butyrate reduces oxidative stress in atherosclerotic lesion sites by a mechanism involving NADPH oxidase down-regulation in endothelial cells. J. Nutr. Biochem. 2016, 34, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.A.; Jackson, J.; Stanton, M.; Rojas-Triana, A.; Bober, L.; Laverty, M.; Yang, X.; Zhu, F.; Liu, J.; Wang, S.; et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 2009, 15, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Menzel, T.; Lührs, H.; Zirlik, S.; Schauber, J.; Kudlich, T.; Gerke, T.; Gostner, A.; Neumann, M.; Melcher, R.; Scheppach, W. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1. Inflamm. Bowel Dis. 2004, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lührs, H.; Gerke, T.; Müller, J.G.; Melcher, R.; Schauber, J.; Boxberge, F.; Scheppach, W.; Menzel, T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Shu, D.; Zheng, M.; Wang, J.; Luo, C.; Wang, Y.; Guo, F.; Zou, X.; Lv, X.; Li, Y.; et al. Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 2016, 6, 24838. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanter, J.E.; Goodspeed, L.; Wang, S.; Kramer, F.; Wietecha, T.; Gomes-Kjerulf, D.; Subramanian, S.; O’Brien, K.D.; Den Hartigh, L.J. 10,12 Conjugated Linoleic Acid-Driven Weight Loss Is Protective against Atherosclerosis in Mice and Is Associated with Alternative Macrophage Enrichment in Perivascular Adipose Tissue. Nutrients 2018, 10, 1416. https://doi.org/10.3390/nu10101416
Kanter JE, Goodspeed L, Wang S, Kramer F, Wietecha T, Gomes-Kjerulf D, Subramanian S, O’Brien KD, Den Hartigh LJ. 10,12 Conjugated Linoleic Acid-Driven Weight Loss Is Protective against Atherosclerosis in Mice and Is Associated with Alternative Macrophage Enrichment in Perivascular Adipose Tissue. Nutrients. 2018; 10(10):1416. https://doi.org/10.3390/nu10101416
Chicago/Turabian StyleKanter, Jenny E., Leela Goodspeed, Shari Wang, Farah Kramer, Tomasz Wietecha, Diego Gomes-Kjerulf, Savitha Subramanian, Kevin D. O’Brien, and Laura J. Den Hartigh. 2018. "10,12 Conjugated Linoleic Acid-Driven Weight Loss Is Protective against Atherosclerosis in Mice and Is Associated with Alternative Macrophage Enrichment in Perivascular Adipose Tissue" Nutrients 10, no. 10: 1416. https://doi.org/10.3390/nu10101416
APA StyleKanter, J. E., Goodspeed, L., Wang, S., Kramer, F., Wietecha, T., Gomes-Kjerulf, D., Subramanian, S., O’Brien, K. D., & Den Hartigh, L. J. (2018). 10,12 Conjugated Linoleic Acid-Driven Weight Loss Is Protective against Atherosclerosis in Mice and Is Associated with Alternative Macrophage Enrichment in Perivascular Adipose Tissue. Nutrients, 10(10), 1416. https://doi.org/10.3390/nu10101416




